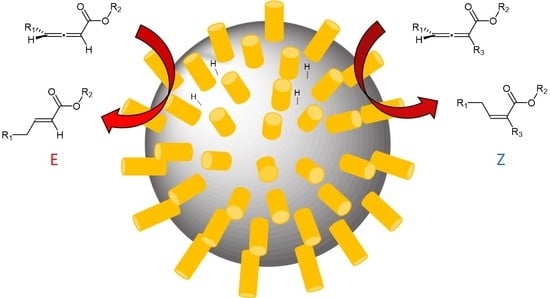
Alkanethiolate-Capped Palladium Nanoparticles for Regio- and Stereoselective Hydrogenation of Allenes
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Sodium S-Octylthiosulfate
3.3. Synthesis of Octanethiolate-Capped Palladium Nanoparticles (C8 PdNP)
3.4. Synthesis of Ethyl but-3-enoate (Compound 26)
3.5. Catalysis Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, T.-B.; Chou, T.-C. Selective hydrogenation of isoprene on eggshell and uniform palladium profile catalysts. Appl. Catal. A Gen. 1994, 108, 7–19. [Google Scholar] [CrossRef]
- Godínez, C.; Cabanes, A.L.; Víllora, G. Experimental study of the tail end selective hydrogenation of steam cracking C2-C3 mixture. Can. J. Chem. Eng. 1996, 74, 84–93. [Google Scholar] [CrossRef]
- Karakhanov, E.; Maximov, A.; Kardasheva, Y.; Semernina, V.; Zolotukhina, A.; Ivanov, A.; Abbott, G.; Rosenberg, E.; Vinokurov, V. Pd nanoparticles in dendrimers immobilized on silica–polyamine composites as catalysts for selective hydrogenation. ACS Appl. Mater. Interfaces 2014, 6, 8807–8816. [Google Scholar] [CrossRef] [PubMed]
- Shorthouse, L.J.; Haq, S.; Raval, R. Hydrocarbons with orthogonal π-systems: Molecular adsorption versus structural isomerisation at surfaces. Surf. Sci. 1996, 368, 296–302. [Google Scholar] [CrossRef]
- Crombie, L.; Jenkins, P.A.; Mitchard, D.A. Heterogeneous catalytic hydrogenation of allenes over supported palladium: Selectivity, stereoselectivity, and regioselectivity. J. Chem. Soc. Perkin Trans. 1975, 0, 1081–1090. [Google Scholar] [CrossRef]
- Mann, R.S.; Shah, A.M. The hydrogenation of allene. IV. The reaction of allene with hydrogen catalyzed by palladium, platinum, iridium, rhodium, ruthenium, and osmium catalysts. Can. J. Chem. 1972, 50, 1793–1796. [Google Scholar] [CrossRef]
- Baudouy, R.; Gore, J. Reduction d’alcools alleniques par l’hydrure et le methoxy hydrure de lithium et d’aluminium. Tetrahedron 1975, 31, 383–476. [Google Scholar] [CrossRef]
- Nagendrappa, G.; Devaprabhakara, D. Diimide reduction of allenes. Tetrahedron Lett. 1970, 11, 4243–4244. [Google Scholar] [CrossRef]
- Meyer, E.F.; Burwell, R.L. The reaction between deuterium and 1-butyne, 1,2-butadiene, and 1,3-butadiene on palladium-on-alumina catalyst. J. Am. Chem. Soc. 1963, 85, 2881–2887. [Google Scholar] [CrossRef]
- Oliver, R.G.; Wells, P.B. The hydrogenation of alkadienes: VIII. Deuterium tracer study of alkane formation in the palladium-catalyzed hydrogenation of propadiene and of 1,2-butadiene and its implications concerning the breakdown of selectivity in ethyne hydrogenation. J. Catal. 1977, 47, 364–370. [Google Scholar] [CrossRef]
- Guo, X.C.; Madix, R.J. Selective hydrogenation and H-D exchange of unsaturated hydrocarbons on Pd(100)-P(1×1)-H(D). J. Catal. 1995, 155, 336–344. [Google Scholar] [CrossRef]
- Carturan, G.; Strukul, G. Activation of H2 with allylpalladium(II) derivatives. Selective catalytic hydrogenation of allene to propene. J. Organomet. Chem. 1978, 157, 475–481. [Google Scholar] [CrossRef]
- Bhagwat, M.M.; Devaprabhakara, D. Selective hydrogenation of allenes with chlorotris-(triphenylphosphine) rhodium catalyst. Tetrahedron Lett. 1972, 13, 1391–1392. [Google Scholar] [CrossRef]
- Guo, H.; Zheng, Z.; Yu, F.; Ma, S.; Holuigue, A.; Tromp, D.S.; Elsevier, C.J.; Yu, Y. [Pd(Ar-BIAN)(alkene)]-catalyzed highly chemo-, regio-, and stereoselective semihydrogenation of 1,2-allenyl phosphonates and related compounds. Angew. Chem. Int. Ed. 2006, 45, 4997–5000. [Google Scholar] [CrossRef] [PubMed]
- Kukhar, V.P.; Hudson, H.R. Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity, 1st ed.; Wiley: Chichester, UK, 2000. [Google Scholar]
- Adler, P.; Comes, F.; Fadel, A.; Rabasso, N. Selective reduction of amino allenephosphonates: Preparation of -amino vinylphosphonates. Eur. J. Org. Chem. 2013, 7546–7555. [Google Scholar] [CrossRef]
- Chen, Z.; Dong, V.M. Enantioselective semireduction of allenes. Nature Commun. 2017, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- San, K.A.; Shon, Y.-S. Synthesis of alkanethiolate-capped metal nanoparticles using alkyl thiosulfate ligand precursors: A method to generate promising reagents for selective catalysis. Nanomaterials 2018, 8, 346. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.S.; Shon, Y.-S. Mechanistic interpretation of selective catalytic hydrogenation and isomerization of alkenes and dienes by ligand deactivated Pd nanoparticles. Nanoscale 2015, 7, 17786–17790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.-A.; Shon, Y.-S. Alkanethioate-capped palladium nanoparticle for selective catalytic hydrogenation of dienes and trienes. Catal. Sci. Technol. 2017, 7, 4823–4829. [Google Scholar] [CrossRef] [PubMed]
- Kahsar, K.R.; Schwartz, D.K.; Medlin, J.W. Selective hydrogenation of polyunsaturated fatty acids using alkanethiol self-assembled monolayer-coated Pd/Al2O3 catalysts. ACS Catal. 2013, 3, 2041–2044. [Google Scholar] [CrossRef]
- Raoult, Y.; Choukroun, R.; Basso-Bert, M.; Gervais, D. Hydrogenation and isomerization of olefins with diphenylphosphinomethyl hydride zirconium, [Cp2ZrH(CH2PPh2)]n, a selective homogeneous catalyst. J. Mol. Catal. 1992, 72, 47–58. [Google Scholar] [CrossRef]
- Chapuis, C.; Jacoby, D. Catalysis in the preparation of fragrances and flavours. Appl. Catal. A Gen. 2001, 221, 93–117. [Google Scholar] [CrossRef]
- Bond, G.C.; Rawle, A.F. Catalytic hydrogenation in the liquid phase. Part 1. Hydrogenation of isoprene catalysed by palladium, palladium-gold and palladium-silver catalysts. J. Mol. Catal. Chem. 1996, 109, 261–271. [Google Scholar] [CrossRef]
- Schrock, R.R.; Osborn, J.A. Catalytic hydrogenation using cationic rhodium complexes. 3. The selective hydrogenation of dienes to monoenes. J. Am. Chem. Soc. 1976, 98, 4450–4455. [Google Scholar] [CrossRef]
- Kumar, G.; Lien, C.-H.; Janik, M.J.; Medlin, J.W. Catalyst site selection via control over noncovalent interactions in self-assembled monolayers. ACS Catal. 2016, 6, 5086–5094. [Google Scholar] [CrossRef]
- Ortuño, M.A.; Lỏpez, N. Creating cavities at palladium-phosphine interfaces for enhanced selectivity in heterogeneous biomass conversion. ACS Catal. 2018, 8, 6138–6145. [Google Scholar] [CrossRef]
- Wang, S.; Vorotnikov, V.; Vlachos, D.G. Coverage-induced conformational effects on activity and selectivity: Hydrogenation and decarbonylation of furfural on Pd(111). ACS Catal. 2015, 5, 104–112. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Nicponski, D.; Kim, B. Total regio- and diastereocontrol in the Aldol reactions of dienolborinates. Org. Lett. 2013, 15, 1398–1401. [Google Scholar] [CrossRef] [PubMed]


| Substrate | Product Composition | |||
|---|---|---|---|---|
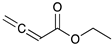 1 | ||||
| Reaction Time | 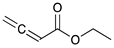 | 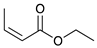 | 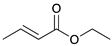 |  |
| 1 | 2 | 3 | 4 | |
| 0.5 h | 86% | 13% | 1% | 0% |
| 1 h | 71% | 26% | 3% | 0% |
| 2 h | 10% | 84% | 6% | 0% |
| 3 h | 0% | 76% | 24% | 0% |
| 4 h | 0% | 67% | 30% | 3% |
| 24 h | 0% | 27% | 69% | 4% |
| 36 h | 0% | 8% | 86% | 6% |
| Substrate Reaction Time | Product Composition | Conv. | ||
|---|---|---|---|---|
| cis or Z | trans or E | Full Hydrogenation | ||
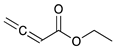 |  | 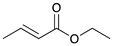 | 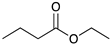 | |
| 1 | 2 | 3 | 4 | |
| 24 h | 27% | 69% | 4% | 100% |
| 36 h | 8% | 86% | 6% | 100% |
 | 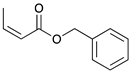 | 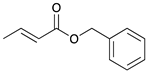 | 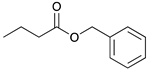 | |
| 5 | 6 | 7 | 8 | |
| 24 h | 26% | 70% | 4% | 100% |
| 36 h | 14% | 82% | 4% | 100% |
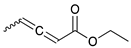 | 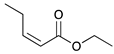 | 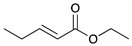 | 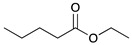 | |
| 9 | 10 | 11 | 12 | |
| 24 h | 20% | 71% | 9% | 100% |
| 48 h | 0% | 82% | 18% | 100% |
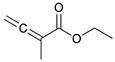 |  | 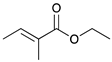 |  | |
| 13 | 14 | 15 | 16 | |
| 24 h | 86% | 14% | 0% | 100% |
| 36 h | 73% | 27% | 0% | 100% |
 | 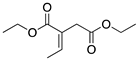 | 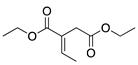 | 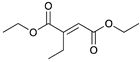 | |
| 17 | 18 | 19 | 20 | |
| 24 h & 48 h | 90% | 10% | 0% | 100% |
| Substrate | Product Composition | ||||
|---|---|---|---|---|---|
 | |||||
| 21 | |||||
| Reaction Time | 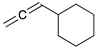 | 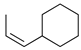 | 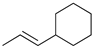 | 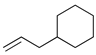 | 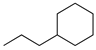 |
| 21 | 22 | 23 | 24 | 25 | |
| 2 h | 42% | 36% | 7% | 17% | 0% |
| 4 h | 0% | 63% | 9% | 28% | 0% |
| 24 h | 0% | 37% | 52% | 0% | 11% |
| Reactant | Product | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Trial 1 | 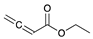 |  | 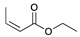 | + | 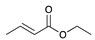 | + |  | ||
| 1a | 2 | 3 | 4 | ||||||
| 0% | 27% | 69% | 4% | ||||||
| Trial 2 | 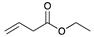 |  | 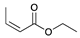 | + | 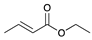 | + | 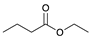 | ||
| 26a | 2 | 3 | 4 | ||||||
| 0% | 7% | 76% | 17% | ||||||
| Trial 3 | 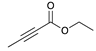 |  | 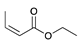 | + | 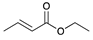 | + | 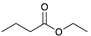 | ||
| 27a | 2 | 3 | 4 | ||||||
| 3% | 86% | 10% | 1% | ||||||
| 0% b | 23% b | 73% b | 4% b | ||||||
| Trial 4 |  | + | 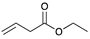 |  | 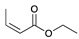 | + | 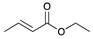 | + | 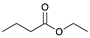 |
| 1c | 26c | 2 | 3 | 4 | |||||
| 0% | 0% | 22% | 67% | 11% | |||||
| Trial 5 |  | + | 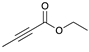 |  | 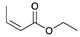 | + | 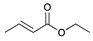 | + |  |
| 1c | 27c | 2 | 3 | 4 | |||||
| 0% | 0% | 66% | 32% | 2% | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-A.; Shon, Y.-S. Alkanethiolate-Capped Palladium Nanoparticles for Regio- and Stereoselective Hydrogenation of Allenes. Catalysts 2018, 8, 428. https://doi.org/10.3390/catal8100428
Chen T-A, Shon Y-S. Alkanethiolate-Capped Palladium Nanoparticles for Regio- and Stereoselective Hydrogenation of Allenes. Catalysts. 2018; 8(10):428. https://doi.org/10.3390/catal8100428
Chicago/Turabian StyleChen, Ting-An, and Young-Seok Shon. 2018. "Alkanethiolate-Capped Palladium Nanoparticles for Regio- and Stereoselective Hydrogenation of Allenes" Catalysts 8, no. 10: 428. https://doi.org/10.3390/catal8100428