Application of Lecitase® Ultra-Catalyzed Hydrolysis to the Kinetic Resolution of (E)-4-phenylbut-3-en-2-yl Esters
Abstract
:1. Introduction
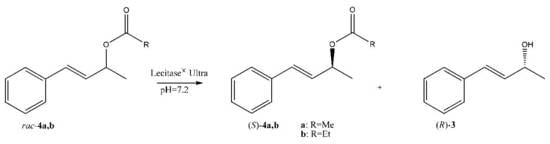
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Analysis
3.3. Synthesis of Esters 4a,b
3.3.1. (E)-4-Phenylbut-3-en-2-one (2)
3.3.2. Rac-(E)-4-Phenylbut-3-en-2-ol (3)
3.3.3. (E)-4-Phenylbut-3-en-3-yl Acetate (4a) and (E)-4-Phenylbut-3-en-3-yl Propionate (4b)
3.4. General Procedure for Enzymatic Hydrolysis of Allyl Esters (4a,b)
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hayes, P.Y.; Chow, S.; Rahm, F.; Bernhardt, P.V.; De Voss, J.J.; Kitching, W. Synthesis of the sponge-derived plakortone series of bioactive compounds. J. Org. Chem. 2010, 75, 6489–6501. [Google Scholar] [CrossRef] [PubMed]
- Grudniewska, A.; Dancewicz, K.; Białońska, A.; Ciunik, Z.; Gabryś, B.; Wawrzeńczyk, C. Synthesis of piperitone-derived halogenated lactones and their effect on aphid probing, feeding, and settling behavior. RSC Adv. 2011, 1, 498–510. [Google Scholar] [CrossRef]
- Mehl, F.; Bombarda, I.; Vanthuyne, N.; Faure, R.; Gaydou, E.M. Hemisynthesis and odour properties of δ-hydroxy-γ-lactones and precursors derived derived from linalool. Food Chem. 2010, 121, 98–104. [Google Scholar] [CrossRef]
- Tashiro, T.; Mori, K. Enzyme-assisted synthesis of (S)-1,3-dihydroxy-3,7-dimethyl-6-octen-2-one, the male-produced aggregation pheromone of the Colorado potato beetle, and its (R)-enantiomer. Tetrahedron Asymmetry 2005, 16, 1801–1806. [Google Scholar] [CrossRef]
- Kamezawa, M.; Raku, T.; Tachibana, H.; Ohtani, T.; Naoshima, Y. Enzymatic hydrolysis of alken- and alkyn-3-ol acetates in an acetone—Water solvent system: Effect of unsaturation on the enantioselectivity of pseudomonas cepacia lipase-catalyzed hydrolysis. Biosci. Biotechnol. Biochem. 1995, 59, 549–551. [Google Scholar] [CrossRef]
- Ohtani, T.; Nakatsukasa, H.; Kamezawa, M.; Tachibana, H.; Naoshima, Y. Enantioselectivity of Candida antarctica lipase for some synthetic substrates including aliphatic secondary alcohols. J. Mol. Catal. B Enzym. 1998, 4, 53–60. [Google Scholar] [CrossRef]
- Singh, A.; Goel, Y.; Rai, A.K.; Banerjee, U.C. Lipase catalyzed kinetic resolution for the production of (S)-3-[5-(4-fluoro-phenyl)-5-hydroxy-pentanoyl]-4-phenyl-oxazolidin-2-one: An intermediate for the synthesis of ezetimibe. J. Mol. Catal. B Enzym. 2013, 85–86, 99–104. [Google Scholar] [CrossRef]
- Angajala, G.; Pavan, P.; Subashini, R. Lipases: An overview of its current challenges and prospectives in the revolution of biocatalysis. Biocatal. Agric. Biotechnol. 2016, 7, 257–270. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Trono, D. Recombinant lipases and phospholipases and their use as biocatalysts for industrial applications. Int. J. Mol. Sci. 2015, 16, 20774–20840. [Google Scholar] [CrossRef] [PubMed]
- De Maria, L.; Vind, J.; Oxenbøll, K.M.; Svendsen, A.; Patkar, S. Phospholipases and their industrial applications. Appl. Microbiol. Biotechnol. 2007, 74, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Vijeeta, T.; Reddy, J.R.C.; Rao, B.V.S.K.; Karuna, M.S.L.; Prasad, R.B.N. Phospholipase-mediated preparation of 1-ricinoleoyl-2-acyl-sn-glycero-3-phosphocholine from soya and egg phosphatidylcholine. Biotechnol. Lett. 2004, 26, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.J.; Wang, X.Y.; Yang, D.; Zhang, H.; Shin, J.A.; Hong, S.T.; Park, S.H.; Lee, K.T. Emulsifying properties of lecithin containing different fatty acids obtained by immobilized Lecitase Ultra-catalyzed reaction. J. Am. Oil Chem. Soc. 2014, 91, 579–590. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Palomo, J.M.; Guisan, J.M.; Fernandez-Lafuente, R. Effect of the immobilization protocol in the activity, stability, and enantioselectivity of Lecitase® Ultra. J. Mol. Catal. B Enzym. 2007, 47, 99–104. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Cabrera, Z.; Godoy, C.; Fernandez-Lafuente, R.; Palomo, J.M.; Guisan, J.M. Interfacially activated lipases against hydrophobic supports: Effect of the support nature on the biocatalytic properties. Process Biochem. 2008, 43, 1061–1067. [Google Scholar] [CrossRef]
- Mishra, M.K.; Kumaraguru, T.; Sheelu, G.; Fadnavis, N.W. Lipase activity of Lecitase® Ultra: Characterization and applications in enantioselective reactions. Tetrahedron Asymmetry 2009, 20, 2854–2860. [Google Scholar] [CrossRef]
- Mishra, M.K.; Harini, M.; Kumaraguru, T.; Lakshmi Prasanna, T.; Fadnavis, N.W. A porous vessel bioreactor for gel entrapped biocatalysts: Kinetic resolution of trans-methyl (4-methoxyphenyl)glycidate by Lecitase® Ultra in gelatin organogel (Gelozyme). J. Mol. Catal. B Enzym. 2011, 71, 56–62. [Google Scholar] [CrossRef]
- Cabrera, Z.; Fernandez-Lorente, G.; Palomo, J.M.; Guisan, J.M.; Fernandez-Lafuente, R. Asymmetric hydrolysis of dimethyl 3-phenylglutarate catalyzed by Lecitase Ultra®. Effect of the immobilization protocol on its catalytic properties. Enzym. Microb. Technol. 2008, 43, 531–536. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Filice, M.; Terreni, M.; Guisan, J.M.; Fernandez-Lafuente, R.; Palomo, J.M. Lecitase® Ultra as regioselective biocatalyst in the hydrolysis of fully protected carbohydrates. Strong modulation by using different immobilization protocols. J. Mol. Catal. B Enzym. 2008, 51, 110–117. [Google Scholar] [CrossRef]
- Brenna, E.; Fuganti, C.; Grasselli, P.; Serra, S. Enzyme-mediated synthesis of (S) and (R)-verapamil. Eur. J. Org. Chem. 2001, 2001, 1349–1357. [Google Scholar] [CrossRef]
- Brenna, E.; Caraccia, N.; Fuganti, C.; Fuganti, D.; Grasselli, P. Enantioselective synthesis of β-substituted butyric acid derivatives via orthoester Claisen rearrangement of enzymatically resolved allylic alcohols: Application to the synthesis of (R)-(−)-baclofen. Tetrahedron Asymmetry 1997, 8, 3801–3805. [Google Scholar] [CrossRef]
- Gładkowski, W.; Skrobiszewski, A.; Mazur, M.; Gliszczyńska, A.; Czarnecka, M.; Pawlak, A.; Obmińska-Mrukowicz, B.; Maciejewska, G.; Białońska, A. Chiral δ-iodo-γ-lactones derived from cuminaldehyde, 2,5-dimethylbenzaldehyde and piperonal: Chemoenzymatic synthesis and antiproliferative activity. Tetrahedron Asymmetry 2016, 27, 227–237. [Google Scholar] [CrossRef]
- Morgan, B.; Oehlschlager, A.C.; Stokest, T.M. Enzyme Reactions in Apolar Solvent. 5. The effect of adjacent unsaturation on the PPL-catalyzed kinetic resolution of secondary alcohols. J. Org. Chem. 1992, 57, 3231–3236. [Google Scholar] [CrossRef]
- Brenna, E.; Fuganti, C.; Gatti, F.G.; Passoni, M.; Serra, S. Enantioselective synthesis of benzylic stereocentres via Claisen rearrangement of enantiomerically pure allylic alcohols: Preparation of (R) and (S)-3-methyl-2-phenylbutylamine. Tetrahedron Asymmetry 2003, 14, 2401–2406. [Google Scholar] [CrossRef]
- Lindner, E.; Ghanem, A.; Warad, I.; Eichele, K.; Mayer, A. Asymmetric hydrogenation of an α,β-unsaturated ketone by diamine (ether–phosphine) ruthenium (II) complexes and lipase-catalyzed kinetic resolution: A consecutive approach. Tetrahedron Asymmetry 2003, 14, 1045–1053. [Google Scholar] [CrossRef]
- Zhu, L.; Kedenburg, J.P.; Xian, M.; Wang, P.G. A systematic strategy for preparation of uncommon sugars through enzymatic resolution and ring-closing metathesis. Tetrahedron Lett. 2005, 46, 811–813. [Google Scholar] [CrossRef]
- Ghanem, A.; Schurig, V. Lipase-catalyzed access to enantiomerically pure (R) and (S)-trans-4-phenyl-3-butene-2-ol. Tetrahedron Asymmetry 2003, 14, 57–62. [Google Scholar] [CrossRef]
- Gładkowski, W.; Gliszczyńska, A.; Siepka, M.; Czarnecka, M.; Maciejewska, G. Kinetic resolution of (E)-4-(2′,5′-dimethylphenyl)-but-3-en-2-ol and (E)-4-(benzo[d][1′,3′]dioxol-5′-yl)-but-3-en-2-ol through lipase-catalyzed transesterification. Tetrahedron Asymmetry 2015, 26, 702–709. [Google Scholar] [CrossRef]
- Gładkowski, W.; Skrobiszewski, A.; Mazur, M.; Siepka, M.; Białońska, A. Convenient chemoenzymatic route to optically active β-aryl-δ-iodo-γ-lactones and β-aryl-γ-iodo-δ-lactones with the defined configurations of stereogenic centers. Eur. J. Org. Chem. 2015, 2015, 605–615. [Google Scholar] [CrossRef]
- Kazlauskas, R.J.; Weissfloch, A.N.E.; Rappaport, A.T.; Cuccia, L.A. A rule to predict which enantiomer of a secondary alcohol reacts faster in reactions catalyzed by cholesterol esterase, lipase from Pseudomonas cepacia, and lipase from Candida rugosa. J. Org. Chem. 1991, 56, 2656–2665. [Google Scholar] [CrossRef]
- Rahman, A.F.M.M.; Ali, R.; Jahng, Y.; Kadi, A.A. A facile solvent free claisen-schmidt reaction: Synthesis of α,α’-bis-(Substituted-benzylidene)cycloalkanones and α,α’-bis-(Substituted-alkylidene)cycloalkanones. Molecules 2012, 17, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Birman, V.B.; Jiang, H. Kinetic resolution of alcohols using a 1,2-dihydroimidazo[1,2-a]quinoline enantioselective acylation catalyst. Org. Lett. 2005, 7, 3445–3447. [Google Scholar] [CrossRef] [PubMed]




| Entry | Substrate | T [°C] | t [h] | c [%] 1 | ees [%] | eep [%] | E2 |
|---|---|---|---|---|---|---|---|
| 1 | 4a | 20 | 168 | 40 | 56 | 85 | 22 |
| 2 | 4a | 30 | 168 | 32 | 38 | 80 | 13 |
| 3 | 4a | 40 | 168 | 40 | 27 | 41 | 3 |
| 4 | 4b | 20 | 120 | 26 | 31 | 90 | 26 |
| 5 | 4b | 30 | 144 | 26 | 32 | 93 | 38 |
| 6 | 4b | 40 | 120 | 27 | 29 | 80 | 12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leśniarek, A.; Chojnacka, A.; Gładkowski, W. Application of Lecitase® Ultra-Catalyzed Hydrolysis to the Kinetic Resolution of (E)-4-phenylbut-3-en-2-yl Esters. Catalysts 2018, 8, 423. https://doi.org/10.3390/catal8100423
Leśniarek A, Chojnacka A, Gładkowski W. Application of Lecitase® Ultra-Catalyzed Hydrolysis to the Kinetic Resolution of (E)-4-phenylbut-3-en-2-yl Esters. Catalysts. 2018; 8(10):423. https://doi.org/10.3390/catal8100423
Chicago/Turabian StyleLeśniarek, Aleksandra, Anna Chojnacka, and Witold Gładkowski. 2018. "Application of Lecitase® Ultra-Catalyzed Hydrolysis to the Kinetic Resolution of (E)-4-phenylbut-3-en-2-yl Esters" Catalysts 8, no. 10: 423. https://doi.org/10.3390/catal8100423