Versatile Synthesis of Pd and Cu Co-Doped Porous Carbon Nitride Nanowires for Catalytic CO Oxidation Reaction
Abstract
:1. Introduction
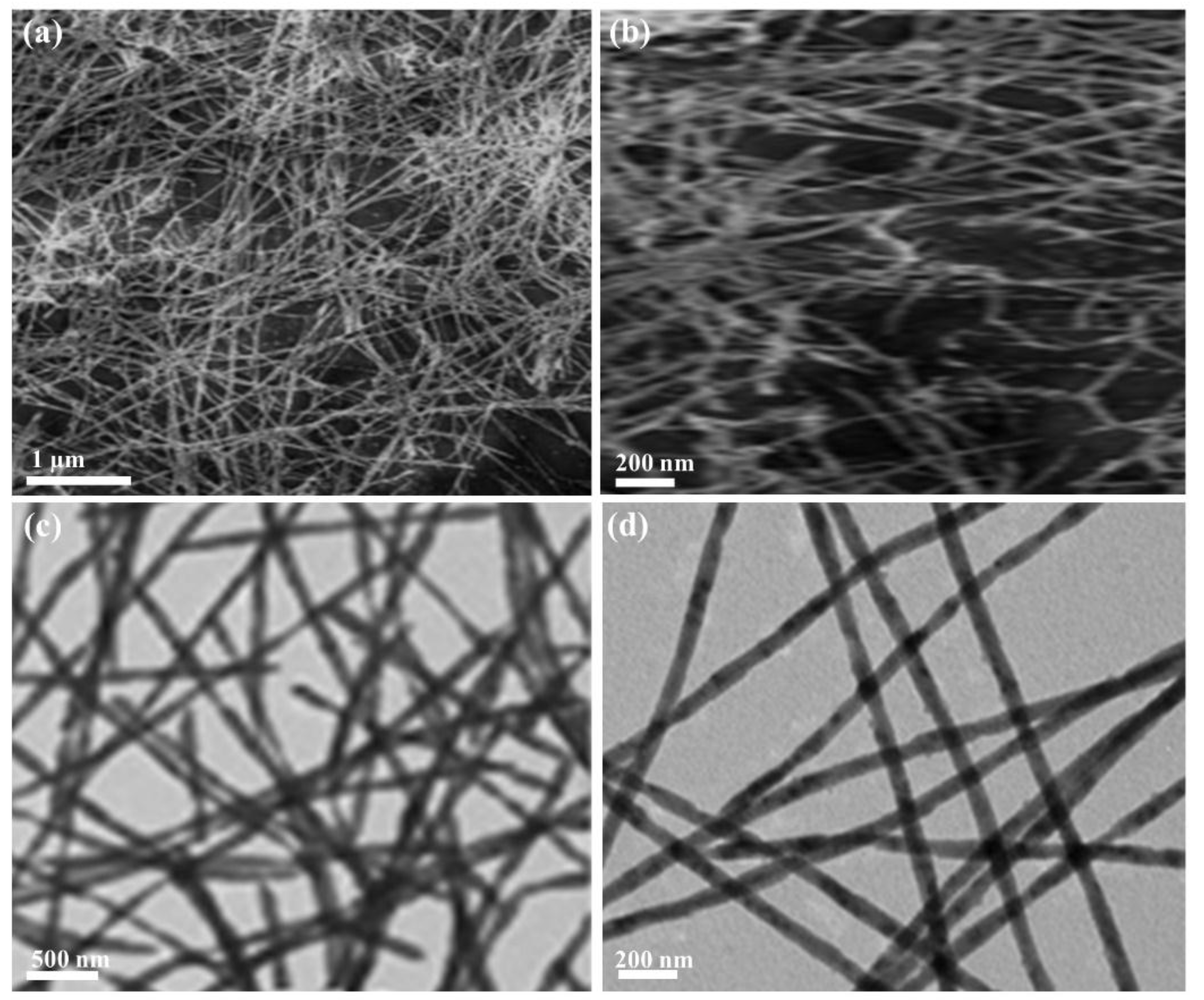
2. Results and Discussion
3. Experimental
3.1. Chemicals and Materials
3.2. Synthesis of Pd/Cu/gCN NWs
3.3. Materials Characterization
3.4. CO Oxidation Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric graphitic carbon nitride for heterogeneous photocatalysis. Acs Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Zhang, H.; Zuo, X.; Tang, H.; Li, G.; Zhou, Z. Origin of photoactivity in graphitic carbon nitride and strategies for enhancement of photocatalytic efficiency: Insights from first-principles computations. Phys. Chem. Chem. Phys. 2015, 17, 6280–6288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, L.; Sheng, X.; Ma, M.; Jung, M.S.; Kim, W.; Lee, H.; Park, J.H. Tunable bandgap energy and promotion of H2O2 oxidation for overall water splitting from carbon nitride nanowire bundles. Adv. Energy Mater. 2016, 6, 1502352. [Google Scholar] [CrossRef]
- Luan, P.; Zhang, Y.; Zhang, X.; Li, Z.; Prathapan, R.; Bach, U.; Zhang, J. Bismuth vanadate with electrostatically anchored 3d carbon nitride nano-networks as efficient photoanodes for water oxidation. ChemSusChem 2018, 11, 2510–2516. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Li, J.; Dong, F.; Sun, Y.; Jiang, G.; Cen, W.; Lee, S.C.; Wu, Z. Highly efficient performance and conversion pathway of photocatalytic NO oxidation on SrO-clusters@amorphous carbon nitride. Environ. Sci. Technol. 2017, 51, 10682–10690. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, S.; Wang, D.; Chen, Q. Interface engineering of Ru–Co3O4 nanocomposites for enhancing CO oxidation. J. Mater. Chem. A 2018, 6, 11037–11043. [Google Scholar] [CrossRef]
- Twigg, M.V. Catalytic control of emissions from cars. Catal. Today 2011, 163, 33–41. [Google Scholar] [CrossRef]
- Heck, R.M.; Farrauto, R.J. Automobile exhaust catalysts. Appl. Catal. A: Gen. 2001, 221, 443–457. [Google Scholar] [CrossRef]
- Wang, C.; Li, B.; Lin, H.; Yuan, Y. Carbon nanotube-supported Pt-Co bimetallic catalysts for preferential oxidation of CO in a H2-rich stream with CO2 and H2O vapor. J. Power Sources 2012, 202, 200–208. [Google Scholar] [CrossRef]
- Liu, K.; Wang, A.; Zhang, T. Recent advances in preferential oxidation of CO reaction over platinum group metal catalysts. ACS Catal. 2012, 2, 1165–1178. [Google Scholar] [CrossRef]
- Morfin, F.; Nassreddine, S.; Rousset, J.; Piccolo, L. Nanoalloying effect in the preferential oxidation of CO over Ir–Pd catalysts. Acs Catal. 2012, 2, 2161–2168. [Google Scholar] [CrossRef]
- Park, E.D.; Lee, D.; Lee, H.C. Recent progress in selective CO removal in a H2-rich stream. Catal. Today 2009, 139, 280–290. [Google Scholar] [CrossRef]
- He, F.; Li, K.; Yin, C.; Wang, Y.; Tang, H.; Wu, Z. Single Pd atoms supported by graphitic carbon nitride, a potential oxygen reduction reaction catalyst from theoretical perspective. Carbon 2017, 114, 619–627. [Google Scholar] [CrossRef]
- Yang, H.; Lv, K.; Zhu, J.; Li, Q.; Tang, D.; Ho, W.; Li, M.; Carabineiro, S.A. Effect of mesoporous g-C3N4 substrate on catalytic oxidation of CO over Co3O4. Appl. Surf. Sci. 2017, 401, 333–340. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, X.; Zhao, J.; Zhou, X.; Zhu, B.; Zhang, S.; Huang, W. Co oxidation over Cu2O deposited on 2d continuous lamellar gC3N4. New J. Chem. 2015, 39, 6642–6648. [Google Scholar] [CrossRef]
- Li, S.-L.; Yin, H.; Kan, X.; Gan, L.-Y.; Schwingenschlögl, U.; Zhao, Y. Potential of transition metal atoms embedded in buckled monolayer gC3N4 as single-atom catalysts. Phys. Chem. Chem. Phys. 2017, 19, 30069–30077. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Wang, Q.; Yan, X.; Zhang, X.; He, C.; Zhou, D.; Tang, Y.; Lu, Z.; Yang, Z. 3d transition metal embedded C2N monolayers as promising single-atom catalysts: A first-principles study. Carbon 2016, 105, 463–473. [Google Scholar] [CrossRef]
- Binder, A.J.; Qiao, Z.-A.; Veith, G.M.; Dai, S. Deposition–precipitation and stabilization of a silica-supported Au catalyst by surface modification with carbon nitride. Catal. Lett. 2013, 143, 1339–1345. [Google Scholar] [CrossRef]
- Leng, K.; Mai, W.; Zhang, X.; Liu, R.; Lin, X.; Huang, J.; Lou, H.; Xie, Y.; Fu, R.; Wu, D. Construction of functional nanonetwork-structured carbon nitride with Au nanoparticle yolks for highly efficient photocatalytic applications. Chem. Commun. 2018, 54, 7159–7162. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Zhao, Y.; Wang, T.; Zhan, Y.; Wang, H.; Li, J.; Thomas, A.; Zhu, J. Polymeric carbon nitride/mesoporous silica composites as catalyst support for Au and Pt nanoparticles. Chem. Eur. J. 2014, 20, 2872–2878. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Feng, Y.; Yu, M.; Wan, Q.; Lin, S. Confined catalysis in the g-C3N4/Pt (111) interface: Feasible molecule intercalation, tunable molecule–metal interaction, and enhanced reaction activity of CO oxidation. ACS Appl. Mater. Interfaces 2017, 9, 33267–33273. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Guan, N.; Li, L. Low temperature CO oxidation on Cu–Cu2O/TiO2 catalyst prepared by photodeposition. Catal. Sci. Technol. 2011, 1, 601–608. [Google Scholar] [CrossRef]
- Mock, S.A.; Zell, E.T.; Hossain, S.T.; Wang, R. Effect of reduction treatment on CO oxidation with CeO2 nanorod-supported cuox catalysts. ChemCatChem 2018, 10, 311–319. [Google Scholar] [CrossRef]
- Slavinskaya, E.; Gulyaev, R.; Zadesenets, A.; Stonkus, O.; Zaikovskii, V.; Shubin, Y.V.; Korenev, S.; Boronin, A. Low-temperature CO oxidation by Pd/CeO2 catalysts synthesized using the coprecipitation method. Appl. Catal. B Environ. 2015, 166, 91–103. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Liu, C. Perspective on CO oxidation over Pd-based catalysts. Catal. Sci. Technol. 2015, 5, 69–81. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, S.; Ning, D.; Zhang, Q.; Han, W.; Wang, Y. The template-free synthesis of CuO@CeO2 nanospheres: Facile strategy, structure optimization, and enhanced catalytic activity toward CO oxidation. Eur. J. Inorg. Chem. 2018, 2018, 2927–2934. [Google Scholar] [CrossRef]
- Ding, J.; Li, L.; Li, H.; Chen, S.; Fang, S.; Feng, T.; Li, G. Optimum preferential oxidation performance of CeO2–CuOx–rGO composites through interfacial regulation. ACS Appl. Mater. Interfaces 2018, 10, 7935–7945. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Ding, T.; Gao, W.; Ma, K.; Tian, Y.; Li, X. CuO/CeO2 catalysts synthesized from Ce-UiO-66 metal-organic framework for preferential CO oxidation. Int. J. Hydrogen Energy 2017, 42, 17457–17465. [Google Scholar] [CrossRef]
- Spezzati, G.; Su, Y.; Hofmann, J.P.; Benavidez, A.D.; DeLaRiva, A.T.; McCabe, J.; Datye, A.K.; Hensen, E.J. Atomically dispersed Pd–O species on CeO2 (111) as highly active sites for low-temperature CO oxidation. ACS Catal. 2017, 7, 6887–6891. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Park, J.-N.; Shon, J.K.; Kim, J.H.; Li, Z.; Park, Y.-K.; Kim, J.M. Low temperature CO oxidation over Pd catalysts supported on highly ordered mesoporous metal oxides. Catal. Today 2012, 185, 183–190. [Google Scholar] [CrossRef]
- Lykaki, M.; Pachatouridou, E.; Carabineiro, S.A.; Iliopoulou, E.; Andriopoulou, C.; Kallithrakas-Kontos, N.; Boghosian, S.; Konsolakis, M. Ceria nanoparticles shape effects on the structural defects and surface chemistry: Implications in CO oxidation by Cu/CeO2 catalysts. Appl. Catal. B Environ. 2018, 230, 18–28. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z.; Chu, W.; Song, L.; Zhang, Z.; Yu, D.; Tian, Y.; Xie, S.; Sun, L. Large-scale synthesis of nitrogen-rich carbon nitride microfibers by using graphitic carbon nitride as precursor. Adv. Mater. 2008, 20, 1777–1781. [Google Scholar] [CrossRef]
- Li, H.-J.; Qian, D.-J.; Chen, M. Templateless infrared heating process for fabricating carbon nitride nanorods with efficient photocatalytic H2 evolution. ACS Appl. Mater. Interfaces 2015, 7, 25162–25170. [Google Scholar] [CrossRef] [PubMed]
- Barrio, J.; Lin, L.; Amo-Ochoa, P.; Tzadikov, J.; Peng, G.; Sun, J.; Zamora, F.; Wang, X.; Shalom, M. Unprecedented centimeter-long carbon nitride needles: Synthesis, characterization and applications. Small 2018, 14, 1800633. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, J.; Zhou, H.; Antonietti, M. Uniform graphitic carbon nitride nanorod for efficient photocatalytic hydrogen evolution and sustained photoenzymatic catalysis. ACS Appl. Mater. Interfaces 2014, 6, 8434–8440. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, X. Facile synthesis of CoO nanorod/C3N4 heterostructure photocatalyst for an enhanced pure water splitting activity. Inorg. Chem. Commun. 2018, 92, 14–17. [Google Scholar] [CrossRef]
- Han, C.; Gao, Y.; Liu, S.; Ge, L.; Xiao, N.; Dai, D.; Xu, B.; Chen, C. Facile synthesis of aupd/g-C3N4 nanocomposite: An effective strategy to enhance photocatalytic hydrogen evolution activity. Int. J. Hydrogen Energy 2017, 42, 22765–22775. [Google Scholar] [CrossRef]
- Le, S.; Jiang, T.; Li, Y.; Zhao, Q.; Li, Y.; Fang, W.; Gong, M. Highly efficient visible-light-driven mesoporous graphitic carbon nitride/ZnO nanocomposite photocatalysts. Appl. Catal. B Environ. 2017, 200, 601–610. [Google Scholar] [CrossRef]
- Lotsch, B.V.; Schnick, W. From triazines to heptazines: Novel nonmetal tricyanomelaminates as precursors for graphitic carbon nitride materials. Chem. Mater. 2006, 18, 1891–1900. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hong, J.; Zhang, W.; Xu, R. Carbon nitride nanosheets for photocatalytic hydrogen evolution: Remarkably enhanced activity by dye sensitization. Catal. Sci. Technol. 2013, 3, 1703–1711. [Google Scholar] [CrossRef]
- Hong, J.; Xia, X.; Wang, Y.; Xu, R. Mesoporous carbon nitride with in situ sulfur doping for enhanced photocatalytic hydrogen evolution from water under visible light. J. Mater. Chem. 2012, 22, 15006–15012. [Google Scholar] [CrossRef]
- Wang, N.; Wang, J.; Hu, J.; Lu, X.; Sun, J.; Shi, F.; Liu, Z.-H.; Lei, Z.; Jiang, R. Design of palladium-doped g- C3N4 for enhanced photocatalytic activity towards hydrogen evolution reaction. ACS Appl. Energy Mater. 2018, 1, 2866–2873. [Google Scholar] [CrossRef]
- Qu, D.; Liu, J.; Miao, X.; Han, M.; Zhang, H.; Cui, Z.; Sun, S.; Kang, Z.; Fan, H.; Sun, Z. Peering into water splitting mechanism of g-C3N4-carbon dots metal-free photocatalyst. Appl. Catal. B Environ. 2018, 227, 418–424. [Google Scholar] [CrossRef]
- Song, X.; Yang, Q.; Yin, M.; Tang, D.; Zhou, L. Highly efficient pollutant removal of graphitic carbon nitride by the synergistic effect of adsorption and photocatalytic degradation. RSC Adv. 2018, 8, 7260–7268. [Google Scholar] [CrossRef] [Green Version]
- Sui, X.-L.; Li, C.-Z.; Zhao, L.; Wang, Z.-B.; Gu, D.-M.; Huang, G.-S. Mesoporous g-C3N4 derived nano-titanium nitride modified carbon black as ultra-fine PtRu catalyst support for methanol electro-oxidation. Int. J. Hydrogen Energy 2018, 43, 5153–5162. [Google Scholar] [CrossRef]
- Lu, N.; Bao, X.; Jiang, N.; Shang, K.; Li, J.; Wu, Y. Non-thermal plasma-assisted catalytic dry reforming of methane and carbon dioxide over g-C3N4-based catalyst. Top. Catal. 2017, 60, 855–868. [Google Scholar] [CrossRef]
- Peterson, E.J.; DeLaRiva, A.T.; Lin, S.; Johnson, R.S.; Guo, H.; Miller, J.T.; Kwak, J.H.; Peden, C.H.; Kiefer, B.; Allard, L.F. Low-temperature carbon monoxide oxidation catalysed by regenerable atomically dispersed palladium on alumina. Nat. Commun. 2014, 5, 4885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Oh, J.-H.; Kim, H.-J.; Jung, J.-H.; Jung, C.-H.; Hong, W.G.; Kim, H.-J.; Park, J.-Y.; Oh, I.-K. Nanohole-structured and palladium-embedded 3d porous graphene for ultrahigh hydrogen storage and co oxidation multifunctionalities. ACS Nano 2015, 9, 7343–7351. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eid, K.; Ahmad, Y.H.; Mohamed, A.T.; Elsafy, A.G.; Al-Qaradawi, S.Y. Versatile Synthesis of Pd and Cu Co-Doped Porous Carbon Nitride Nanowires for Catalytic CO Oxidation Reaction. Catalysts 2018, 8, 411. https://doi.org/10.3390/catal8100411
Eid K, Ahmad YH, Mohamed AT, Elsafy AG, Al-Qaradawi SY. Versatile Synthesis of Pd and Cu Co-Doped Porous Carbon Nitride Nanowires for Catalytic CO Oxidation Reaction. Catalysts. 2018; 8(10):411. https://doi.org/10.3390/catal8100411
Chicago/Turabian StyleEid, Kamel, Yahia H. Ahmad, Assem T. Mohamed, Anas G. Elsafy, and Siham Y. Al-Qaradawi. 2018. "Versatile Synthesis of Pd and Cu Co-Doped Porous Carbon Nitride Nanowires for Catalytic CO Oxidation Reaction" Catalysts 8, no. 10: 411. https://doi.org/10.3390/catal8100411





