Kinetic Modelling and Experimental Studies for the Effects of Fe2+ Ions on Xylan Hydrolysis with Dilute-Acid Pretreatment and Subsequent Enzymatic Hydrolysis
Abstract
:1. Introduction
- XH = xylan in biomass;
- XO = xylooligomer;
- X = xylose;
- F = furfural;
- D = degradation products.
2. Results
2.1. Effect of Ferrous Ion on Xylan Hydrolysis during Acid Pretreatment
2.2. Modelling Xylan Hydrolysis Kinetics
2.2.1. Model Prediction and Data for Xylan Hydrolysis Using 0.5% H2SO4
2.2.2. Model Prediction and Data for Xylan Hydrolysis Using 0.5% H2SO4 and 0.75 mM FeSO4
2.3. Effect of Ferrous Ions on Enzymatic Hydrolysis
3. Discussion
3.1. Dilute-Acid Pretreatments under Different Temperatures and Cost Issues
3.2. Technology Development for Iron Dilute-Acid Pretreatment: from Post-Harvest Supplementation of Iron to In-Planta Iron Accumulation
3.3. Effects of Iron Dilute-Acid Pretreatment and Its Enhancement of Xylose Monomer Yield
3.4. Potential Mechanisms of Iron-Based Dilute-Acid Pretreatment and Enhancement of Xylose Monomer Yield
4. Materials and Methods
4.1. Raw Material and Its Compositional Analysis
4.2. Process Water
4.3. Impregnation
4.4. Bench-Scale Pretreatment
4.5. High-Solids Enzymatic Hydrolysis of Unwashed Solids
4.6. Low-Solids Enzymatic Hydrolysis of Washed Solids
4.7. Analyses of Liquors and Solid Residues
4.8. Data Modelling
- T = temperature (K);
- B = pre-exponential factor (min−1);
- c = acid concentration (wt %);
- m = acid concentration exponent (unitless);
- E = activation energy (kJ/mol);
- R = 8.314 J/mol⋅K.
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fengel, D.; Wegener, G. Wood—Chemistry, Ultrastructure, Reactions; Walter de Gruyter: New York, NY, USA, 1984. [Google Scholar]
- Aden, A.; Ruth, M.; Ibsen, K.; Jechura, J.; Neeves, K.; Sheehan, J.; Wallace, B.; Montague, L.; Slayton, A.; Lukas, J. Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing Co-Current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis for Corn Stover; NREL/TP-510-32438; National Renewable Energy Laboratory: Golden, CO, USA, 2002.
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; NREL/TP-510-47764; National Renewable Energy Laboratory: Golden, CO, USA, 2011.
- Wyman, C.E. What is (and is not) vital to advancing cellulosic ethanol. Trends Biotechnol. 2007, 25, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.; Lee, K.; Tribe, D. Kinetics of alcohol production by zymomonas mobilis at high sugar concentrations. Biotechnol. Lett. 1979, 1, 165–170. [Google Scholar] [CrossRef]
- Schell, D.J.; Farmer, J.; Newman, M.; McMillan, J.D. Dilute-sulfuric acid pretreatment of corn stover in pilot-scale reactor: Investigation of yields, kinetics, and enzymatic digestibilities of solids. Appl. Biochem. Biotechnol. 2003, 105, 69–86. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ruiz, J.C.; West, R.M.; Dumesic, J.A. Catalytic conversion of renewable biomass resources to fuels and chemicals. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, Z.; Zhang, K.; Si, M.; Liu, M.; Chai, L.; Liu, X.; Shi, Y. Bacteria-enhanced dilute-acid pretreatment of lignocellulosic biomass. Bioresour. Technol. 2017, 245, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.B.; Kalif, M.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Mild-temperature dilute-acid pretreatment for integration of first and second generation ethanol processes. Bioresour. Technol. 2017, 245(Part A), 145–151. [Google Scholar] [CrossRef] [PubMed]
- Sievers, D.A.; Kuhn, E.M.; Tucker, M.P.; McMillan, J.D. Effects of dilute-acid pretreatment conditions on filtration performance of corn stover hydrolyzate. Bioresour. Technol. 2017, 243, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Teramura, H.; Sasaki, K.; Kawaguchi, H.; Matsuda, F.; Kikuchi, J.; Shirai, T.; Sazuka, T.; Yamasaki, M.; Takumi, S.; Ogino, C. Differences in glucose yield of residues from among varieties of rice, wheat, and sorghum after dilute-acid pretreatment. Biosci. Biotechnol. Biochem. 2017, 81, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Deshavath, N.N.; Mohan, M.; Veeranki, V.D.; Goud, V.V.; Pinnamaneni, S.R.; Benarjee, T. Dilute-acid pretreatment of sorghum biomass to maximize the hemicellulose hydrolysis with minimized levels of fermentative inhibitors for bioethanol production. 3 Biotech. 2017, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Lehto, J.; Louhelainen, J.; Huttunen, M.; Alen, R. Spectroscopic analysis of hot-water- and dilute-acid-extracted hardwood and softwood chips. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 184, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.-B.; Zhu, M.-J. Direct hydrogen production from dilute-acid pretreated sugarcane bagasse hydrolysate using the newly isolated Thermoanaerobacterium thermosaccharolyticum MJ1. Microb. Cell Fact. 2017, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Azizi, N.; Najafpour, G.; Younesi, H. Acid pretreatment and enzymatic saccharification of brown seaweed for polyhydroxybutyrate (phb) production using cupriavidus necator. Int. J. Biol. Macromol. 2017, 101, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Soam, S.; Agrawal, R.; Gupta, R.P.; Tuli, D.K.; Kumar, R. Pilot scale dilute-acid pretreatment of rice straw and fermentable sugar recovery at high solid loadings. Bioresour. Technol. 2017, 224, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kuhn, E.; Jennings, E.W.; Nelson, R.; Tao, L.; Zhang, M.; Tucker, M.P. Dmr (deacetylation and mechanical refining) processing of corn stover achieves high monomeric sugar concentrations (230 g l-1) during enzymatic hydrolysis and high ethanol concentrations (>10% v/v) during fermentation without hydrolysate purification or concentration. Energy Environ. Sci. 2016, 9, 1237–1245. [Google Scholar]
- Chen, X.; Shekiro, J.; Pschorn, T.; Sabourin, M.; Tao, L.; Elander, R.; Park, S.; Jennings, E.; Nelson, R.; Trass, O.; et al. A highly efficient dilute alkali deacetylation and mechanical (disc) refining process for the conversion of renewable biomass to lower cost sugars. Biotechnol. Biofuels 2014, 7, 98. [Google Scholar] [CrossRef]
- Amirkhani, H.; Yunus, R.; Rashid, U.; Salleh, S.F.; Radhiah, A.D.; Syam, S. Low-temperature dilute acid hydrolysis of oil palm frond. Chem. Eng. Commun. 2015, 202, 1235–1244. [Google Scholar] [CrossRef]
- Hong, E.; Kim, J.; Rhie, S.; Ha, S.-J.; Kim, J.; Ryu, Y. Optimization of dilute sulfuric acid pretreatment of corn stover for enhanced xylose recovery and xylitol production. Biotechnol. Bioprocess Eng. 2016, 21, 612–619. [Google Scholar] [CrossRef]
- Nguyen, Q.A.; Tucker, M. Dilute Acid/Metal Salt Hydrolysis of Lignocellulosics. U.S. Patent 6,423,145, 23 July 2002. [Google Scholar]
- Liu, L.; Sun, J.; Cai, C.; Wang, S.; Pei, H.; Zhang, J. Corn stover pretreatment by inorganic salts and its effects on hemicellulose and cellulose degradation. Bioresour. Technol. 2009, 100, 5865–5871. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wyman, C.E. The enhancement of xylose monomer and xylotriose degradation by inorganic salts in aqueous solutions at 180 °C. Carbohydr. Res. 2006, 341, 2550–2556. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, R.; Fu, S. Fecl3 pretreatment of three lignocellulosic biomass for ethanol production. ACS Sustain. Chem. Eng. 2015, 3, 1794–1800. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, G.; Wei, Y.; Li, X.; Zhang, A.; Xie, J. Enhanced enzymatic hydrolysis of sugarcane bagasse with ferric chloride pretreatment and surfactant. Bioresour. Technol. 2017, 229, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Yu, A.; Han, M.; Choi, G.W.; Chung, B. Ethanosolv pretreatment of barley straw with iron(III) chloride for enzymatic saccharification. Chem. Technol. Biotechnol. 2010, 85, 1494–1498. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, J.Y.; Fu, S.Y. Effects of lignin−metal complexation on enzymatic hydrolysis of cellulose. J. Agric. Food Chem. 2010, 58, 7233–7238. [Google Scholar] [CrossRef] [PubMed]
- Degenstein, J.; Reddy Kamireddy, S.; Tucker, M.P.; Ji, Y. Oligomer saccharide reduction during dilute-acid pretreatment co-catalyzed with lewis acids on corn stover biomass. Int. J. Agric. Biol. Eng. 2013, 6, 54–62. [Google Scholar]
- Saeman, J.F. Kinetics of wood saccharification—Hydrolysis of cellulose and decomposition of sugars in dilute acid at high temperature. Ind. Eng. Chem. 1945, 37, 43–52. [Google Scholar] [CrossRef]
- Morinelly, J.E.; Jensen, J.R.; Browne, M.; Co, T.B.; Shonnard, D.R. Kinetic characterization of xylose monomer and oligomer concentrations during dilute-acid pretreatment of lignocellulosic biomass from forests and switchgrass. Ind. Eng. Chem. Res. 2009, 48, 9877–9884. [Google Scholar] [CrossRef]
- Conner, A.H. Kinetic modeling of hardwood prehydrolysis. Part I. Xylan removal by water prehydrolysis. Wood Fiber Sci. 1984, 16, 268–277. [Google Scholar]
- Adler, E. Lignin chemistry—Past, present and future. Wood Sci. Technol. 1977, 11, 169–218. [Google Scholar] [CrossRef]
- Dammstrom, S.; Salmen, L.; Gatenholm, P. On the interactions between cellulose and xylan, a biomimetic simulation of the hardwood cell wall. Bioresources 2009, 4, 3–14. [Google Scholar]
- Reis, D.l.; Vian, B. Helicoidal pattern in secondary cell walls and possible role of xylans in their construction. Comptes Rendus Biol. 2004, 327, 785–790. [Google Scholar] [CrossRef]
- Abatzoglov, N.; Bouchard, J.; Chornet, E.; Overend, R.P. Dilute acid depolymerization of cellulose in aqueous phase: Experimental evidence of the significant presence of soluble oligomeric intermediates. Can. J. Chem. Eng. 1986, 64, 781–786. [Google Scholar] [CrossRef]
- Garrote, G.; Domı́nguez, H.; Parajó, J.C. Interpretation of deacetylation and hemicellulose hydrolysis during hydrothermal treatments on the basis of the severity factor. Process Biochem. 2002, 37, 1067–1073. [Google Scholar] [CrossRef]
- Garrote, G.; Dominguez, H.; Parajo, J. Autohydrolysis of corncob: Study of non-isothermal operation for xylooligosaccharide production. J. Food Eng. 2002, 52, 211–218. [Google Scholar] [CrossRef]
- Schell, D.J.; Farmer, J.; Newman, M.; McMillan, J.D. Dilute-sulfuric acid pretreatment of corn stover in pilot-scale reactor. In Biotechnology for Fuels and Chemicals; Springer: New York, NY, USA, 2003; pp. 69–85. [Google Scholar]
- Wyman, C.E.; Yang, B. Combined severity factor for predicting sugar recovery in acid-catalyzed pretreatment followed by enzymatic hydrolysis. In Hydrothermal Processing in Biorefineries; Springer: New York, NY, USA, 2017; pp. 161–180. [Google Scholar]
- Zelinka, S.L.; Gleber, S.-C.; Vogt, S.; Rodriguez Lopez, G.M.; Jakes, J.E. Threshold for ion movements in wood cell walls below fiber saturation observed by X-ray fluorescence microscopy (XFM). Holzforschung 2015, 69, 441–448. [Google Scholar] [CrossRef]
- Wei, H.; Yang, H.; Ciesielski, P.N.; Donohoe, B.S.; McCann, M.C.; Murphy, A.S.; Peer, W.A.; Ding, S.-Y.; Himmel, M.E.; Tucker, M.P. Transgenic ferritin overproduction enhances thermochemical pretreatments in arabidopsis. Biomass Bioenerg. 2015, 72, 55–64. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Jakes, J.E.; Donohoe, B.S.; Ciesielski, P.N.; Yang, H.; Gleber, S.-C.; Vogt, S.; Ding, S.-Y.; Peer, W.A.; Murphy, A.S.; et al. Directed plant cell-wall accumulation of iron: embedding cocatalyst for efficient biomass conversion. Biotechnol. Biofuels 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wei, H.; Ma, G.; Antunes, M.S.; Vogt, S.; Cox, J.; Zhang, X.; Liu, X.; Bu, L.; Charlotte, G.S.; et al. Cell wall targeted in planta iron accumulation enhances biomass conversion and seed iron concentration in arabidopsis and rice. Plant Biotechnol. J. 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Donohoe, B.S.; Vinzant, T.B.; Ciesielski, P.N.; Wang, W.; Gedvilas, L.M.; Zeng, Y.; Johnson, D.K.; Ding, S.Y.; Himmel, M.E.; et al. Elucidating the role of ferrous ion cocatalyst in enhancing dilute-acid pretreatment of lignocellulosic biomass. Biotechnol. Biofuels 2011, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Vigneshwaran, N. Effect of Fenton’s pretreatment on cotton cellulosic substrates to enhance its enzymatic hydrolysis response. Bioresour. Technol. 2012, 103, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Bauer, I.; Knölker, H.-J. Iron catalysis in organic synthesis. Chem. Rev. 2015, 115, 3170–3387. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hyman, D.; Payne, C.; Wolfe, J. Determination of Insoluble Solids in Pretreated Biomass Material Laboratory Analytical Procedure (LAP); Technical report NREL/TP-510-42627; National Renewable Energy Laboratory: Golden, CO, USA, 2008. Available online: https://www.nrel.gov/docs/gen/fy08/42627.pdf (accessed on 18 January 2018).
- Tucker, M.P.; Kim, K.H.; Newman, M.M.; Nguyen, Q.A. Effects of temperature and moisture on dilute-acid steam explosion pretreatment of corn stover and cellulase enzyme digestibility. Appl. Biochem. Biotechnol. 2003, 105, 165–178. [Google Scholar] [CrossRef]
- Selig, M.; Weiss, N.; Ji, Y. Enzymatic Saccharification of Lignocellulosic Biomass. Laboratory Analytical Procedure (LAP); NERL/TP-510-42629; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; National Renewable Energy Laboratory: Golden, CO, USA, 2006.
- Hyman, D.; Sluiter, A.; Crocker, D.; Johnson, D.; Sluiter, J.; Black, S.; Scarlata, C. Determination of Acid Soluble Lignin Concentration Curve by UV-Vis Spectroscopy Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008; 13p.
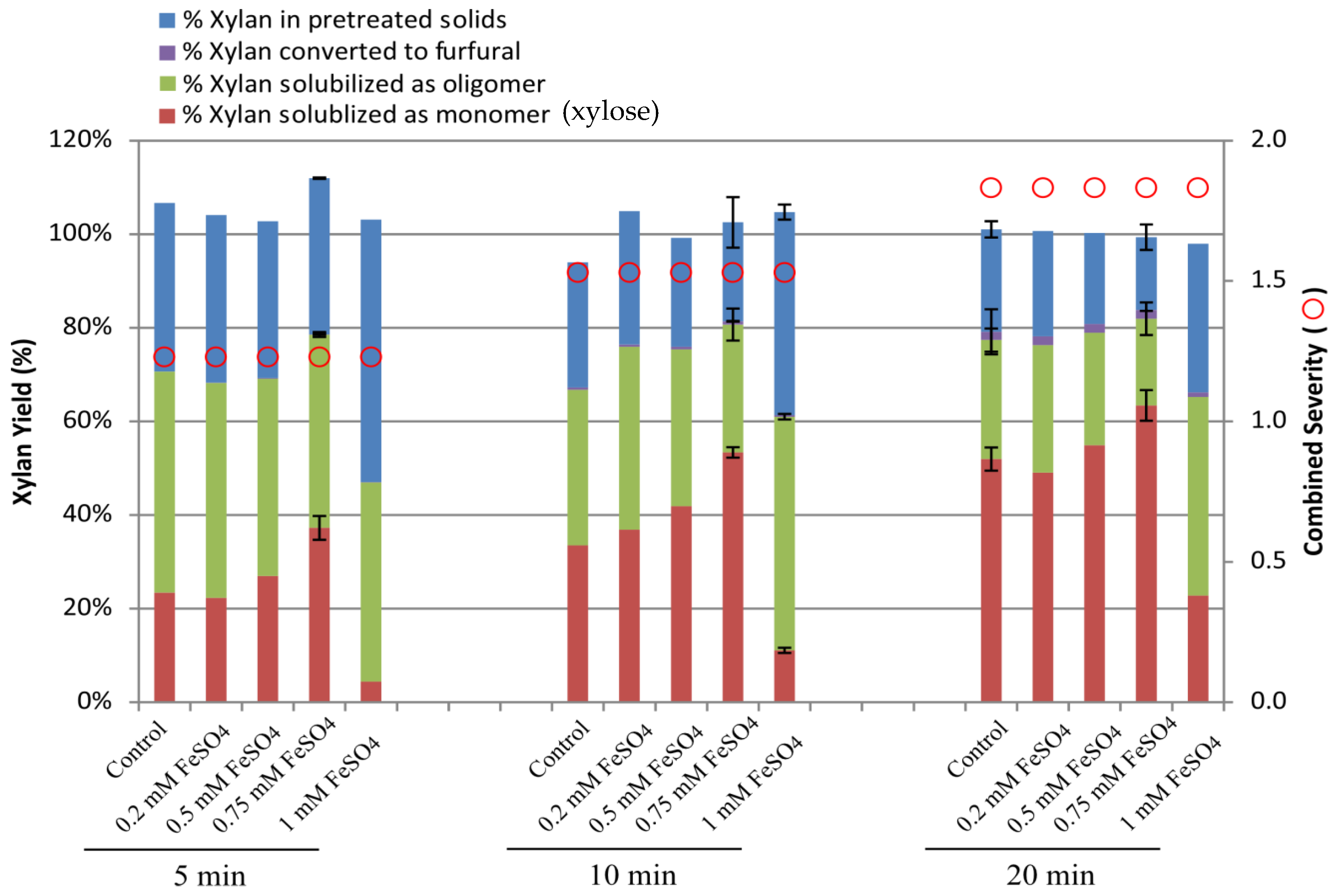
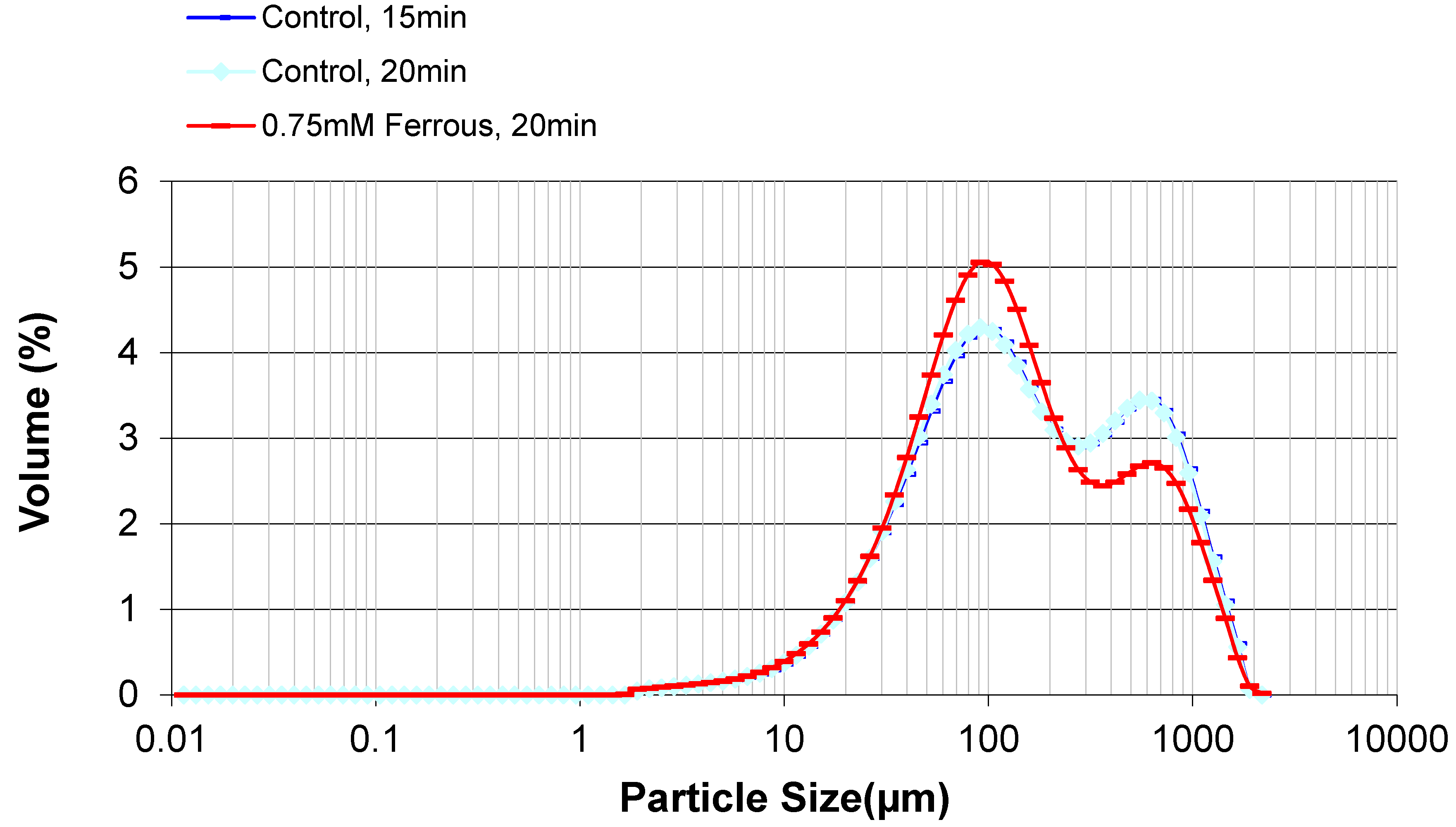
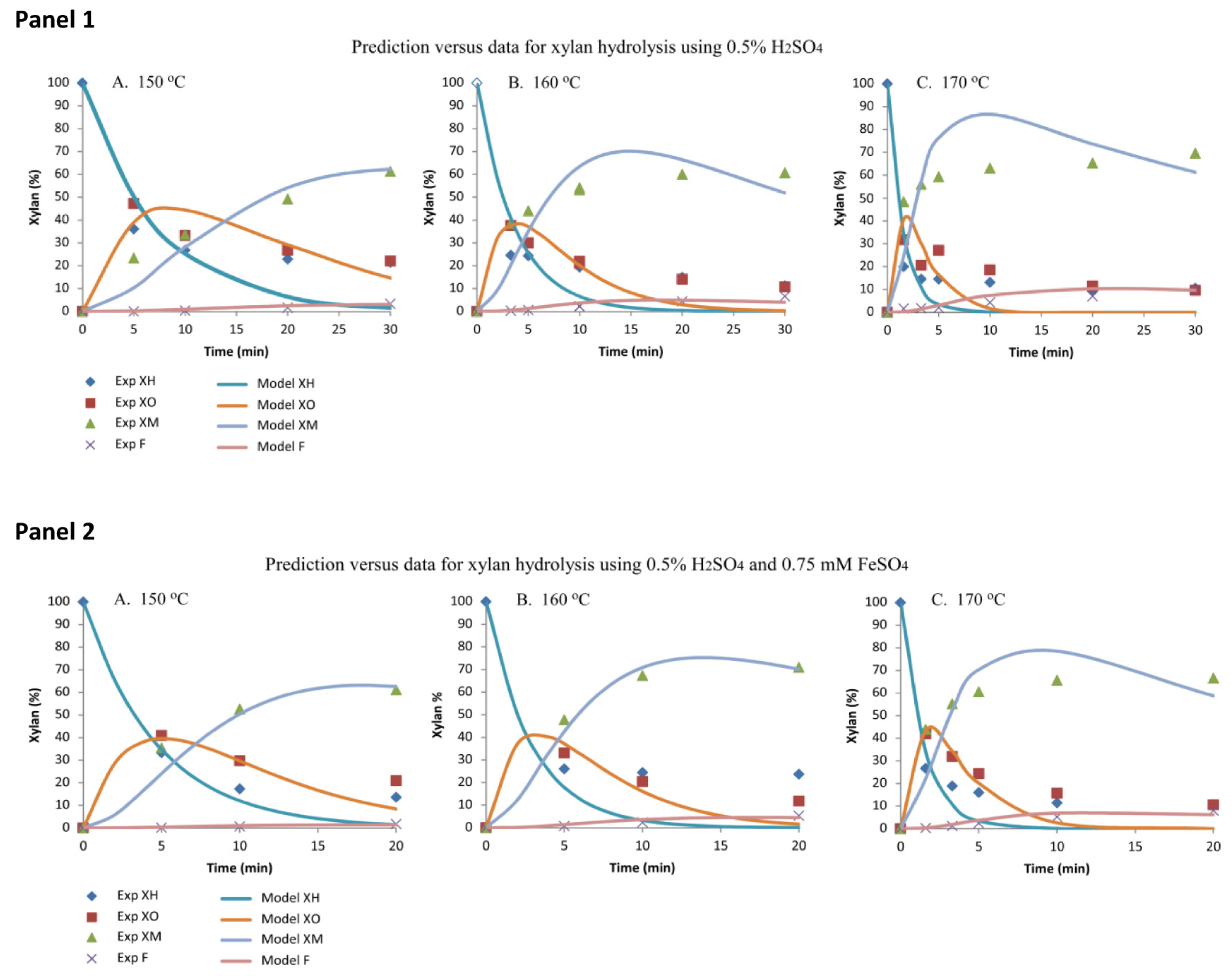
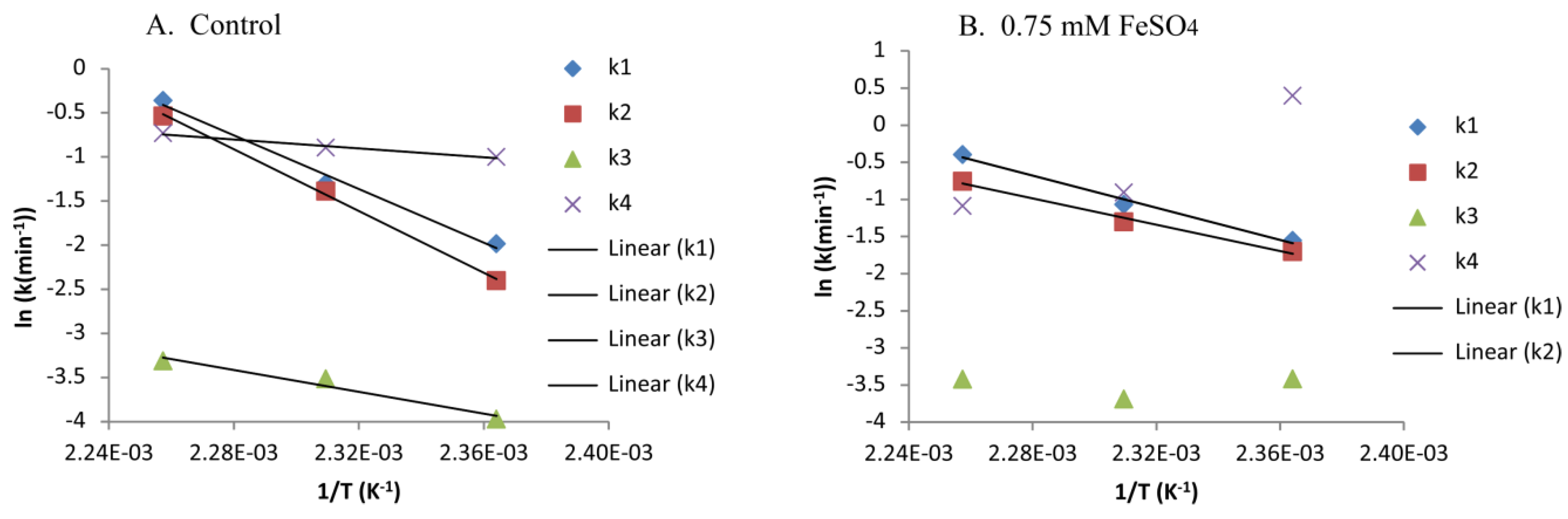


| Sample ID | Ash (%) | Lignin (%) | Glucan (%) | Xylan (%) | Galactan (%) | Arabinan (%) | Acetate (%) | Total (%) |
|---|---|---|---|---|---|---|---|---|
| 0.5% H2SO4 only | 5.5 | 18.2 | 38.4 | 25.0 | 1.9 | 4.3 | 3.1 | 96.3 |
| 0.5% H2SO4, 0.75 mM FeSO4 | 3.2 | 18.5 | 38.3 | 26.6 | 1.9 | 4.0 | 3.3 | 95.8 |
| 0.5% H2SO4, 1 mM FeSO4 | 5.2 | 18.4 | 38.9 | 24.8 | 2.6 | 5.8 | 3.7 | 99.3 |
| 150 °C Control | 150 °C 0.75 mM Fe2+ | 160 °C Control | 160 °C 0.75 mM Fe2+ | 170 °C Control | 170 °C 0.75 mM Fe2+ | |
|---|---|---|---|---|---|---|
| k1 | 0.137 | 0.212 | 0.272 | 0.343 | 0.698 | 0.674 |
| k2 | 0.090 | 0.182 | 0.250 | 0.272 | 0.584 | 0.470 |
| k3 | 0.019 | 0.033 | 0.030 | 0.025 | 0.036 | 0.033 |
| k4 | 0.367 | 1.488 | 0.408 | 0.403 | 0.481 | 0.337 |
| Ea for Control (0.5% H2SO4 Only) (kJ/mol) | Arrhenius Fit, R2 for Control (0.5% H2SO4) | Ea for 0.75 mM FeSO4 with 0.5% H2SO4 (kJ/mol) | Arrhenius Fit, R2 for 0.75 mM FeSO4 with 0.5% H2SO4 | |
|---|---|---|---|---|
| k1 | 128 | 0.9889 | 90 | 0.9883 |
| k2 | 146 | 0.9986 | 74 | 0.9900 |
| k3 | 51 | 0.9593 | NA | NA |
| k4 | 21 | 0.9807 | NA | NA |
| Feedstock | Structural Protein (%) | Sucrose (%) | Water-Extractable Others (%) | Ethanol Extractives (%) | Lignin (%) | Glucan (%) | Xylan (%) | Galactan (%) | Arabinan (%) | Mannan (%) | Uronic Acid (%) | Acetyl (%) | Total (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corn stover 33A14 | 1.6 | 4.0 | 6.1 | 2.2 | 12.3 | 34.0 | 22.0 | 1.6 | 3.1 | 0.0 | 3.7 | 2.9 | 99.1 |
| Equations | No. | Boundary Conditions (Sugar Yield) |
|---|---|---|
| Equation (3) | XH(0) = 100% | |
| Equation (4) | XO(0) = 0% | |
| Equation (5) | X(0) = 0% | |
| Equation (6) | F(0) = 0% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Chen, X.; Shekiro, J.; Kuhn, E.; Wang, W.; Ji, Y.; Kozliak, E.; Himmel, M.E.; Tucker, M.P. Kinetic Modelling and Experimental Studies for the Effects of Fe2+ Ions on Xylan Hydrolysis with Dilute-Acid Pretreatment and Subsequent Enzymatic Hydrolysis. Catalysts 2018, 8, 39. https://doi.org/10.3390/catal8010039
Wei H, Chen X, Shekiro J, Kuhn E, Wang W, Ji Y, Kozliak E, Himmel ME, Tucker MP. Kinetic Modelling and Experimental Studies for the Effects of Fe2+ Ions on Xylan Hydrolysis with Dilute-Acid Pretreatment and Subsequent Enzymatic Hydrolysis. Catalysts. 2018; 8(1):39. https://doi.org/10.3390/catal8010039
Chicago/Turabian StyleWei, Hui, Xiaowen Chen, Joseph Shekiro, Erik Kuhn, Wei Wang, Yun Ji, Evguenii Kozliak, Michael E. Himmel, and Melvin P. Tucker. 2018. "Kinetic Modelling and Experimental Studies for the Effects of Fe2+ Ions on Xylan Hydrolysis with Dilute-Acid Pretreatment and Subsequent Enzymatic Hydrolysis" Catalysts 8, no. 1: 39. https://doi.org/10.3390/catal8010039






