Inhibiting Fe–Al Spinel Formation on a Narrowed Mesopore-Sized MgAl2O4 Support as a Novel Catalyst for H2 Production in Chemical Looping Technology
Abstract
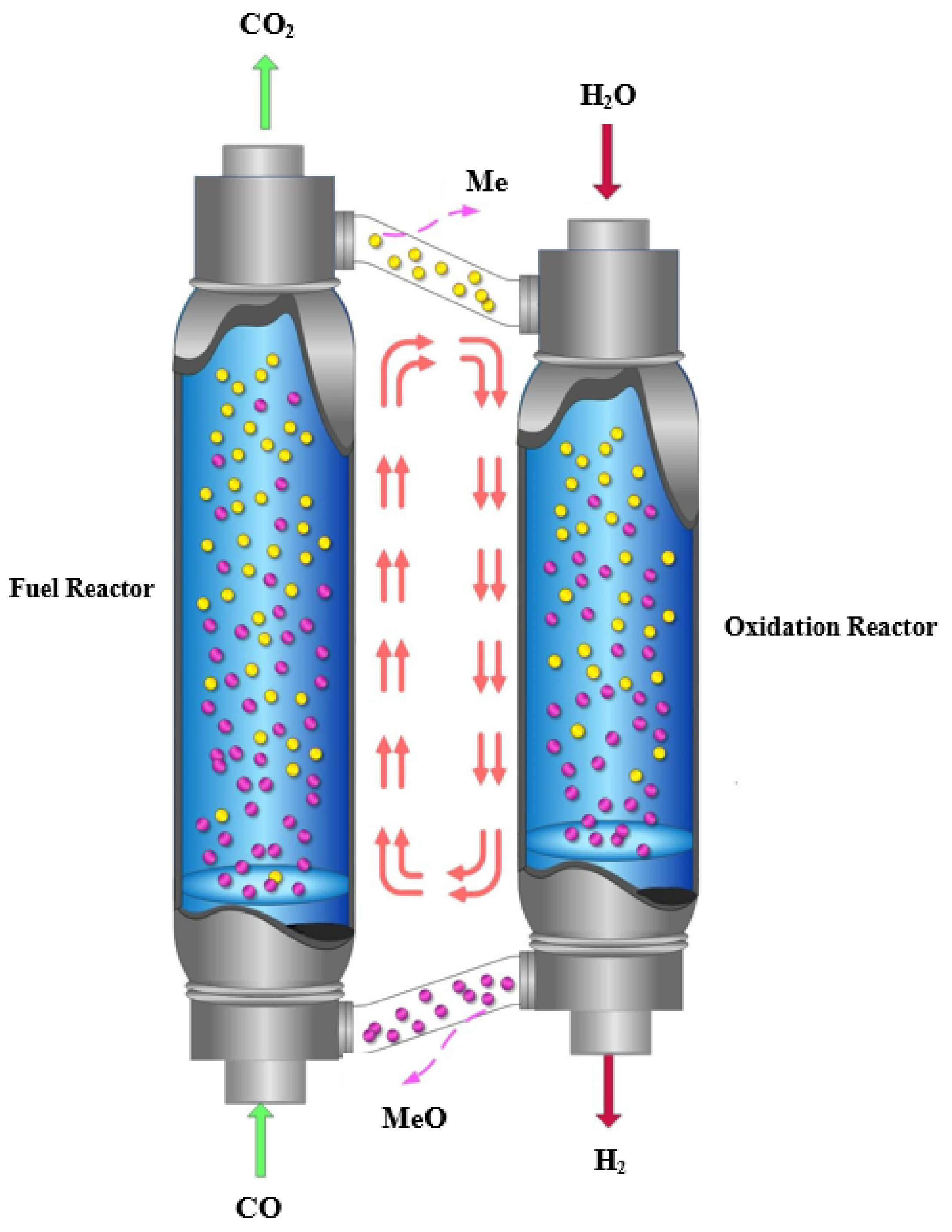
:1. Introduction
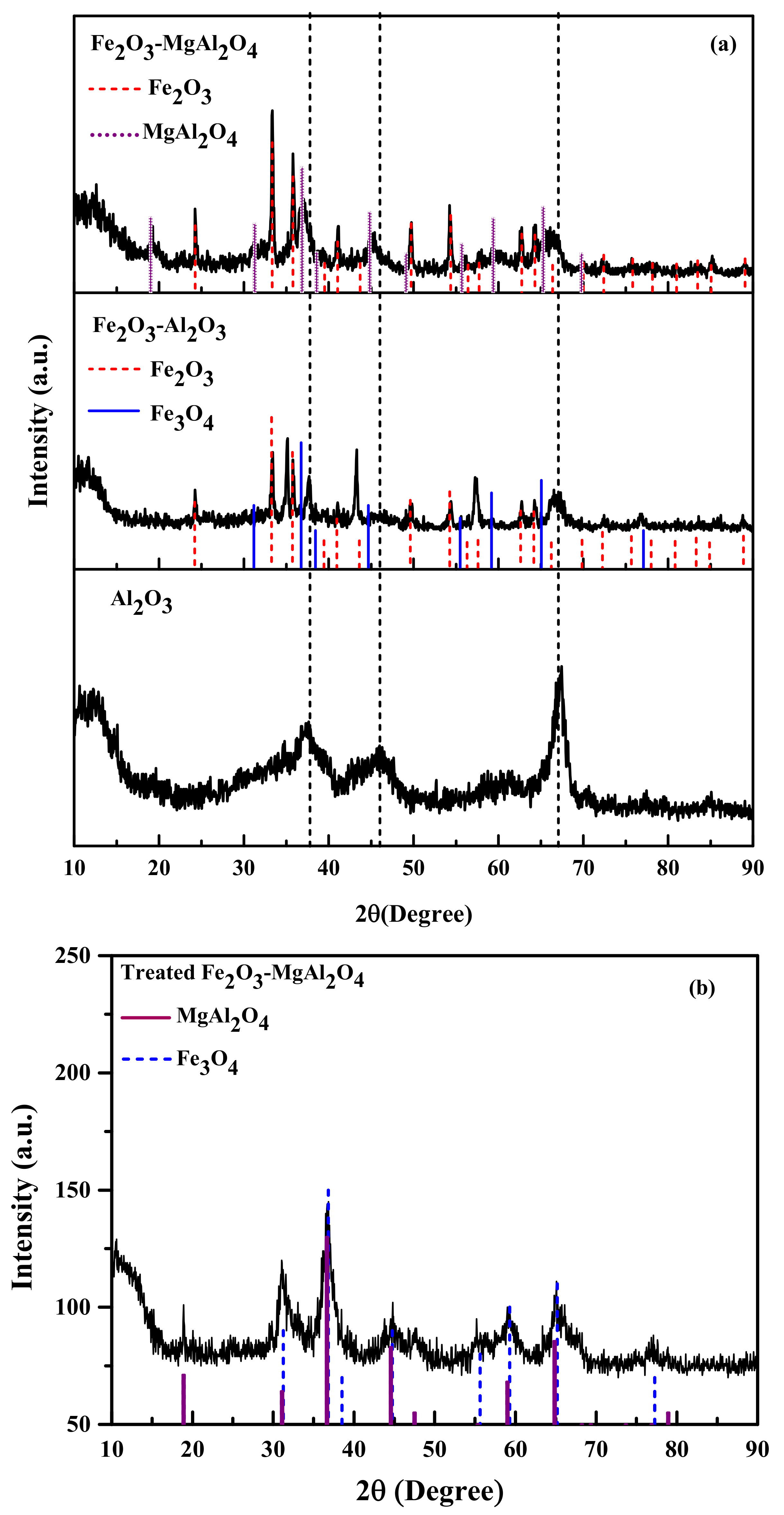
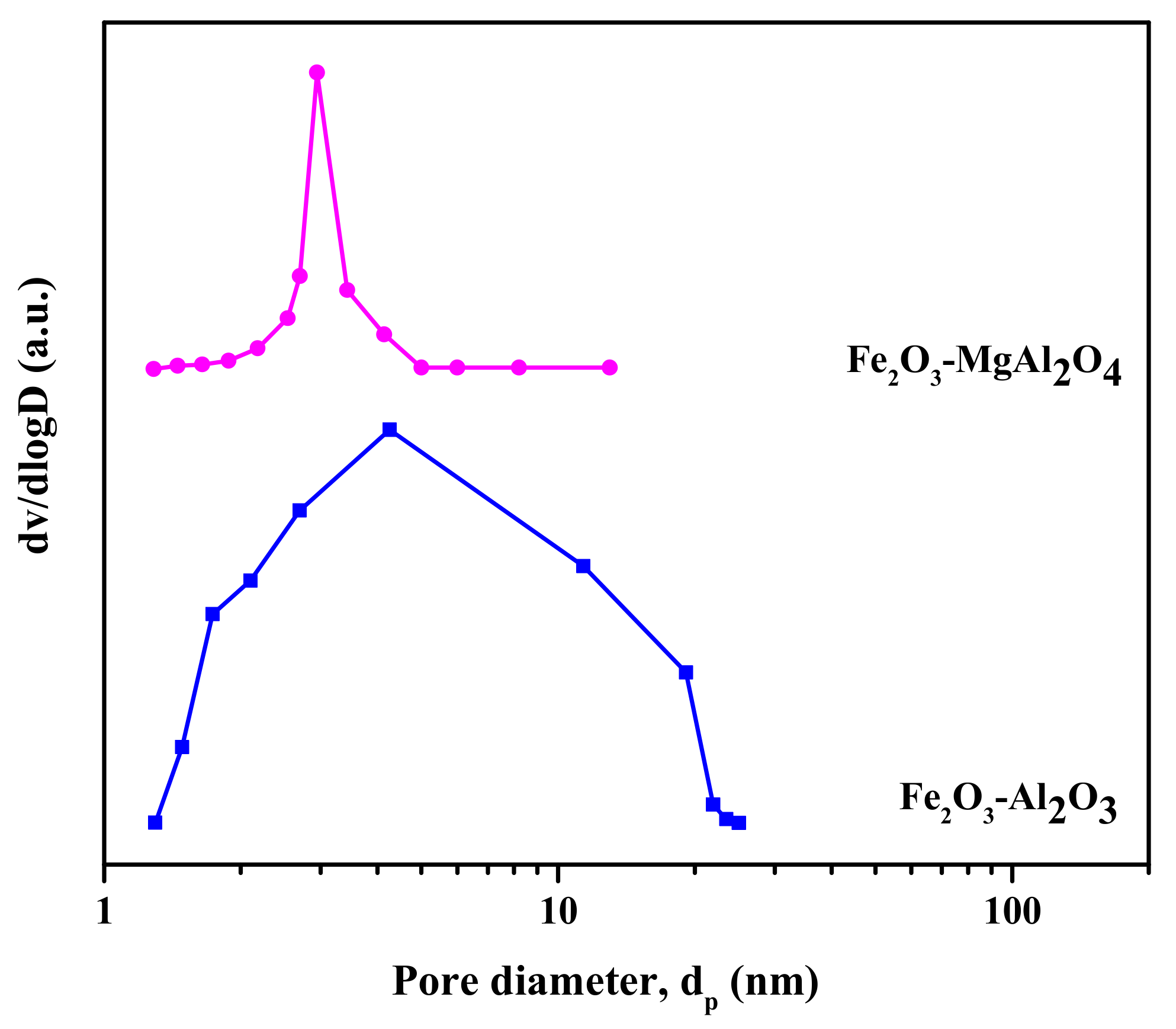
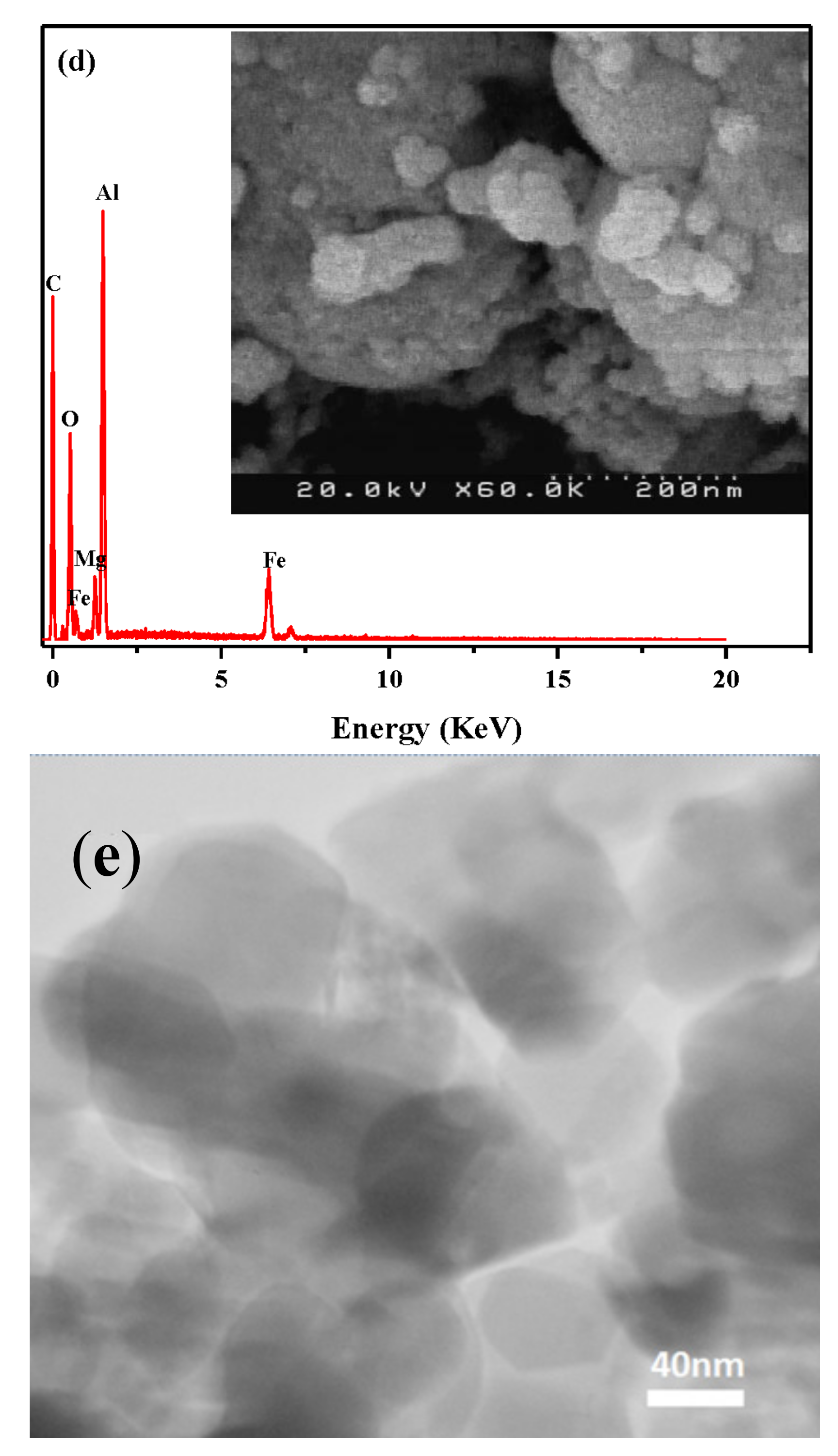
2. Results and Discussion
2.1. Sample Characterization
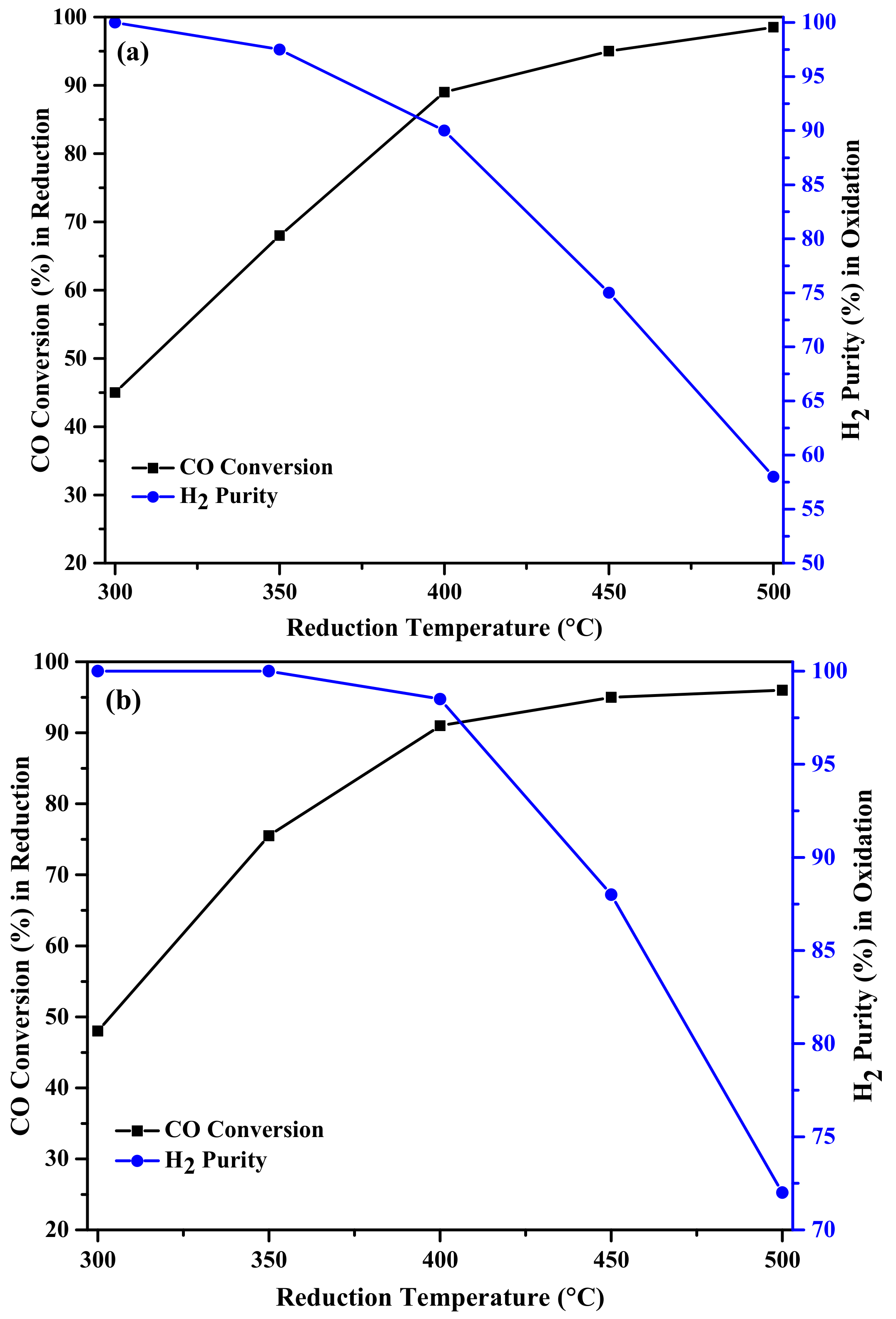
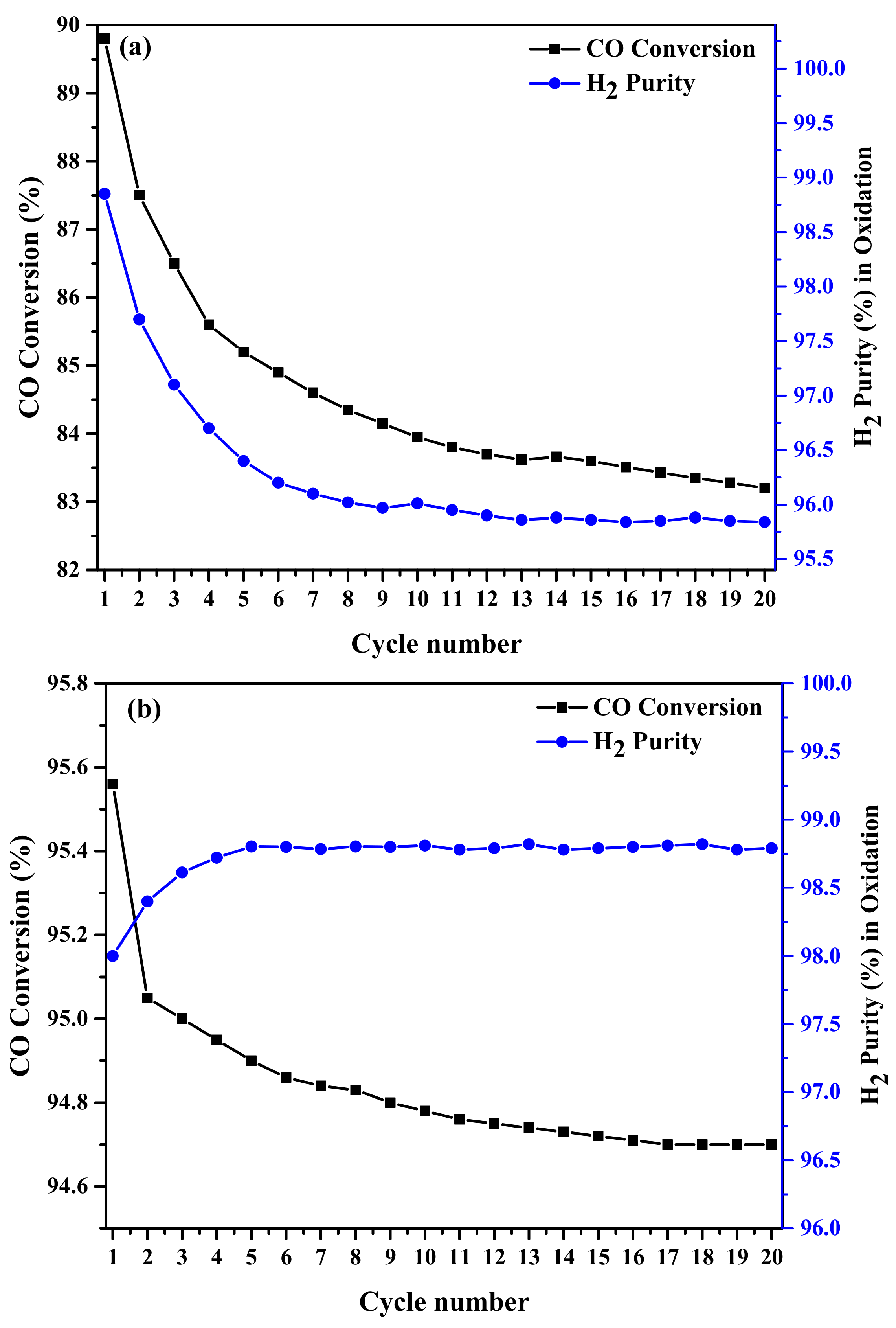
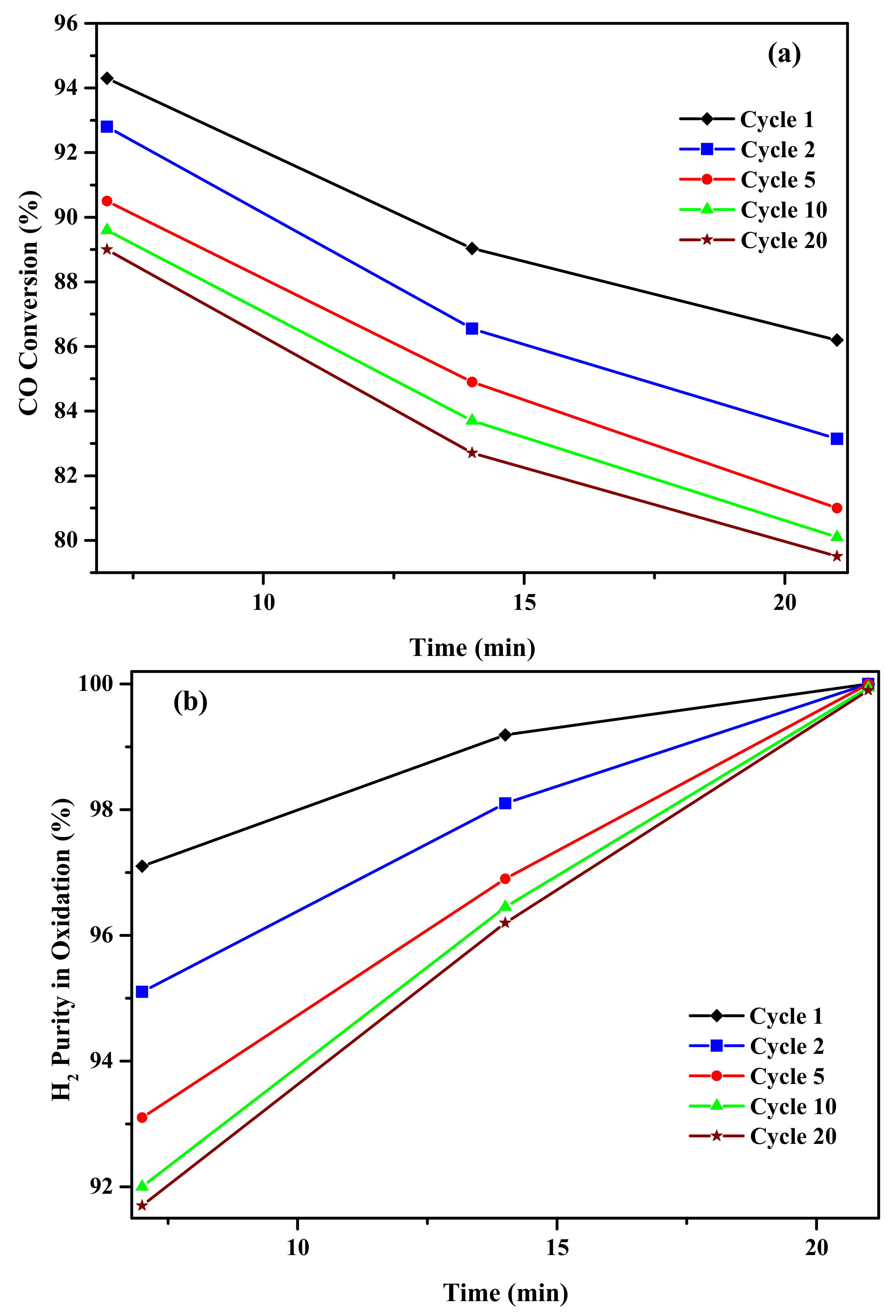
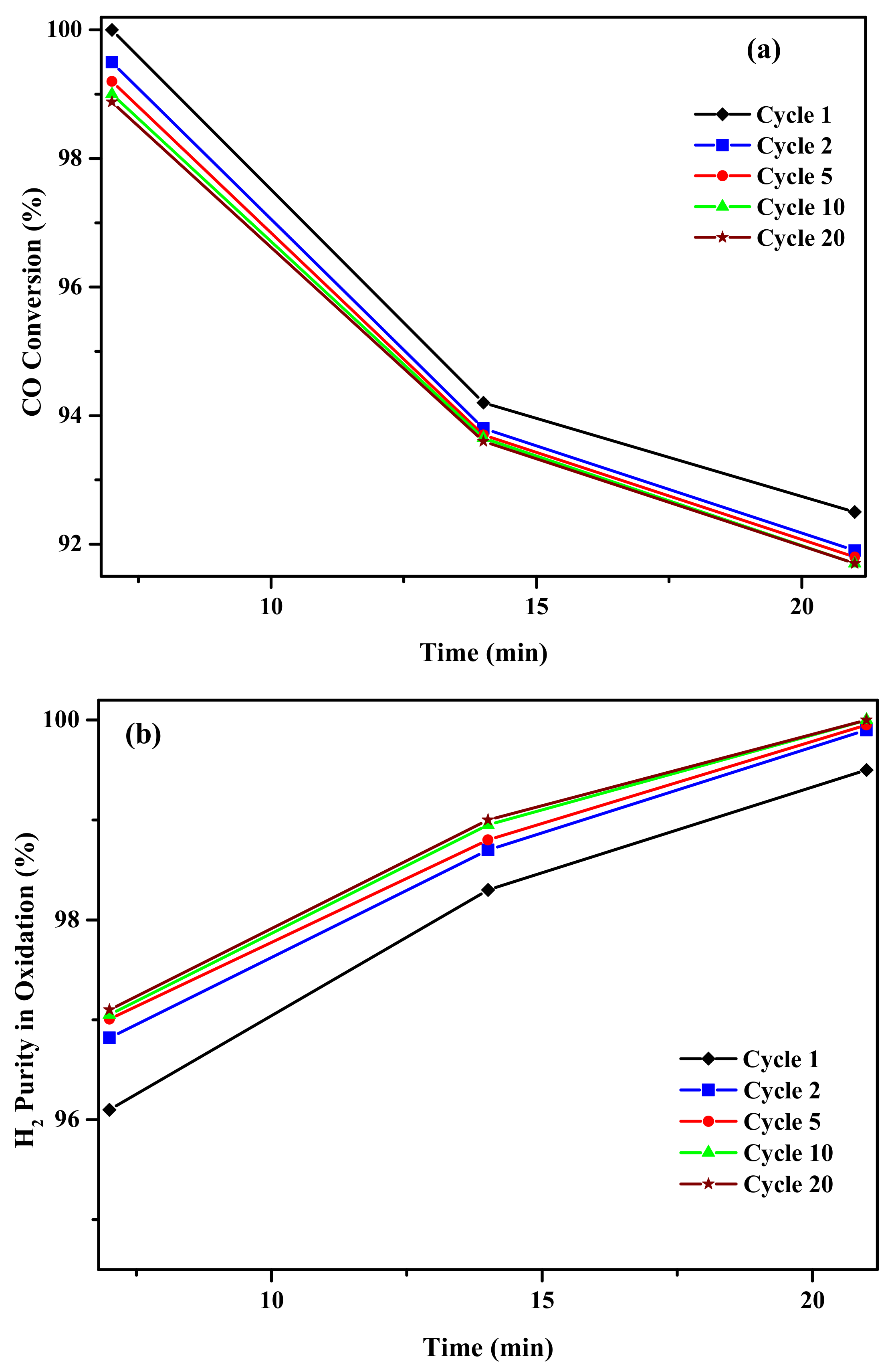
2.2. Activity Results
3. Experimental Methods
3.1. Oxygen Carrier Preparation
3.2. Oxygen Carrier Characterization
3.3. Process Activity
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Sarshar, Z.; Kleitz, F.; Kaliaguine, S. Novel oxygen carriers for chemical looping combustion: La1−xCexBO3 (B = Co, Mn) perovskites synthesized by reactive grinding and nanocasting. Energy Environ. Sci. 2012, 4, 4258–4269. [Google Scholar] [CrossRef]
- Hafizi, A.; Rahimpour, M.R.; Hassanajili, S. Hydrogen production via chemical looping steam methane reforming process: Effect of cerium and calcium promoters on the performance of Fe2O3/Al2O3 oxygen carrier. Appl. Energy 2016, 165, 685–694. [Google Scholar] [CrossRef]
- Mohsenzadeh, A.; Richards, T.; Bolton, K. DFT study of the water gas shift reaction on Ni(111), Ni(100) and Ni(110) surfaces. Surf. Sci. 2016, 644, 53–63. [Google Scholar] [CrossRef]
- Tang, Q.-L.; Chen, Z.-X.; He, X. A theoretical study of the water gas shift reaction mechanism on Cu(111) model system. Surf. Sci. 2009, 603, 2138–2144. [Google Scholar] [CrossRef]
- Alihoseinzadeh, A.; Khodadadi, A.A.; Mortazavi, Y. Enhanced catalytic performance of Au/CuO–ZnO catalysts containing low CuO content for preferential oxidation of carbon monoxide in hydrogen-rich streams for PEMFC. Int. J. Hydrogen Energy 2014, 39, 2056–2066. [Google Scholar] [CrossRef]
- Tosti, S.; Zerbo, M.; Basile, A.; Calabrò, V.; Borgognoni, F.; Santucci, A. Pd-based membrane reactors for producing ultra-pure hydrogen: Oxidative reforming of bio-ethanol. Int. J. Hydrogen Energy 2013, 38, 701–707. [Google Scholar] [CrossRef]
- Akbari-Emadabadi, S.; Rahimpour, M.R.; Hafizi, A.; Keshavarz, P. Promotion of Ca-Co bifunctional catalyst/sorbent with yttrium for hydrogen production in modified chemical looping steam methane reforming. Catalysts 2017, 7, 270. [Google Scholar] [CrossRef]
- Liu, W.; Ismail, M.; Dunstan, M.T.; Hu, W.; Zhang, Z. Fennell PS Inhibiting the interaction between FeO and Al2O3 during chemical looping production of hydrogen. RSC Adv. 2015, 5, 1759–1771. [Google Scholar] [CrossRef]
- Akbari-Emadabadi, S.; Rahimpour, M.R.; Hafizi, A.; Keshavarz, P. Production of hydrogen-rich syngas using Zr modified Ca-Co bifunctional catalyst-sorbent in chemical looping steam methane reforming. Appl. Energy 2017, 206, 51–62. [Google Scholar] [CrossRef]
- Alirezaei, I.; Hafizi, A.; Rahimpour, M.R. Syngas production in chemical looping reforming process over ZrO2 promoted Mn-based catalyst. J. CO2 Util. 2017, 23, 105–116. [Google Scholar] [CrossRef]
- Hafizi, A.; Rahimpour, M.R.; Hassanajili, S. Calcium promoted Fe/Al2O3 oxygen carrier for hydrogen production via cyclic chemical looping steam methane reforming process. Int. J. Hydrogen Energy 2015, 40, 16159–16168. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, L.; Zheng, Y.; Wang, H.; Wei, Y.; Li, K. CeO2 modified Fe2O3 for the chemical hydrogen storage and production via cyclic water splitting. Int. J. Hydrogen Energy 2014, 39, 13381–13388. [Google Scholar] [CrossRef]
- Alirezaei, I.; Hafizi, A.; Rahimpour, M.R.; Raeissi, S. Application of zirconium modified Cu-based oxygen carrier in chemical looping reforming. J. CO2 Util. 2016, 14, 112–121. [Google Scholar] [CrossRef]
- Ortiz, M.; de Diego, L.F.; Abad, A.; García-Labiano, F.; Gayán, P.; Adánez, J. Catalytic Activity of Ni-Based Oxygen-Carriers for Steam Methane Reforming in Chemical-Looping Processes. Energy Fuels 2012, 26, 791–800. [Google Scholar] [CrossRef]
- Forutan, H.; Karimi, E.; Hafizi, A.; Rahimpour, M.R.; Keshavarz, P. Expert representation chemical looping reforming: A comparative study of Fe, Mn, Co and Cu as oxygen carriers supported on Al2O3. J. Ind. Eng. Chem. 2015, 21, 900–911. [Google Scholar] [CrossRef]
- Hacker, V.; Faleschini, G.; Fuchs, H.; Fankhauser, R.; Simader, G.; Ghaemi, M.; Spreitz, B.; Friedrich, M. Usage of biomass gas for fuel cells by the SIR process. J. Power Sources 1998, 71, 226–230. [Google Scholar] [CrossRef]
- Hacker, V.; Fankhauser, R.; Faleschini, G.; Fuchs, H.; Friedrich, K.; Muhr, M.; Kordesch, K. Hydrogen production by steam–iron process. J. Power Sources 2000, 86, 531–535. [Google Scholar] [CrossRef]
- Hafizi, A.; Rahimpour, M.R.; Hassanajili, S. High purity hydrogen production via sorption enhanced chemical looping reforming: Application of 22Fe2O3/MgAl2O4 and 22Fe2O3/Al2O3 as oxygen carriers and cerium promoted CaO as CO2 sorbent. Appl. Energy 2016, 169, 629–641. [Google Scholar] [CrossRef]
- Li, F.; Sun, Z.; Luo, S.; Fan, L.S. Ionic diffusion in the oxidation of iron—Effect of support and its implications to chemical looping applications. Energy Environ. Sci. 2011, 4, 876–880. [Google Scholar] [CrossRef]
- Xu, B.-Q.; Wei, J.-M.; Wang, H.-Y.; Sun, K.-Q.; Zhu, Q.-M. Nano-MgO: Novel preparation and application as support of Ni catalyst for CO2 reforming of methane. Catal. Today 2001, 68, 217–225. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Hafizi, A.; Rahimpour, M.R.; Hassanajili, S. Hydrogen production by chemical looping steam reforming of methane over Mg promoted iron oxygen carrier: Optimization using design of experiments. J. Taiwan Inst. Chem. Eng. 2016, 62, 140–149. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.; Sahebdelfar, S.; Bai, P.; Liu, X.; Yan, Z.-F. CO2 reforming of CH4 over nanocrystalline zirconia-supported nickel catalysts. Appl. Catal. B Environ. 2008, 77, 346–354. [Google Scholar] [CrossRef]
- Wang, N.; Yu, X.; Shen, K.; Chu, W.; Qian, W. Synthesis, characterization and catalytic performance of MgO-coated Ni/SBA-15 catalysts for methane dry reforming to syngas and hydrogen. Int. J. Hydrogen Energy 2013, 38, 9718–9731. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Sutthisripok, W.; Assabumrungrat, S. Synthesis gas production from dry reforming of methane over CeO2 doped Ni/Al2O3: Influence of the doping ceria on the resistance toward carbon formation. Chem. Eng. J. 2005, 112, 13–22. [Google Scholar] [CrossRef]
- Huang, L.; Xie, J.; Chen, R.; Chu, D.; Chu, W.; Hsu, A.T. Effect of iron on durability of nickel-based catalysts in auto-thermal reforming of ethanol for hydrogen production. Int. J. Hydrogen Energy 2008, 33, 7448–7456. [Google Scholar] [CrossRef]
- Hafizi, A.; Jafari, M.; Rahimpour, M.R.; Hassanajili, S. Experimental investigation of sorption enhanced chemical looping reforming for high purity hydrogen production using CeO2–CaO CO2 sorbent and 15Fe–5Ca/Al2O3 oxygen carrier. J. Taiwan Inst. Chem. Eng. 2016, 65, 185–196. [Google Scholar] [CrossRef]
- Li, D.; Zeng, L.; Li, X.; Wang, X.; Ma, H.; Assabumrungrat, S.; Gong, J. Ceria-promoted Ni/SBA-15 catalysts for ethanol steam reforming with enhanced activity and resistance to deactivation. Appl. Catal. B Environ. 2015, 176, 532–541. [Google Scholar] [CrossRef]
- Jabbour, K.; Massiani, P.; Davidson, A.; Casale, S.; Hassan, N. Ordered mesoporous “one-pot” synthesized Ni-Mg(Ca)-Al2O3 as effective and remarkably stable catalysts for combined steam and dry reforming of methane (CSDRM). Appl. Catal. B Environ. 2017, 201, 527–542. [Google Scholar] [CrossRef]
- Jiang, B.; Dou, B.; Wang, K.; Song, Y.; Chen, H.; Zhang, C.; Xu, Y.; Li, M. Hydrogen production from chemical looping steam reforming of glycerol by Ni based Al-MCM-41 oxygen carriers in a fixed-bed reactor. Fuel 2016, 183, 170–176. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, L.; Tang, C.; Dong, L.; Li, J. Construction of hierarchical MgAl2O4 spinel as catalytic supports. Mater. Lett. 2015, 159, 204–206. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Fierro, J. Ni-based catalysts for reforming of methane with CO2. Int. J. Hydrogen Energy 2012, 37, 15966–15975. [Google Scholar] [CrossRef]
- Wang, P.; Tanabe, E.; Ito, K.; Jia, J.; Morioka, H.; Shishido, T.; Takehira, K. Filamentous carbon prepared by the catalytic pyrolysis of CH4 on Ni/SiO2. Appl. Catal. A Gen. 2002, 231, 35–44. [Google Scholar] [CrossRef]


| Sample | Fe a (wt %) | Mg a (wt %) | Al a (wt %) | C a (wt %) | O a (wt %) | BET Surface Area (m2 g−1) | Average Pore Size (nm) | Pore Volume (cm3 g−1) |
|---|---|---|---|---|---|---|---|---|
| Fresh Fe2O3-Al2O3 | 17.72 | - | 39.82 | - | 42.46 | 174.3 | 4.3 | 0.416 |
| Fresh Fe2O3-MgAl2O4 | 16.42 | 10.81 | 21.94 | - | 50.83 | 113.1 | 2.3 | 0.389 |
| Used Fe2O3-Al2O3 | 16.97 | - | 40.01 | 5.83 | 37.29 | - | - | - |
| Used Fe2O3-MgAl2O4 | 14.86 | 9.25 | 33.46 | 1.20 | 41.23 | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafizi, A.; Rahimpour, M.R. Inhibiting Fe–Al Spinel Formation on a Narrowed Mesopore-Sized MgAl2O4 Support as a Novel Catalyst for H2 Production in Chemical Looping Technology. Catalysts 2018, 8, 27. https://doi.org/10.3390/catal8010027
Hafizi A, Rahimpour MR. Inhibiting Fe–Al Spinel Formation on a Narrowed Mesopore-Sized MgAl2O4 Support as a Novel Catalyst for H2 Production in Chemical Looping Technology. Catalysts. 2018; 8(1):27. https://doi.org/10.3390/catal8010027
Chicago/Turabian StyleHafizi, Ali, and Mohammad Reza Rahimpour. 2018. "Inhibiting Fe–Al Spinel Formation on a Narrowed Mesopore-Sized MgAl2O4 Support as a Novel Catalyst for H2 Production in Chemical Looping Technology" Catalysts 8, no. 1: 27. https://doi.org/10.3390/catal8010027
APA StyleHafizi, A., & Rahimpour, M. R. (2018). Inhibiting Fe–Al Spinel Formation on a Narrowed Mesopore-Sized MgAl2O4 Support as a Novel Catalyst for H2 Production in Chemical Looping Technology. Catalysts, 8(1), 27. https://doi.org/10.3390/catal8010027




