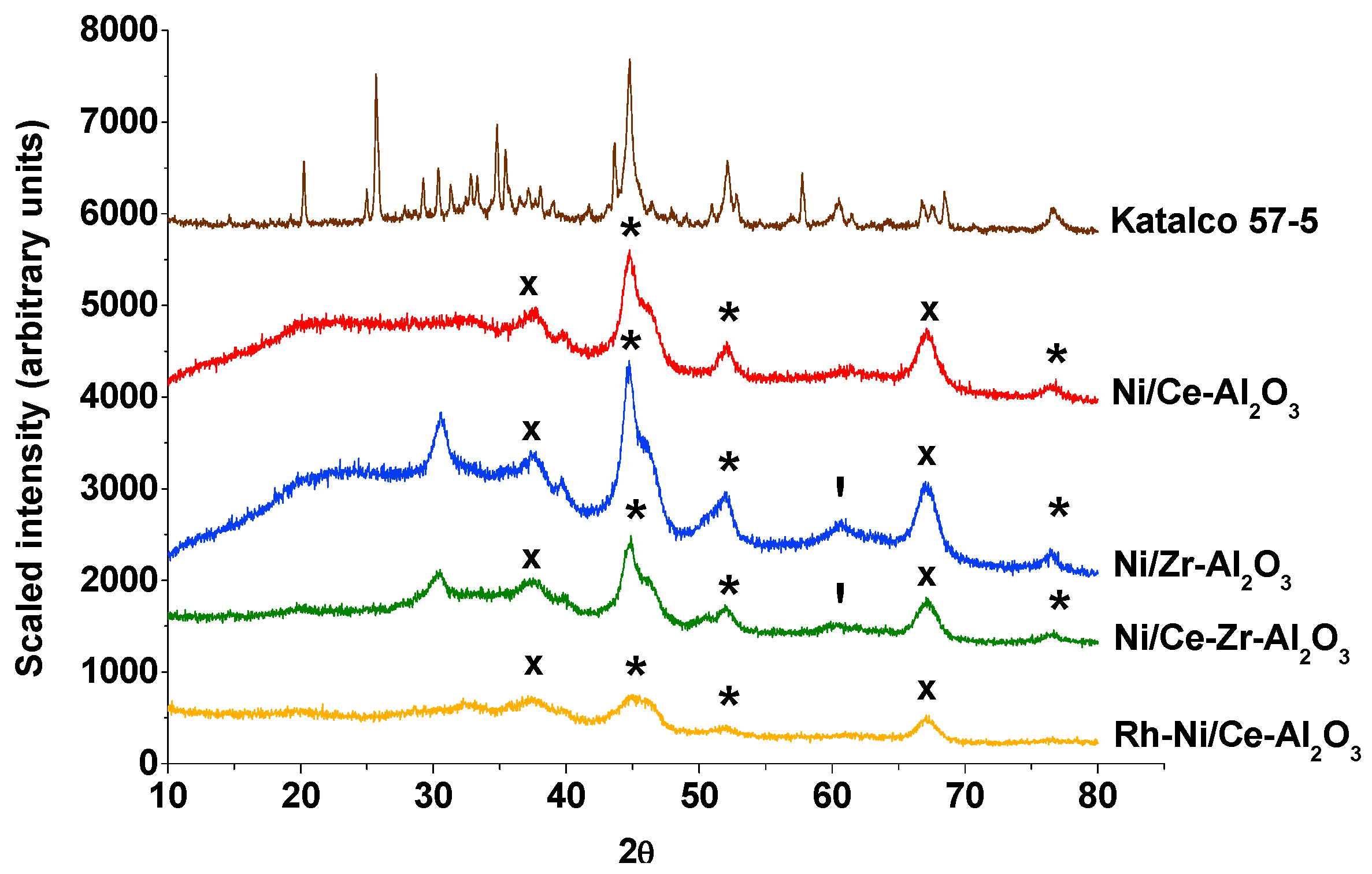
2.1. Fresh and Reduced Catalysts Characterization
Regarding the physico-chemical properties of the catalysts, a specific surface area (SSA) of 255 m
2/g was measured for the commercial bare γ-Al
2O
3·support. As it is observed in
Table 1, when modifiers were incorporated in the γ-Al
2O
3·support, the SSA decreased. Then, when metals were added in order to prepare the corresponding catalysts, the SSA value decreased even more. However, regarding the measured pore diameter (PD), the values of both unreduced catalysts and supports were similar, which could be because of the relatively low metal loadings (~10 wt. %) and good dispersion of the metals on the supports. In
Table 1, the textural characterization of the catalysts under investigation is shown.
Reduced catalysts’ physico-chemical properties were also determined for the Ni/Ce-Al
2O
3, Ni/Ce-Zr-Al
2O
3, and Rh-Ni/Ce-Al
2O
3 (see
Table 1) in order to observe possible differences between calcined and reduced catalysts. For that purpose, the samples were reduced following the same procedure as in the experiments (see
Section 3.2). In general, when catalysts were reduced the surface area, pore volume and pore diameter decreased significantly. However, the surface area of the Ni/Ce-Zr-Al
2O
3 catalyst decrased only slightly, and its pore diameter increased while its pore volume remained constant.
In
Table 2, calcined catalysts elemental compositions (ICP-OES), H
2-chemisorption, and XRD results are summarized. Regarding the metal composition determined by ICP-OES, similar values to the intended ones were measured for all the catalysts prepared. However, the largest differences were more evident with the catalysts containing Ce. This can be related to dissolution processes of the samples previous to analysis by ICP-OES. For ICP-OES analysis, a solution that consisted of HCl, HNO
3, and HF was employed for Ni, Rh, Zr and Ca quantification. In the case of Ce, however, it was not possible to detect it due to CeF
2 precipitate formation. For Ce quantification, a solution consisting of H
2O
2 and HNO
3 was used instead [
22]. After preparing the corresponding solutions, the catalysts were dissolved using a digester and thereafter analyzed in an ICP-OES instrument. Despite this, small particles of the catalysts could still remain in solid state (not being perceptible), and therefore, not measured.
Using the hydrogen chemisorption technique, the highest metal surface area (MSA) and dispersion (D) were measured for the bimetallic catalysts. It has been reported that the addition of a small amount of noble metals to non-noble metal catalysts improves the previously mentioned properties due to the spillover effect [
23,
24], and this was evident with the Rh-Ni/Ce-Al
2O
3 catalyst. Therefore, higher catalytic activity is expected with this catalyst. Finally, from XRD, smaller Ni crystal sizes were measured for all the catalysts under investigation, when compared to the ones measured by H
2 chemisorption. The observed differences could be related to Ni particles sintering during the reduction at high temperature for the samples analyzed by H
2 chemisorption. It is well known that the dispersion of the particles onto the surface of the supports increases when small particles are obtained. Therefore, the highest metal dispersion and surface area measured for the bimetallic catalysts indicates the formation of smaller particles in this catalyst. Therefore, it is expected that the Rh-Ni/Ce-Al
2O
3 catalyst would have more active sites available for conversion of CH
4, CO
2, and CO to produce hydrogen.
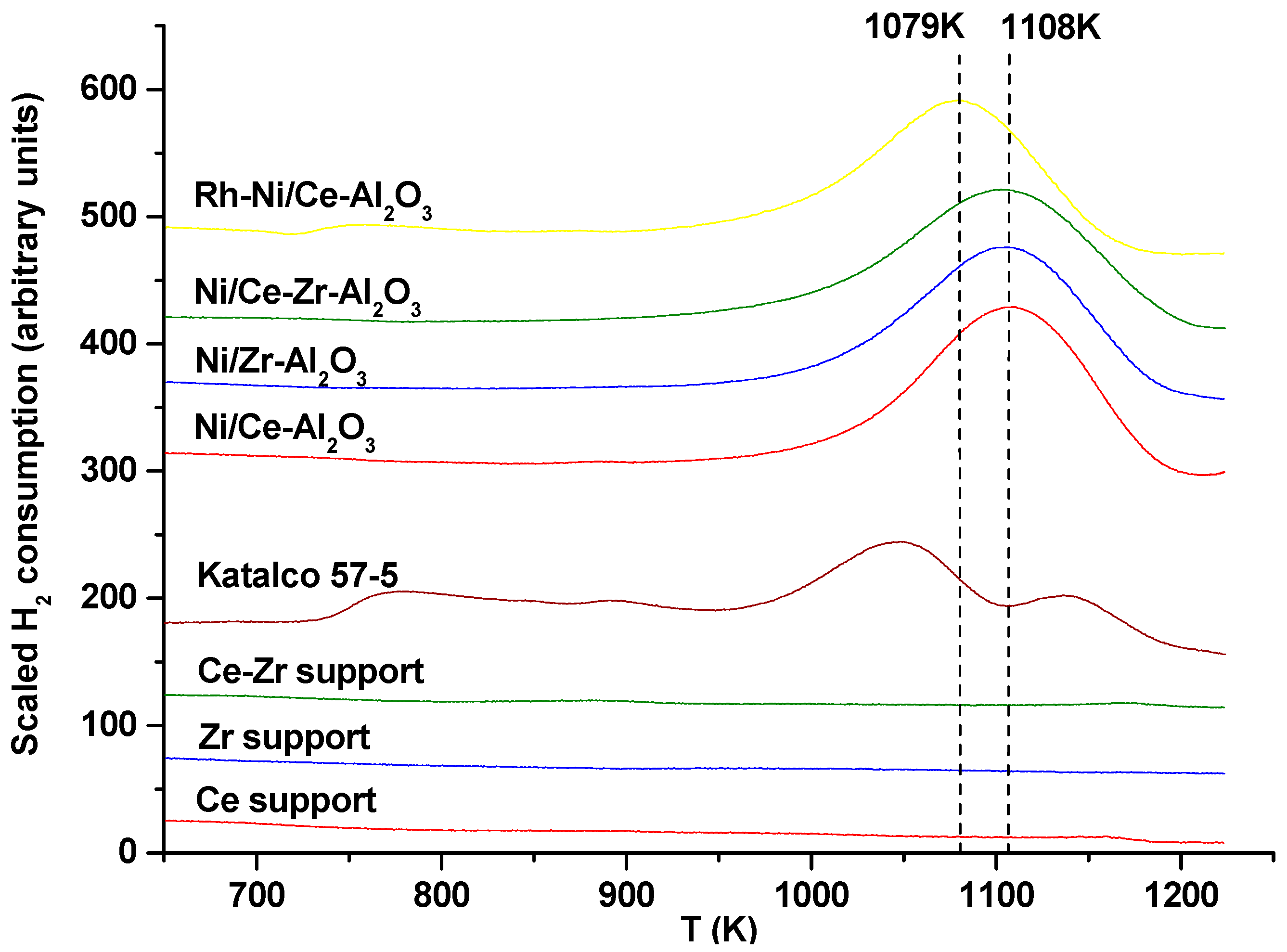
In addition to the above techniques, TPR profiles for all the catalysts have also been previously published [
19,
25] (see
Figure A2), showing slight differences among the catalysts under investigation. One significant difference is that with the commercial catalyst, Katalco 57-5, four different reduction peaks were detected, the biggest one being at 1048 K. For the rest of the monometallic and bimetallic catalysts, the main reduction peaks appeared in the range 900 K to 1200 K. Different reduction peaks were observed for the promoted supports. For Ce-Zr-Al
2O
3, Ce-Al
2O
3 and Zr-Al
2O
3 supports contributions were measured at 690 K, 880 K, 1000 K and 1170 K. For all of them very small peaks were observed. When calcined catalysts and calcined supports profiles are compared, lower H
2 consumption is observed for the calcined supports.
The main peaks of Ni/Zr-Al
2O
3, Ni/Ce-Zr-Al
2O
3 and Ni/Ce-Al
2O
3 catalysts appeared at 1103 K, 1105 K and 1108 K, respectively. Thus, this is a possible indication of Ce reduction taking place at higher temperatures than Zr reduction. If Ni/Ce-Al
2O
3 and Rh-Ni/Ce-Al
2O
3 catalysts are compared, a lower reduction temperature was needed for the latter (1079 K). This could be related to the “spill over” effect, which improved the nickel reducibility [
26]. According to the literature, these broad peaks can be attributed to the contribution of three different species. The peaks around 950 K are attributed to the reduction of NiO-Al species weakly interacting with the alumina support, while the reduction peaks reported at higher temperatures are related to the reduction of highly dispersed non-stoichiometric amorphous nickel aluminate spinels (at around 1050 K) and to a diluted NiAl
2O
4-like phase (at 1100 K) [
27].
2.2. Activity Results
Once the characterization of the fresh catalysts was performed, the activity experiments were carried out using a Microactivity plant (see
Section 3.2). The experimental conditions of the tests were selected based on the results obtained in a previous work of the authors [
7], steam to carbon, H
2O/C = 1.0 and oxygen to carbon, O/C = 0.25 (molar ratio). These results were again reproduced by all the catalysts under investigation by reaching very high conversions, near to the ones predicted by thermodynamic equilibrium (at 800 °C and 1 atm: X
CH4 = 99.6, X
CO2 = 34.7, H
2 yield = 75.2). The Katalco 57-5 catalyst was used to establish the appropriate hydrogen sulfide concentration to the feed that would allow study of the deactivation phenomenon. To achieve this, a slow deactivation was targeted, in order to observe the deactivation curve and the resistance of the catalysts. Therefore, after one hour of stable operation under tri-reforming conditions, different H
2S concentrations were continuously added to the system.
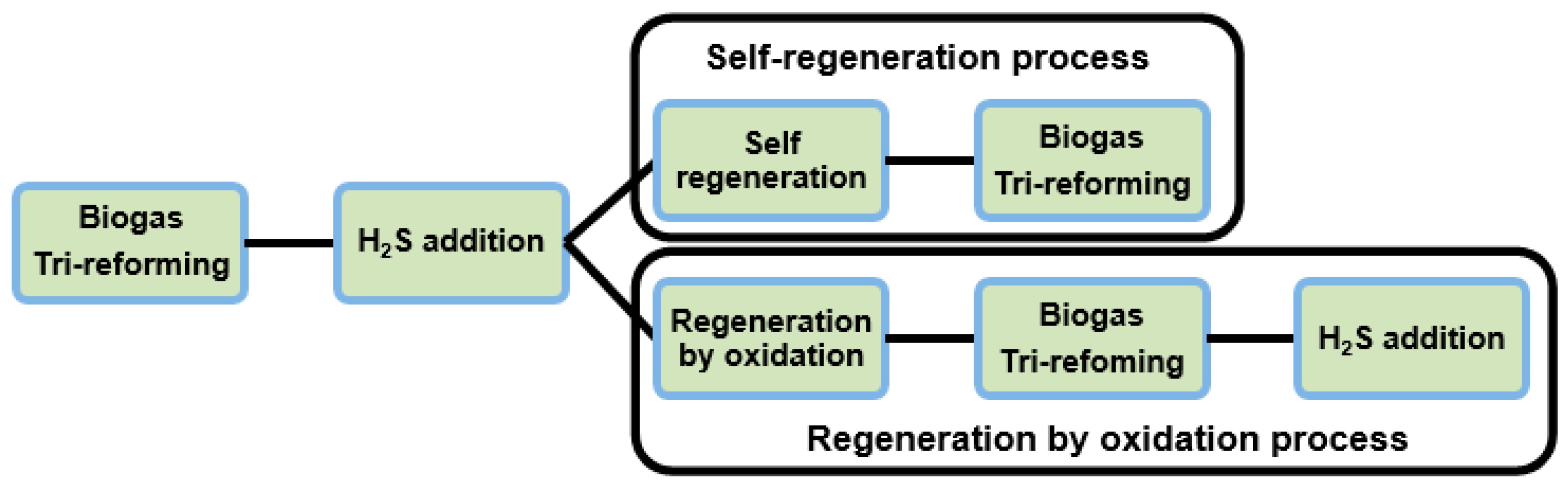
When 1.0%, 0.5%, 0.1%, 100 ppm, and 50 ppm (vol %) of H2S were constantly added to the system, it was not possible to observe the deactivation curves because this process was very fast. Finally, it was possible to observe the catalysts deactivation phenomena by adding continuously 25 ppm of H2S to the system. Therefore, the H2S concentration of 25 ppm was selected to provoke the deactivation by sulfur for the rest of the catalysts under investigation. Once the catalysts were fully deactivated, the self-regeneration and oxidation regeneration processes were studied.
The self-regeneration consisted of just removing the continuously fed H
2S from the system until a stable activity was measured. For the regeneration by oxidation, the temperature was set at 873 K to feed a synthetic air stream for 1 h to the system after switching off all the reactant gases. Then, the experiments continued under the same initial tri-reforming conditions, free of H
2S. The activity results obtained are presented in
Figure 1,
Figure 2 and
Figure 3, and which are described below.
For all the catalysts under investigation, higher methane conversion was measured than carbon dioxide conversion because carbon dioxide is only consumed though the DR reaction, which is the most endothermic reaction, and SMR, CPO and WGS are more favorable, which is consistent with the observed high H2/CO ratios. This was expected from the thermodynamic equilibrium trends.
2.2.1. Katalco 57-5 Ni-Al2O3 Catalyst
In the case of Katalco 57-5 catalyst, see
Figure 1, a stable and high activity (X
CH4 = 96.6, X
CO2 = 32.3, H
2 yield = 74.6) was measured operating under tri-reforming conditions. When H
2S was introduced to the system, the activity of the catalyst dropped. However, no regeneration was observed after the deactivation by any of the two regeneration processes studied.
During deactivation, the H2/CO ratio increased, which suggests that the main reactions occurring could be CPO and WGS. After deactivation, the negative CO2 conversion is also consistent with the WGS reaction. The previously mentioned reasons justify the absence of O2 as a reaction product and the H2/CO ratio higher than 2, assuming that the contribution of DR and SMR reactions is negligible.
2.2.2. Ni/Zr-Al2O3 Catalyst
The catalytic activity measured for this catalyst operating under tri-reforming conditions was also high (X
CH4 = 99.4, X
CO2 = 34.3, H
2 yield = 74.5), as shown in
Figure 2. When H
2S was continuously added to the system, quick catalyst deactivation was observed. Afterwards, as happened for Katalco 57-5 Ni-Al
2O
3 catalyst, no catalyst regeneration took place after both self-regeneration and regeneration by oxidation processes. However, the Ni/Zr-Al
2O
3 catalyst showed a slightly higher activity once being deactivated, compared to the Katalco 57-5 Ni-Al
2O
3 catalyst, as it is observed by its higher methane conversion and lower CO
2 conversion. This could be due to the DR reaction.
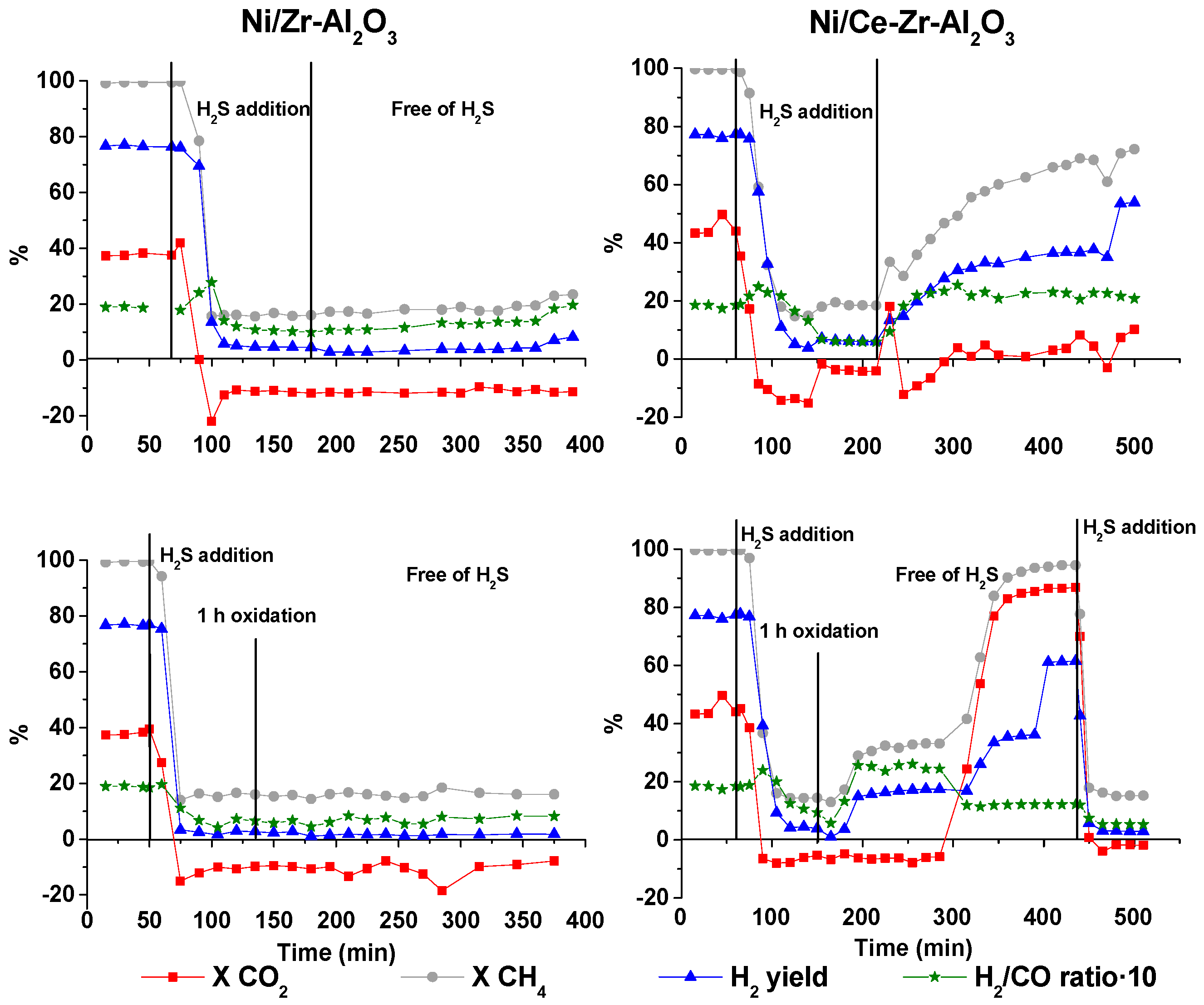
2.2.3. Ni/Ce-Zr-Al2O3 Catalyst
The same experimental schedule (see
Figure 4) was carried out for the Ni/Ce-Zr-Al
2O
3 catalyst. Although this catalyst recovered part of its activity after the regeneration processes, the conversion values measured after the self-regeneration process were lower than the ones obtained at the beginning of the experiment. The presence of H
2S and subsequent deactivation could alter the morphology of the surface since no catalytic reactions occurred during the period in which the catalyst was fully deactivated. Then, when the continuous H
2S addition was stopped, the catalyst recovered most of its activity, but the very low CO
2 conversion indicates a combined poor selectivity to the DR reaction and concomitant presence of WGS reaction.
On the other hand, this catalyst recovered the catalytic activity after being self-regenerated (presented at the top of
Figure 4). This regeneration process successfully allowed recovery of the initial conversion values. However, through the regeneration by the oxidation at low temperature (presented at the bottom of
Figure 4), significantly higher carbon dioxide conversions were measured compared to the ones measured after the self-regeneration process and before deactivation. After regeneration by oxidation, the main reaction is SMR, but after 300 min of time on stream, the selectivity to DR reaction increased significantly, as indicated by the increase in the CH
4 and CO
2 conversion, which is also consistent with the preponderance of this reaction with regard to the WGS. The last step of this study corresponded to a second H
2S addition, in which a quicker deactivation was noticed compared to the first one.
2.2.4. Ni/Ce-Al2O3 Catalyst
In the case of the catalyst modified only with CeO2, a similar behavior to the one observed for the Ni/Ce-Zr-Al2O3 was noticed with respect to the self-regeneration process. However, for the Ni/Ce-Al2O3 catalyst the activity recovered was slightly higher because the methane and carbon dioxide conversions achieved were almost the same as the ones measured at the beginning of the experiment. Regarding the regeneration process by oxidation at low temperature, after two hours of fully deactivated operation, this catalyst recovered its reforming capacity. As a result, CH4 conversion reached values similar to the ones obtained before the sulfur addition and CO2 conversion reached higher values than the ones initially measured.
Thus, after the self-regeneration process, higher conversions were achieved by the catalyst containing both support modifiers, CeO
2 and ZrO
2, especially for carbon dioxide (86.8%,
Figure 2, right), than for the catalyst containing only CeO
2 (79.5%,
Figure 3, right). In the case of the only CeO
2-containing catalyst, with respect to the regeneration process by the oxidation at low temperature, it needed longer time to be regenerated. Moreover, for this catalyst, quicker deactivation occurred when H
2S was added to the system a second time.
2.2.5. Rh-Ni/Ce-Al2O3 Catalyst
The main difference when operating with this catalyst is its higher resistance against deactivation. Thus, apparently the presence of a small amount of Rh increased the deactivation resistance. From the catalytic results, the carbon dioxide conversions achieved were positive and the methane conversions quite significant, contributing to hydrogen yields around 30%. Therefore, this was the only catalyst showing relevant activity operating with 25 ppm of H2S.
When the self-regeneration process was carried out, the catalyst recovered its activity obtaining values close to the ones measured at the beginning of the experiment (
Figure 3, top left). This catalyst was the one showing the highest activity after the self-regeneration process. In the study of the catalyst regeneration by the low temperature oxidation, this catalyst showed a very quick activity recovery compared to the previous ones. Additionally, lower carbon dioxide conversion was measured but at much higher hydrogen yield. Therefore, after regeneration by the low temperature oxidation, this catalyst did not show the previously mentioned selectivity to the reverse WGS reaction. This indicates that the Rh-containing catalysts resist the addition of H
2S as shown here by the tendency of this catalyst to recover its initial activity.
Thus, this was a remarkable catalyst achieving similar conversion values before deactivation and after regenerations because of its high recovery capacity without being influenced by the rWGS reaction. Furthermore, this catalyst was the only one showing significant activity after the second H2S addition, while the rest of the catalysts suffered an initial quick and complete deactivation, with a posterior second deactivation being even more drastic. For the bimetallic catalyst, hydrogen yields around 20% and methane conversions near 30% were measured after the second H2S addition.
2.3. Fresh and Tested Catalysts Characterization by XPS
In order to compare the characteristics of the fresh and tested catalyst surfaces, a characterization using the XPS technique was carried out for all the catalysts. Therefore, apart from the characterization results obtained for the fresh catalysts, the corresponding ones were also compiled for each catalyst after operating under the abovementioned conditions in order to detect possible differences between both regeneration processes studied.
It is important to highlight that in the case of the regeneration process by oxidation at low temperature (a process that lasted 1 h), the catalysts completely deactivated after the last H
2S addition. Characterization of the deactivated catalysts was carried out at this point. The comparison given in
Table 3 is between fresh and calcined catalysts with the samples obtained after finishing all the procedure, as specified in
Figure 4.
According to the results compiled in
Table 3, the highest Ni/Al ratio measured for Katalco 57-5 catalyst can be related to the higher Ni content present in this catalyst (see
Table 2). Focusing on the rest of γ-Al
2O
3-based catalysts, lower Ni/Al ratios than the measured for Katalco 57-5 catalyst were detected, the ratio measured for the Ni/Ce-Zr-Al
2O
3 catalyst being especially low. Then, the presence of both CeO
2 and ZrO
2 affected to the Ni/Al ratio. However, for the bimetallic catalyst, Rh-Ni/Ce-Al
2O
3, a ratio of 0.16 was determined, and this high value could be a result of a better dispersion of the metallic phase as confirmed by H
2-chemisorption results.
The Ni/Al ratios measured for the catalysts tested after the self-regeneration process (self-regeneration + tri-reforming) were generally lower than fresh calcined catalysts, which can be directly related to the carbon deposition detected and determined by the high C/Al ratios (see
Table 3). However, the catalysts containing CeO
2 and CeO
2-ZrO
2 improved their Ni dispersion, which could be due to more C deposited onto Al
2O
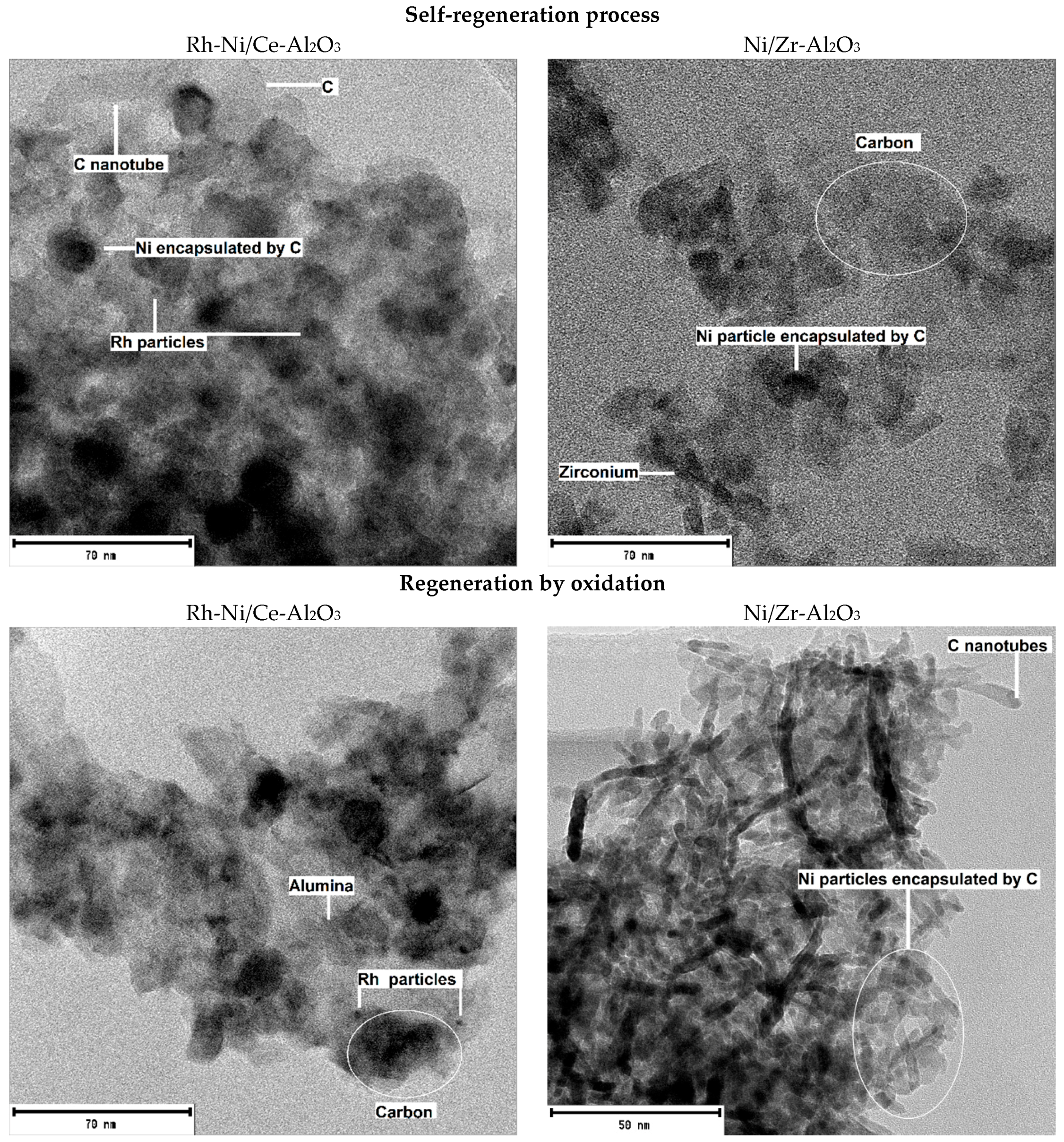
3 than onto Ni, as observed in the Transmission Electron Microscope (TEM) pictures (
Figure 5). In addition, higher carbon deposition was measured by XPS for the catalysts tested under the self-regeneration process. This fact can be explained by the influence of the low-temperature oxidation process, which contributed to remove part of the carbon deposited onto the catalyst surfaces.
Furthermore, the sulfur/alumina (S/Al) ratio was also measured for all the catalysts under investigation, with special interest paid to the catalysts tested under the regeneration process by oxidation at low temperature (regeneration by oxidation + tri-reforming + H2S addition), because for the completely deactivated catalysts, some sulfur deposition onto the catalytic surfaces was expected. However, only for the Katalco 57-5 Ni-Al2O3 catalyst some deposition of sulfur was detected, S/Al = 0.08, and corresponded to the catalyst tested after the self-regeneration process (self-regeneration + tri-reforming). The non-detection of sulfur in the rest of catalysts could be due to the low amount of sulfur deposited onto the surface of the catalysts, which could not be detected by this technique, but sufficient to deactivate catalyst active sites.
In order to determine the reasons why some catalysts suffered from deactivation and others recovered partially or almost all their initial activity, TEM images were acquired for the catalysts showing the best, Rh-Ni/Ce-Al
2O
3, (left of
Figure 5) and the worst, Ni/Zr-Al
2O
3, (right of
Figure 5) activity results. For both, the images correspond to the catalysts after the self-regeneration process and after the regeneration by oxidation processes. The corresponding micrographs can be observed in
Figure 5.
These pictures are the most representative ones among all the micrographs obtained by this technique. For all the catalysts and areas analyzed no sulfur was detected by the Energy Dispersive X-ray Spectroscopy (EDX), which is in good agreement with the results obtained by XPS for the Rh-Ni/Ce-Al
2O
3 and Ni/Zr-Al
2O
3 catalysts. In addition, the presence of carbon can easily be observed, which is also in concordance with XPS results. Furthermore, the type of the carbon deposited onto the surface can be analyzed by TEM. As can be observed in
Figure 5, encapsulated carbon was predominant, although some carbon filaments were also observed. The latter carbon appears as carbon nanotubes, which grow from the Ni particle.
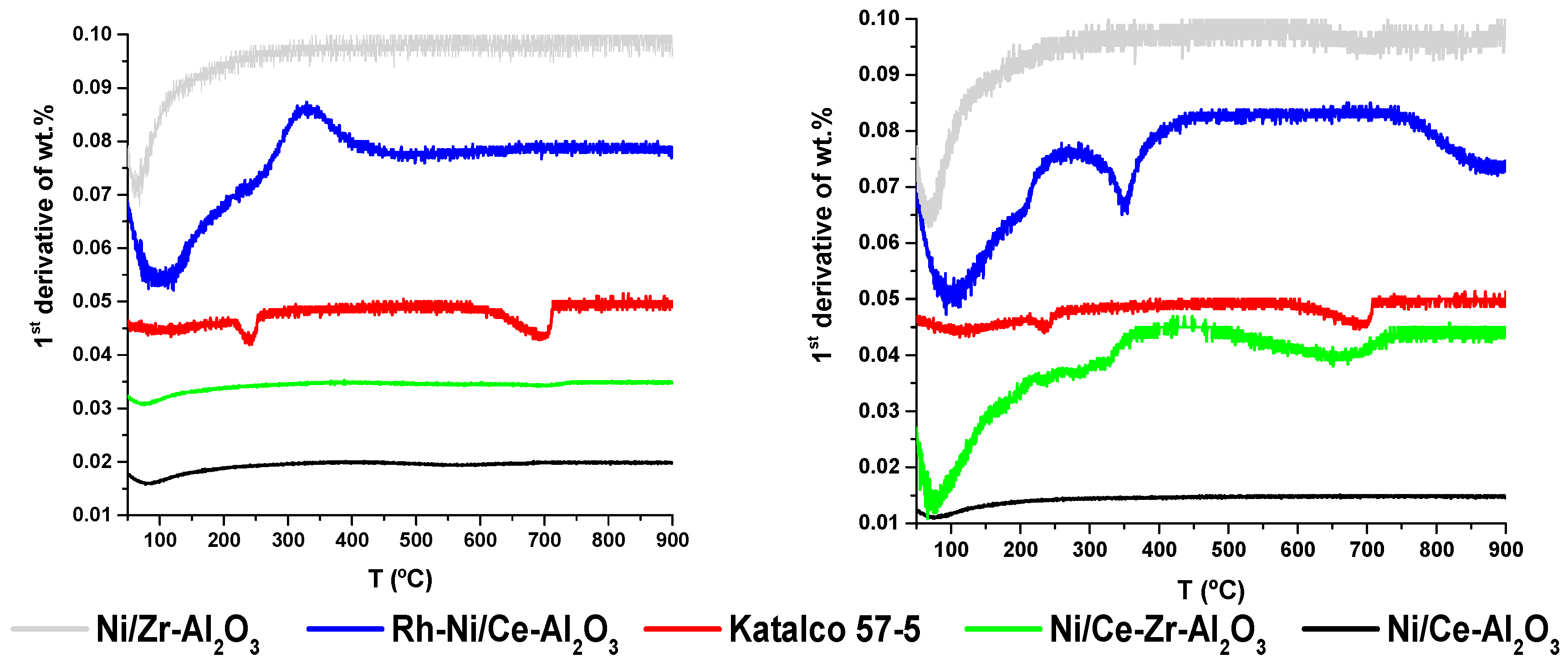
In order to quantify the presence of the C deposited onto the catalysts surfaces, Temperature Programmed Oxidation (TPO) experiments were performed by Thermal Gravimetric Analyzer (TGA). The results shown in
Figure 6 represent the evolution of the temperature with the first derivative of the percentage weight loss, in order to detect easily the negative or positive peaks related to the weight loss or gain, respectively. Additionally, the last column of
Table 3 summarizes the total weight loss percentages.
Regarding the type of C, it has been reported that the oxidation of filamentous carbon associated with nickel particles occurs between 300 °C and 527 °C while the oxidation of carbon with different degrees of graphitization takes place above 527 °C [
28,
29,
30]. From
Figure 6 and the results compiled in
Table 3 it can be seen that in general, very low weight losses were measured. Focusing on the left of
Figure 6, there are negative peaks at low temperature, until 150 °C, attributed to the evaporation of water [
31], specially detected for the Rh-Ni/Ce-Al
2O
3 and Ni/Zr-Al
2O
3 samples. Then, at increasing temperatures, these samples gain weight due to the oxidation at low temperature of the reduced Rh and Ni surface species. Regarding the carbon deposition, a negative peak was detected for Katalco 57-5 catalyst in the temperature range between 600 °C and 700 °C, which could correspond to the oxidation of carbon with different degrees of graphitization. This weight loss corresponds to 0.16% of the mass of the catalyst, and fits properly with the results obtained by XPS. Therefore, the C measured by XPS for the rest of catalysts, which shows a much lower ratio compared to the Katalco 57-5 one, could be considered negligible because no significant peaks were detected for these catalysts by TPO analyses in the same temperature range.
In the characterization of the catalysts tested after the regeneration by oxidation process, the initial large peaks at low temperature, below 100 °C, also correspond to moisture in the samples. Then, Rh and Ni particle oxidation takes place, mainly for the Rh-Ni/Ce-Al2O3 and Ni/Ce-Zr-Al2O3 catalysts, when the catalysts gain weight. The main difference compared to the previously described catalysts lies in the weight loss detected at the temperature around 350 °C for the Rh-Ni/Ce-Al2O3 and the negative peak for the Ni/Ce-Zr-Al2O3 catalyst in the temperature range between 500 °C and 700 °C. The weight loss for this last catalyst is around 0.52%. Then, in the temperature range between 800 °C and 900 °C, a significant peak was detected for the Rh-Ni/Ce-Al2O3 catalysts, which is attributed to the oxidation of carbon with different degrees of graphitization. The weight loss in this case corresponds to the 1.78% of the catalyst weight.
The low carbon deposition measured and the quick deactivation observed lead to the conclusions that the deactivation was mainly due to the addition of small amounts of sulfur as low as 25 ppm. As has been reported by other authors, the deactivation by sulfur can be described by the following general reaction Equation (8) [
32,
33], which is proposed to operate at high temperature:
As is shown above, all the CeO2-containing catalysts partially recovered their initial activity by both regeneration processes. This effect could be related to the oxygen fed in the regeneration process by oxidation at low temperature as well as the oxygen fed together with the other reactants for the self-regeneration process, which reacts according to reaction Equation (9).
For the catalysts tested under regeneration by oxidation process, a second sulfur addition was applied. At these conditions, no sulfur was detected, with the exception only of the Katalco 57-5 catalyst. There could be two effects to explain the non-detection of sulfur or SO2. The first one is the poor detection limit of the µ-GC and the characterization techniques used. The second deals with the possible sulfur desorption when the system was cooled down from 800 °C to atmospheric temperature. Therefore, the results of this work strongly suggest that catalyst deactivation mainly occurs via a mechanism of deposition of small amounts of sulfur on the catalysts active sites, which are successfully regenerated by sulfur oxidation with oxygen in presence of highly dispersed Ni and a noble metal.