Halide-Enhanced Catalytic Activity of Palladium Nanoparticles Comes at the Expense of Catalyst Recovery
Abstract
:1. Introduction
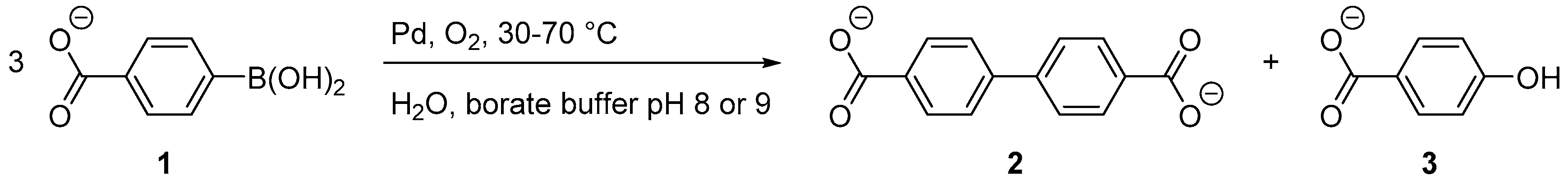
1.1. The Oxidative Homocoupling Reaction of Arylboronic Acids
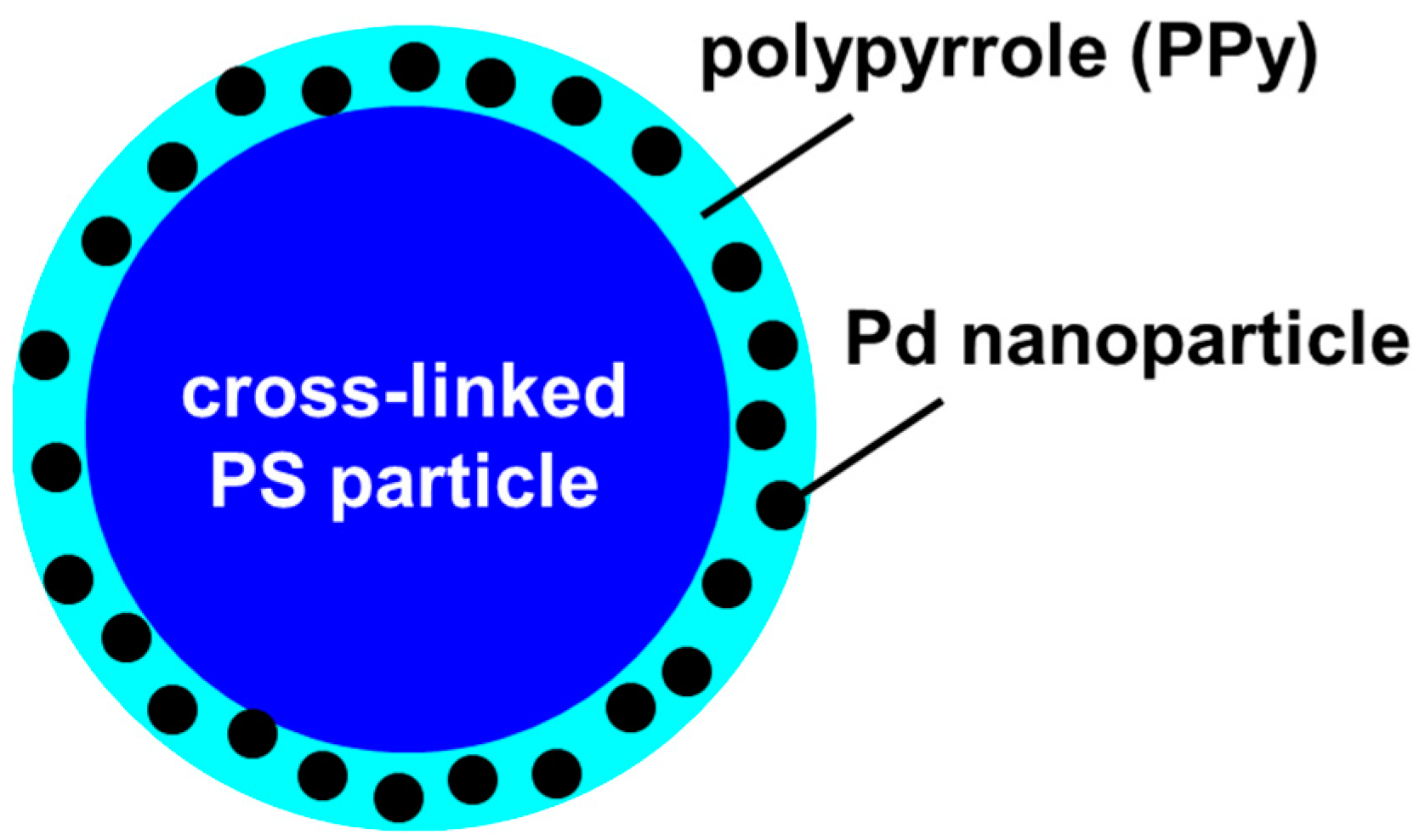
1.2. Nanoparticle Catalysis
1.3. The Suzuki-Miyaura cross Coupling Reaction and the Oxidative Homocoupling Reaction or Arylboronic Acids Catalyzed by Pd NPs
1.4. The Mechanism of Catalysis by Nanoparticles—On the Surface or in Solution?
1.5. Catalysis in Aqueous Solutions
1.6. Aims
2. Results and Discussion
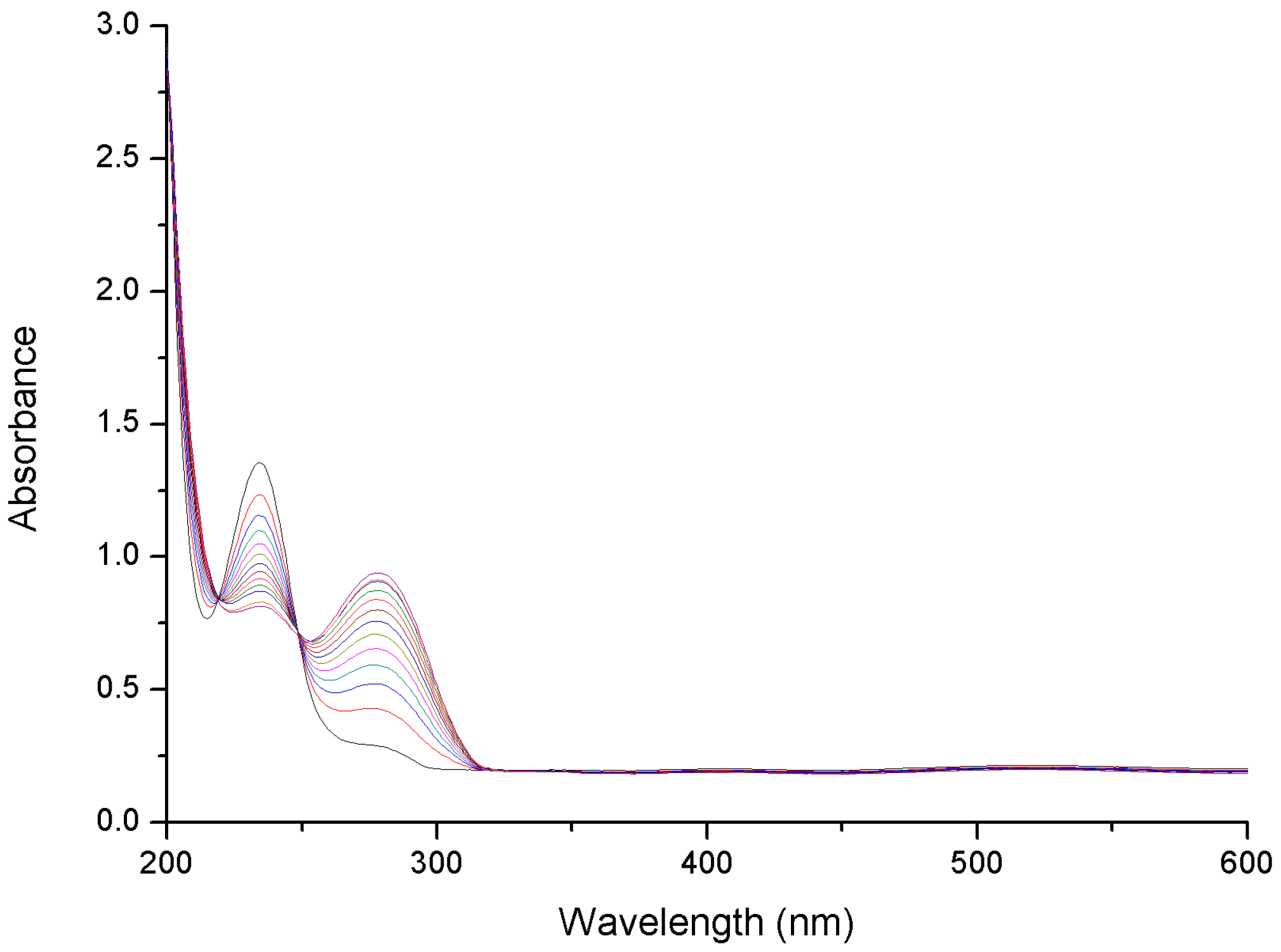
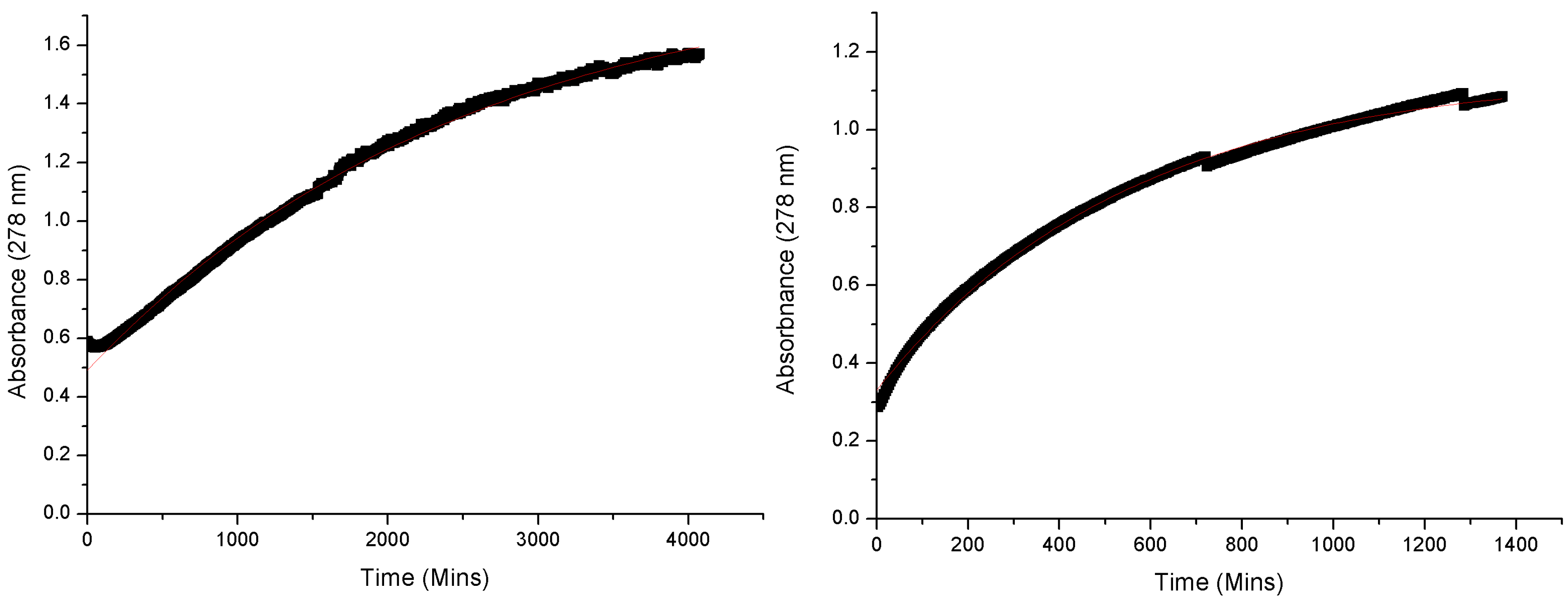
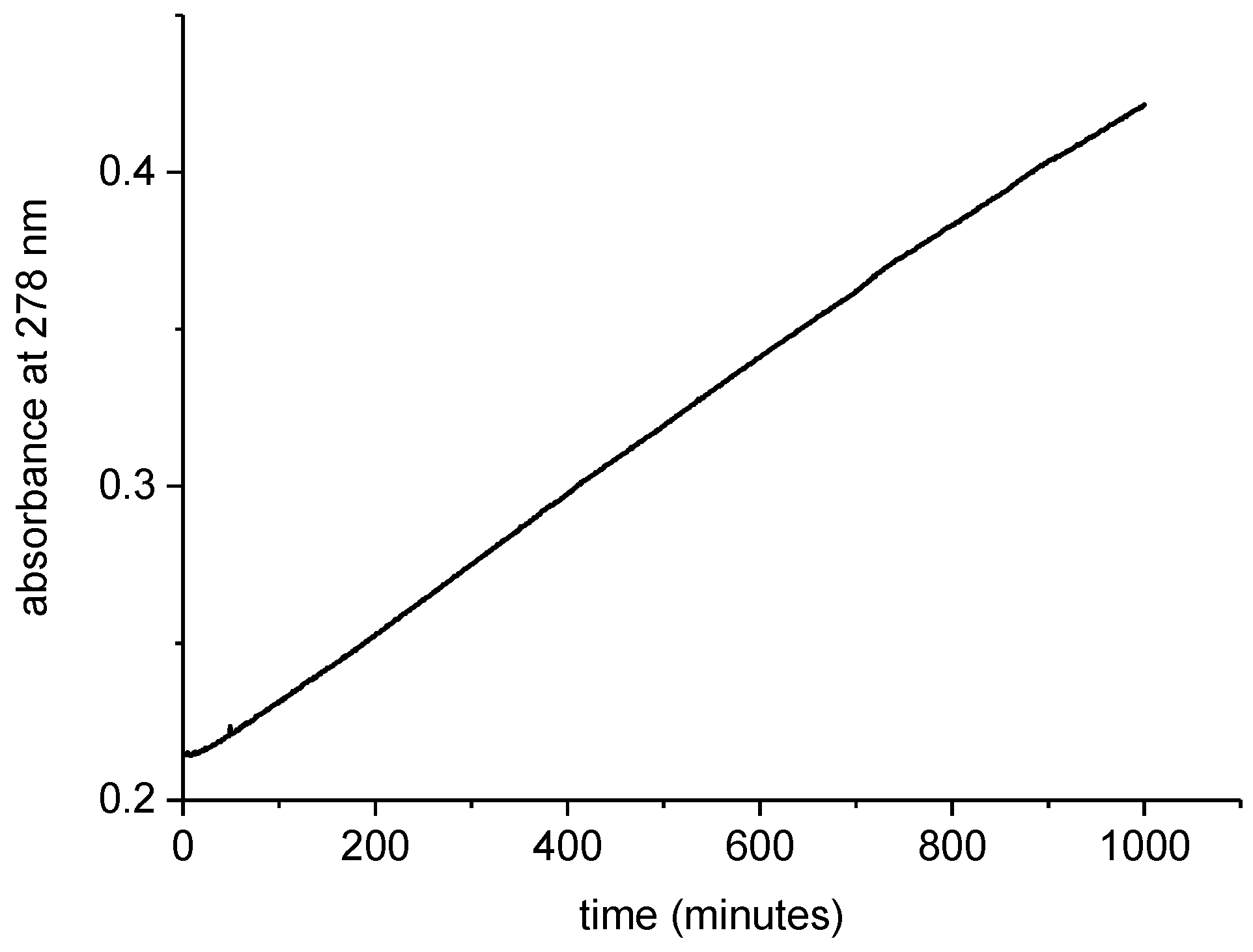
2.1. Reaction Parameters
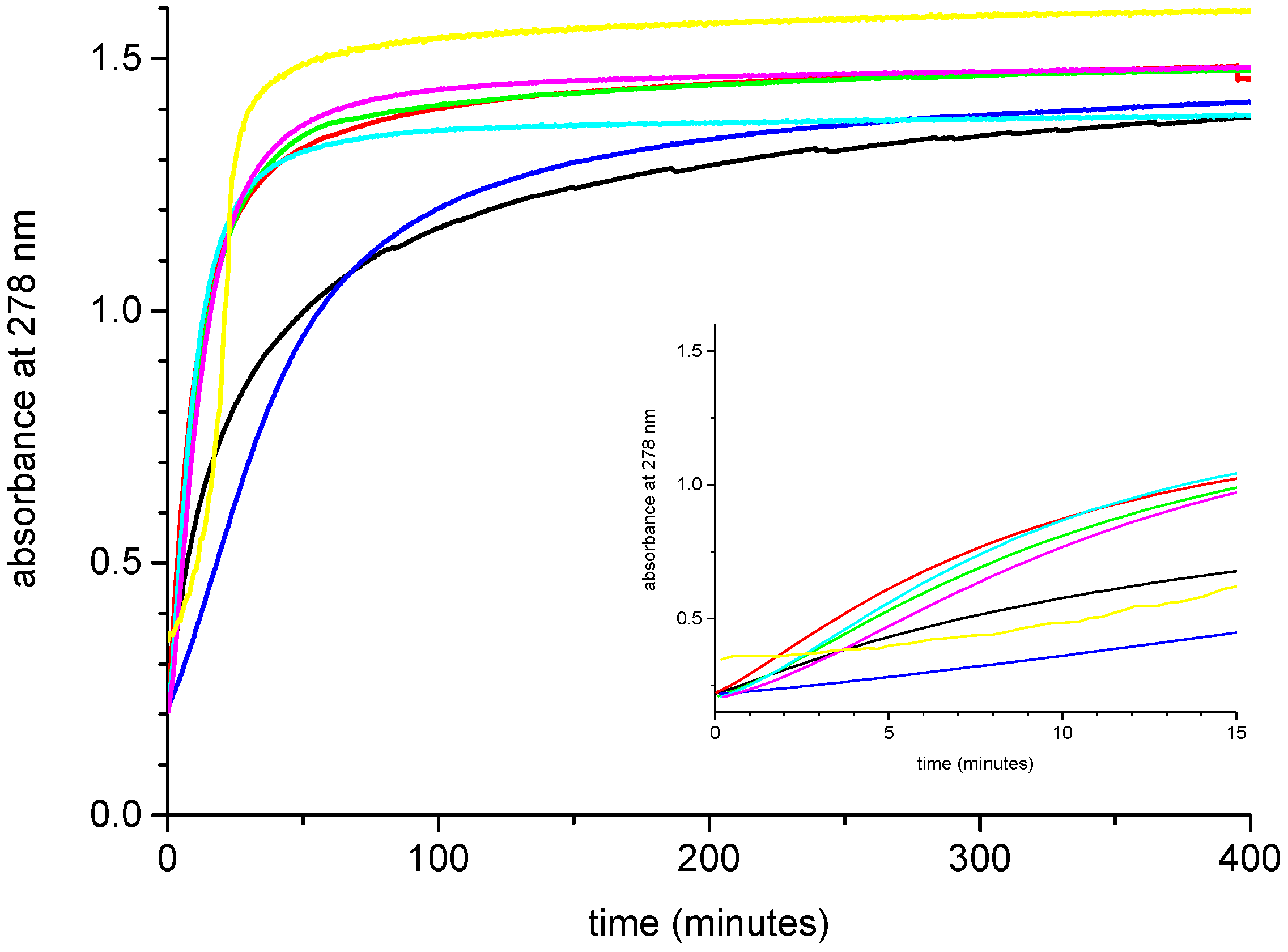
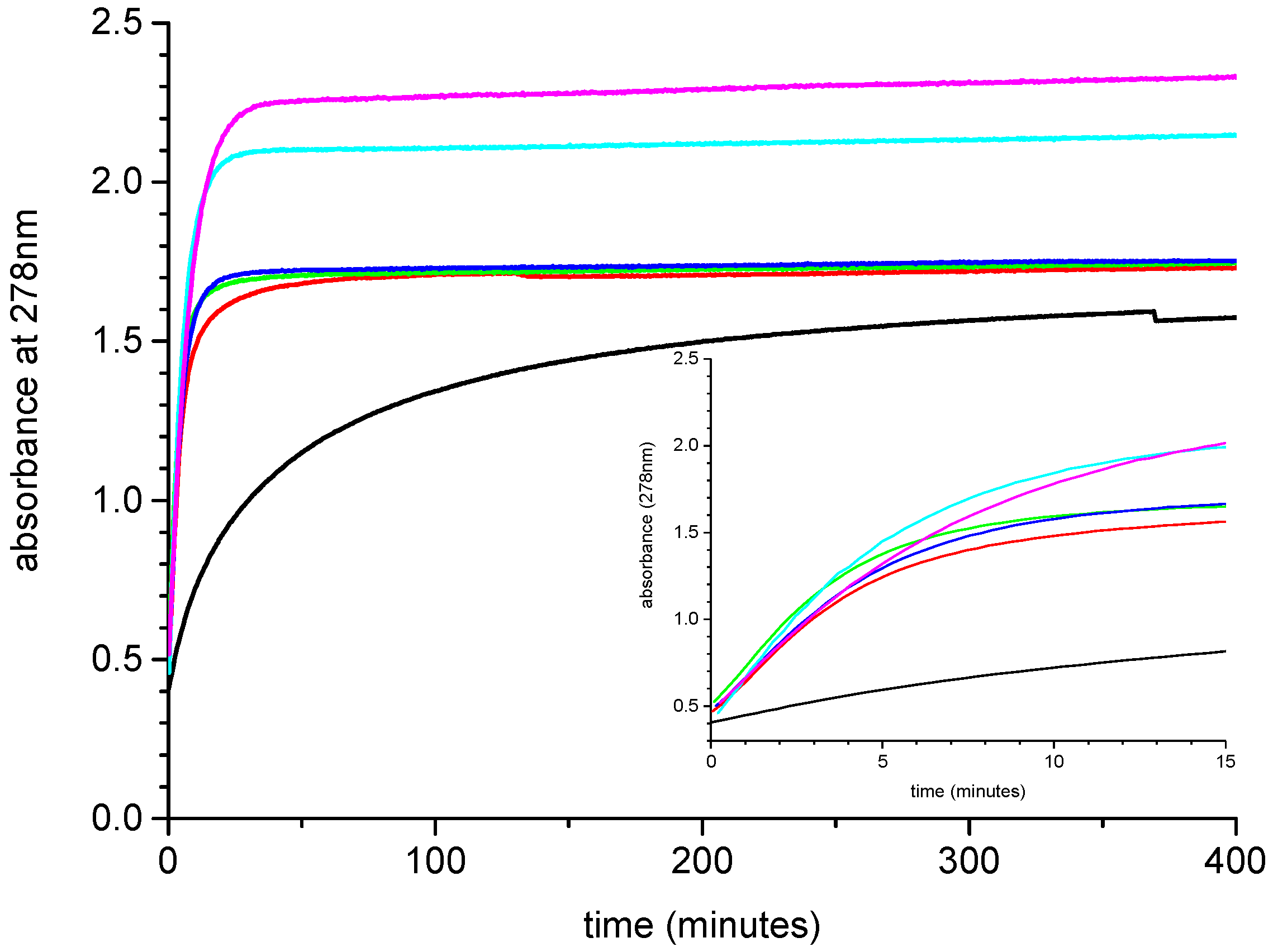
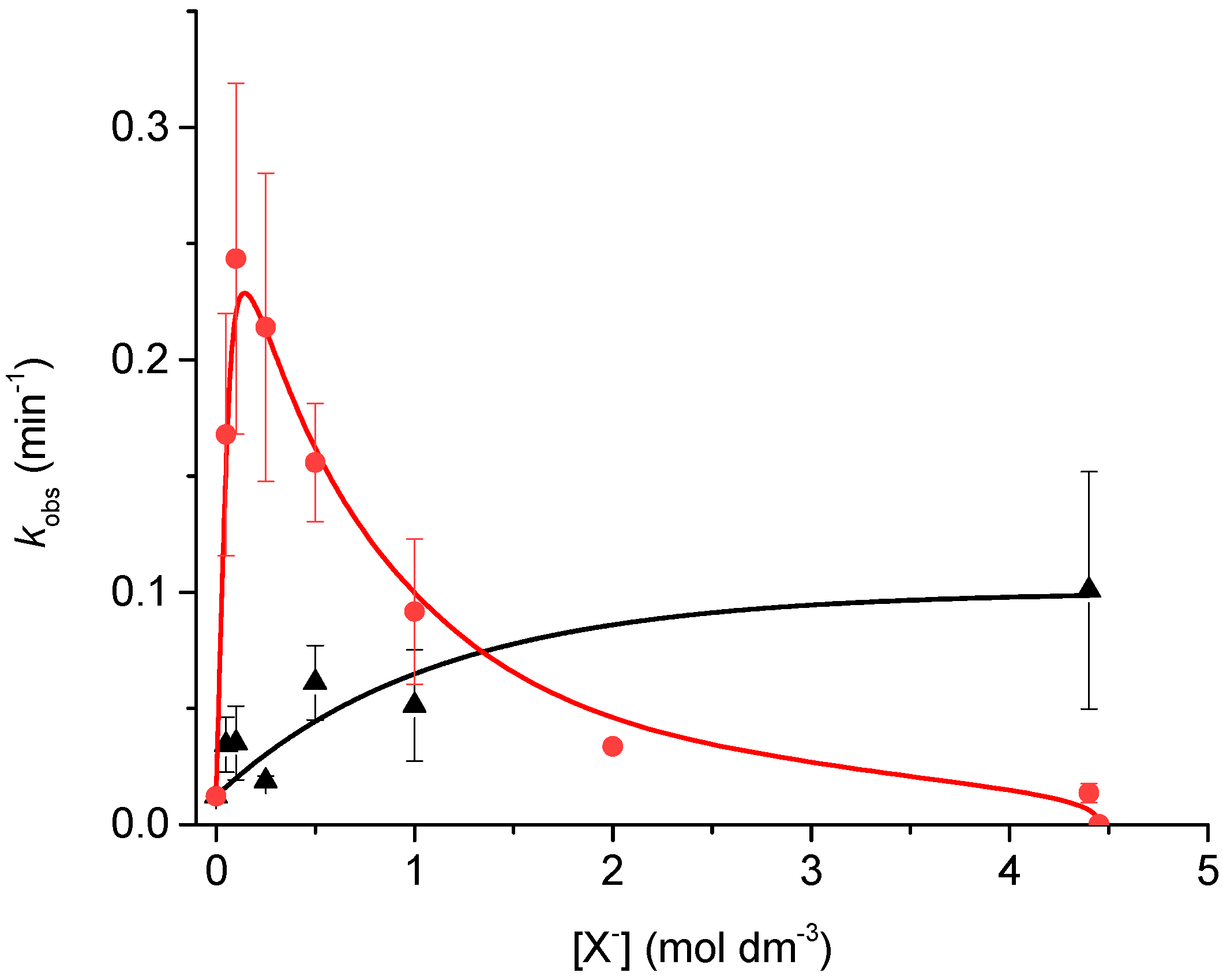
2.2. Effect of Added Halide on the Catalytic Activity of Pd-PPy-PS Nanocomposites
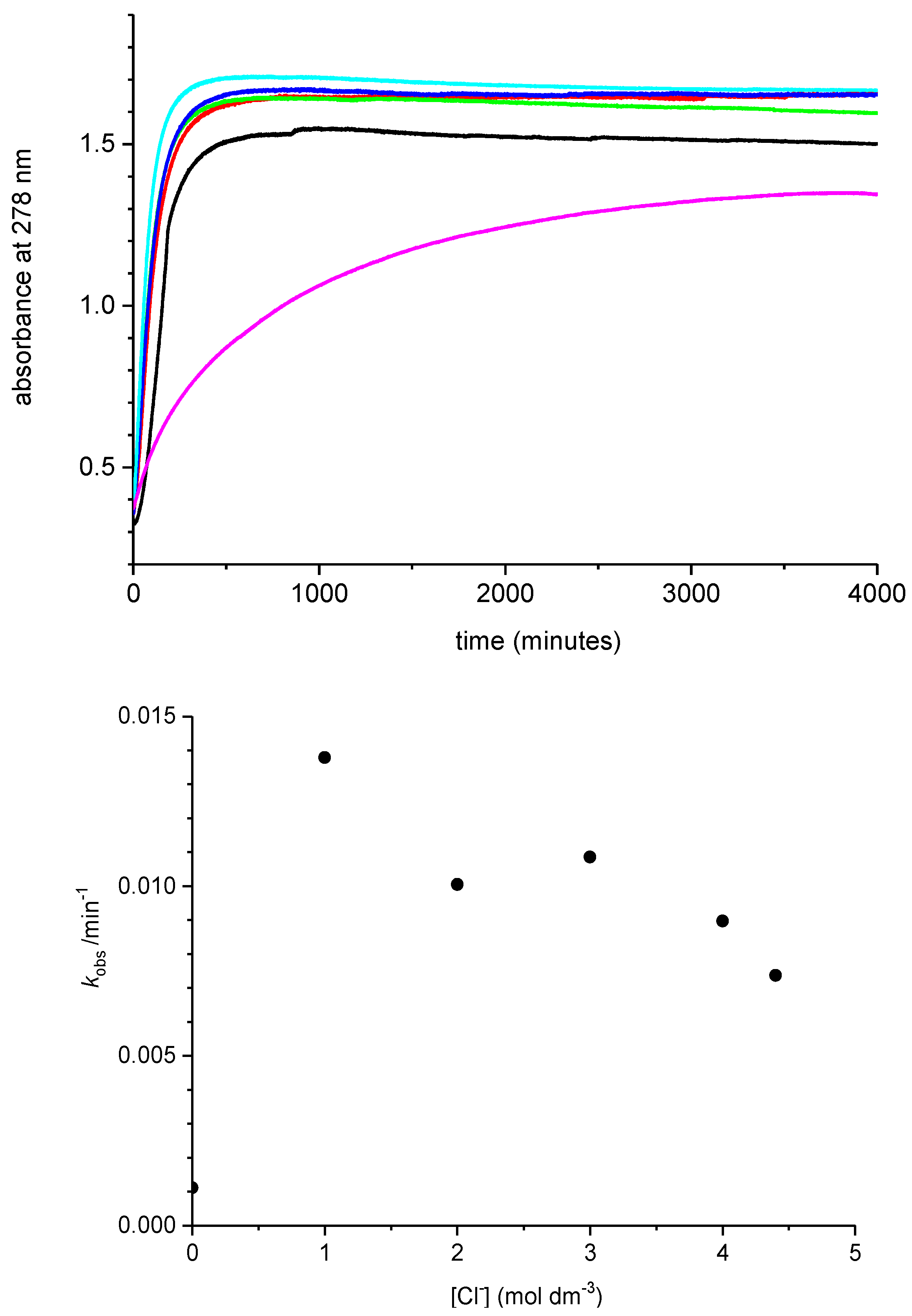
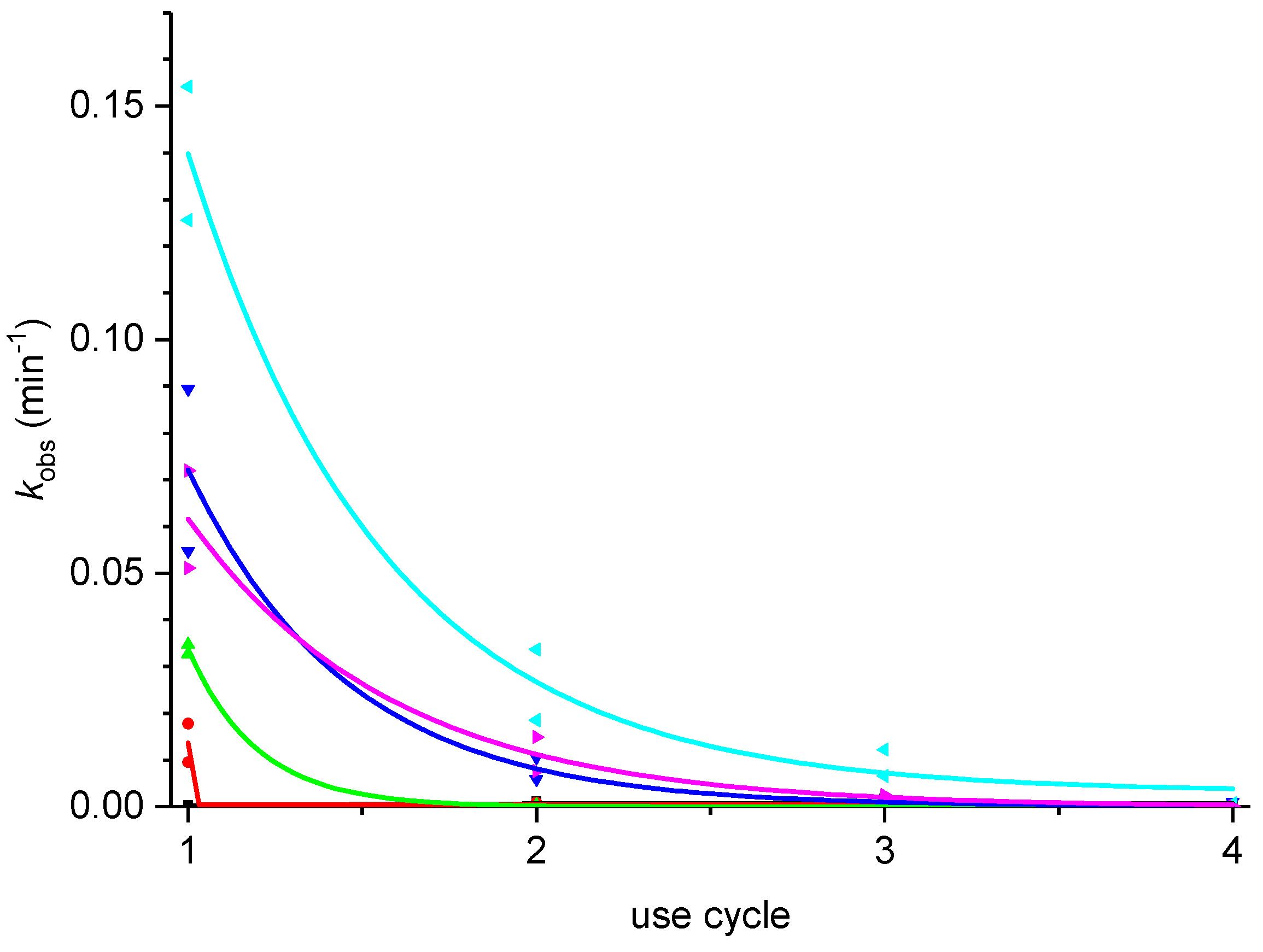
2.3. Effect of Added Halide on the Recovery of Catalytic Activity
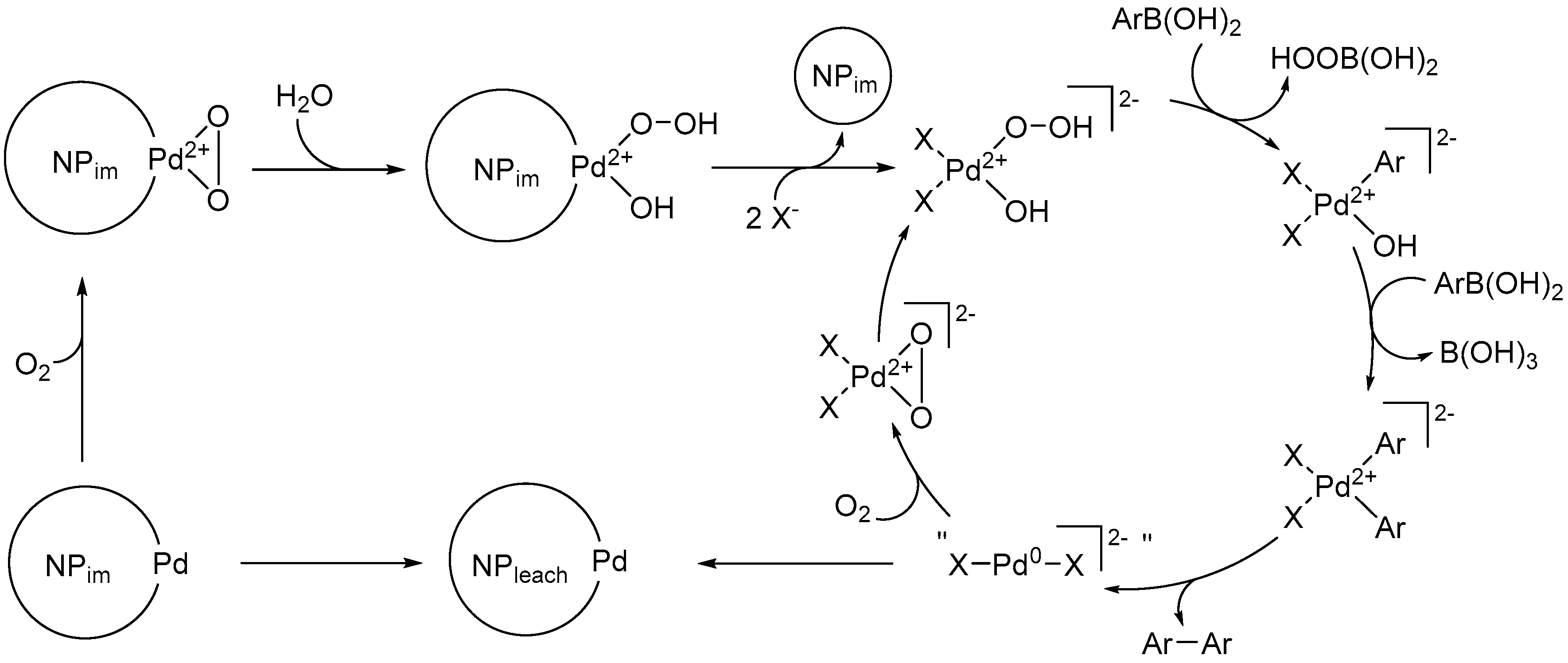
2.4. Proposed Mechanism for Enhanced Catalysis and Increased Loss of Activity
3. Materials and Methods
3.1. Chemicals
3.2. Kinetic Experiments
3.3. Catalyst Recovery
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adamo, C.; Amatore, C.; Ciofini, I.; Jutand, A.; Lakmini, H. Mechanism of the palladium-catalyzed homocoupling of arylboronic acids: Key involvement of a palladium peroxo complex. J. Am. Chem. Soc. 2006, 128, 6829–6836. [Google Scholar] [CrossRef] [PubMed]
- Lakmini, H.; Ciofini, I.; Jutand, A.; Amatore, C.; Adamo, C. Pd-catalyzed homocoupling reaction of arylboronic acid: Insights from density functional theory. J. Phys. Chem. A 2008, 112, 12896–12903. [Google Scholar] [CrossRef] [PubMed]
- Amatore, C.; Jutand, A.; Le Duc, G. Kinetic data for the transmetalation/reductive elimination in palladium-catalyzed Suzuki-Miyaura reactions: Unexpected triple role of hydroxide ions used as base. Chem. Eur. J. 2011, 17, 2492–2503. [Google Scholar] [CrossRef] [PubMed]
- Amatore, C.; Jutand, A. Anionic Pd(0) and Pd(II) intermediates in palladium-catalyzed heck and cross-coupling reactions. Acc. Chem. Res. 2000, 33, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Fagnou, K.; Lautens, M. Halide effects in transition metal catalysis. Angew. Chem. Int. Ed. 2002, 41, 26–47. [Google Scholar] [CrossRef]
- Kozuch, S.; Amatore, C.; Jutand, A.; Shaik, S. What makes for a good catalytic cycle? A theoretical study of the role of an anionic palladium(0) complex in the cross-coupling of an aryl halide with an anionic nucleophile. Organometallics 2005, 24, 2319–2330. [Google Scholar] [CrossRef]
- Darzi, E.R.; White, B.M.; Loventhal, L.K.; Zakharov, L.N.; Jasti, R. An operationally simple and mild oxidative homocoupling of aryl boronic esters to access conformationally constrained macrocycles. J. Am. Chem. Soc. 2017, 139, 3106–3114. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Palladium nanoparticles as efficient green homogeneous and heterogeneous carbon-carbon coupling precatalysts: A unifying view. Inorg. Chem. 2007, 46, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as recyclable catalysts: The frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Compton, R.G. Size effects in nanoparticle catalysis at nanoparticle modified electrodes: The interplay of diffusion and chemical reactions. J. Phys. Chem. C 2017, 121, 2521–2528. [Google Scholar] [CrossRef]
- Hervés, P.; Pérez-Lorenzo, M.; Liz-Marzán, L.M.; Dzubiella, J.; Lu, Y.; Ballauff, M. Catalysis by metallic nanoparticles in aqueous solution: Model reactions. Chem. Soc. Rev. 2012, 41, 5577–5587. [Google Scholar] [CrossRef] [PubMed]
- Taladriz-Blanco, P.; Hervés, P.; Pérez-Juste, J. Supported pd nanoparticles for carbon-carbon coupling reactions. Top. Catal. 2013, 56, 1154–1170. [Google Scholar] [CrossRef]
- Pérez-Lorenzo, M. Palladium nanoparticles as efficient catalysts for Suzuki cross-coupling reactions. J. Phys. Chem. Lett. 2012, 3, 167–174. [Google Scholar] [CrossRef]
- Karimi, B.; Behzadnia, H.; Farhangi, E.; Jafari, E.; Zamani, A. Recent application of polymer supported metal nanoparticles in heck, Suzuki and Sonogashira coupling reactions. Curr. Org. Synth. 2010, 7, 543–567. [Google Scholar] [CrossRef]
- Mahouche-Chergui, S.; Guerrouache, M.; Carbonnier, B.; Chehimi, M.M. Polymer-immobilized nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 43–68. [Google Scholar] [CrossRef]
- Ley, S.V.; Mitchell, C.; Pears, D.; Ramarao, C.; Yu, J.Q.; Zhou, W. Recyclable polyurea-microencapsulated pd(0) nanoparticles: An efficient catalyst for hydrogenolysis of epoxides. Org. Lett. 2003, 5, 4665–4668. [Google Scholar] [CrossRef] [PubMed]
- Kidambi, S.; Dai, J.; Li, J.; Bruening, M.L. Selective hydrogenation by Pd nanoparticles embedded in polyelectrolyte multilayers. J. Am. Chem. Soc. 2004, 126, 2658–2659. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.M.; Gulgun, M.A.; Menceloglu, Y.Z.; Erman, B.; Abramchuk, S.S.; Makhaeva, E.E.; Khokhlov, A.R.; Matveeva, V.G.; Sulman, M.G. Palladium nanoparticles by electrospinning from poly(acrylonitrile-co-acrylic acid)-PdCl2 solutions. Relations between preparation conditions, particle size, and catalytic activity. Macromolecules 2004, 37, 1787–1792. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, B.P.S.; Rathore, J.S.; Bandoo, T. “Polysiloxane-Pd” nanocomposites as recyclable chemoselective hydrogenation catalysts. J. Am. Chem. Soc. 2004, 126, 8493–8500. [Google Scholar] [CrossRef] [PubMed]
- Sanji, T.; Ogawa, Y.; Nakatsuka, Y.; Tanaka, M.; Sakurai, H. Metal nanoparticles derived from polysilane shell cross-linked micelle templates. Chem. Lett. 2003, 32, 980–981. [Google Scholar] [CrossRef]
- Sawoo, S.; Srimani, D.; Dutta, P.; Lahiri, R.; Sarkar, A. Size controlled synthesis of Pd nanoparticles in water and their catalytic application in C-C coupling reactions. Tetrahedron 2009, 65, 4367–4374. [Google Scholar] [CrossRef]
- Mastrorilli, P.; Dell’Anna, M.; Rizzuti, A.; Mali, M.; Zapparoli, M.; Leonelli, C. Resin-immobilized palladium nanoparticle catalysts for organic reactions in aqueous media: Morphological aspects. Molecules 2015, 20, 18661–188684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dell’Anna, M.M.; Mali, M.; Mastrorilli, P.; Rizzuti, A.; Ponzoni, C.; Leonelli, C. Suzuki-Miyaura coupling under air in water promoted by polymer supported palladium nanoparticles. J. Mol. Catal. A Chem. 2013, 366, 186–194. [Google Scholar] [CrossRef]
- Cotugno, P.; Casiello, M.; Nacci, A.; Mastrorilli, P.; Dell’Anna, M.M.; Monopoli, A. Suzuki coupling of iodo and bromoarenes catalyzed by chitosan-supported Pd-nanoparticles in ionic liquids. J. Organomet. Chem. 2014, 752, 1–5. [Google Scholar] [CrossRef]
- Fujii, S.; Matsuzawa, S.; Hamasaki, H.; Nakamura, Y.; Bouleghlimat, A.; Buurma, N.J. Polypyrrole-palladium nanocomposite coating of micrometer-sized polymer particles toward a recyclable catalyst. Langmuir 2012, 28, 2436–2447. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Kaefer, K.; Mourdikoudis, S.; Polavarapu, L.; Vaz, B.; Cartmell, S.E.; Bouleghlimat, A.; Buurma, N.J.; Yate, L.; De Lera, A.R.; et al. Palladium nanoparticle-loaded cellulose paper: A highly efficient, robust, and recyclable self-assembled composite catalytic system. J. Phys. Chem. Lett. 2015, 6, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Onel, L.; Buurma, N.J. Reactivity in organised assemblies. Annu. Rep. Prog. Chem. Sect. B 2009, 105, 363–379. [Google Scholar] [CrossRef]
- Onel, L.; Buurma, N.J. Reactivity in organised assemblies. Annu. Rep. Prog. Chem. Sect. B 2010, 106, 344–375. [Google Scholar] [CrossRef]
- Buurma, N.J. Reactivity in organised assemblies. Annu. Rep. Prog. Chem. Sect. B 2011, 107, 328–348. [Google Scholar] [CrossRef]
- Buurma, N.J. Reactivity in organised assemblies. Annu. Rep. Prog. Chem. Sect. B 2012, 108, 316–333. [Google Scholar] [CrossRef]
- Carregal-Romero, S.; Buurma, N.J.; Pérez-Juste, J.; Liz-Marzán, L.M.; Hervés, P. Catalysis by au@pnipam nanocomposites: Effect of the cross-linking density. Chem. Mater. 2010, 22, 3051–3059. [Google Scholar] [CrossRef]
- Selvan, S.T.; Spatz, J.P.; Klok, H.A.; Möller, M. Gold-polypyrrole core-shell particles in diblock copolymer micelles. Adv. Mater. 1998, 10, 132–134. [Google Scholar] [CrossRef]
- Han, J.; Wang, M.; Hu, Y.; Zhou, C.; Guo, R. Conducting polymer-noble metal nanoparticle hybrids: Synthesis mechanism application. Prog. Polym. Sci. 2017, 70, 52–91. [Google Scholar] [CrossRef]
- Khan, M.A.; Perruchot, C.; Armes, S.P.; Randall, D.P. Synthesis of gold-decorated latexes via conducting polymer redox templates. J. Mater. Chem. 2001, 11, 2363–2372. [Google Scholar] [CrossRef]
- Selvan, S.T.; Hayakawa, T.; Nogami, M.; Möller, M. Block copolymer mediated synthesis of gold quantum dots and novel gold-polypyrrole nanocomposites. J. Phys. Chem. B 1999, 103, 7441–7448. [Google Scholar] [CrossRef]
- Lu, F.; Ruiz, J.; Astruc, D. Palladium-dodecanethiolate nanoparticles as stable and recyclable catalysts for the Suzuki-Miyaura reaction of aryl halides under ambient conditions. Tetrahedron Lett. 2004, 45, 9443–9445. [Google Scholar] [CrossRef]
- Balanta, A.; Godard, C.; Claver, C. Pd nanoparticles for C-C coupling reactions. Chem. Soc. Rev. 2011, 40, 4973–4985. [Google Scholar] [CrossRef] [PubMed]
- Favier, I.; Madec, D.; Teuma, E.; Gómez, M. Palladium nanoparticles applied in organic synthesis as catalytic precursors. Curr. Org. Chem. 2011, 15, 3127–3174. [Google Scholar] [CrossRef]
- Han, W.; Liu, C.; Jin, Z. Aerobic ligand-free Suzuki coupling reaction of aryl chlorides catalyzed by in situ generated palladium nanoparticles at room temperature. Adv. Synth. Catal. 2008, 350, 501–508. [Google Scholar] [CrossRef]
- Han, W.; Liu, C.; Jin, Z.L. In situ generation of palladium nanoparticles: A simple and highly active protocol for oxygen-promoted ligand-free Suzuki coupling reaction of aryl chlorides. Org. Lett. 2007, 9, 4005–4007. [Google Scholar] [CrossRef] [PubMed]
- Desmarets, C.; Omar-Amrani, R.; Walcarius, A.; Lambert, J.; Champagne, B.; Fort, Y.; Schneider, R. Naphthidine di(radical cation)s-stabilized palladium nanoparticles for efficient catalytic Suzuki-Miyaura cross-coupling reactions. Tetrahedron 2008, 64, 372–381. [Google Scholar] [CrossRef]
- Diallo, A.K.; Ornelas, C.; Salmon, L.; Aranzaes, J.R.; Astruc, D. “Homeopathic” catalytic activity and atom-leaching mechanism in Miyaura-Suzuki reactions under ambient conditions with precise dendrimer-stabilized Pd nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 8644–8648. [Google Scholar] [CrossRef] [PubMed]
- Gallon, B.J.; Kojima, R.W.; Kaner, R.B.; Diaconescu, P.L. Palladium nanoparticles supported on polyaniline nanofibers as a semi-heterogeneous catalyst in water. Angew. Chem. Int. Ed. 2007, 46, 7251–7254. [Google Scholar] [CrossRef] [PubMed]
- Prastaro, A.; Ceci, P.; Chiancone, E.; Boffi, A.; Cirilli, R.; Colone, M.; Fabrizi, G.; Stringaro, A.; Cacchi, S. Suzuki-Miyaura cross-coupling catalyzed by protein-stabilized palladium nanoparticles under aerobic conditions in water: Application to a one-pot chemoenzymatic enantioselective synthesis of chiral biaryl alcohols. Green Chem. 2009, 11, 1929–1932. [Google Scholar] [CrossRef]
- Willis, N.G.; Guzman, J. Influence of the support during homocoupling of phenylboronic acid catalyzed by supported gold. Appl. Catal. A Gen. 2008, 339, 68–75. [Google Scholar] [CrossRef]
- Prastaro, A.; Ceci, P.; Chiancone, E.; Boffi, A.; Fabrizi, G.; Cacchi, S. Homocoupling of arylboronic acids and potassium aryltrifluoroborates catalyzed by protein-stabilized palladium nanoparticles under air in water. Tetrahedron Lett. 2010, 51, 2550–2552. [Google Scholar] [CrossRef]
- Pagliaro, M.; Pandarus, V.; Ciriminna, R.; Béland, F.; DemmaCarà, P. Heterogeneous versus homogeneous palladium catalysts for cross-coupling reactions. ChemCatChem 2012, 4, 432–445. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Kurokhtina, A.A. Distinguishing between the homogeneous and heterogeneous mechanisms of catalysis in the mizoroki-heck and Suzuki-Miyaura reactions: Problems and prospects. Kinet. Catal. 2012, 53, 714–730. [Google Scholar] [CrossRef]
- Bej, A.; Ghosh, K.; Sarkar, A.; Knight, D.W. Palladium nanoparticles in the catalysis of coupling reactions. RSC Adv. 2016, 6, 11446–11453. [Google Scholar] [CrossRef]
- Eremin, D.B.; Ananikov, V.P. Understanding active species in catalytic transformations: From molecular catalysis to nanoparticles, leaching, “cocktails” of catalysts and dynamic systems. Coord. Chem. Rev. 2017, 346, 2–19. [Google Scholar] [CrossRef]
- Biffis, A.; Zecca, M.; Basato, M. Palladium metal catalysts in heck C-C coupling reactions. J. Mol. Catal. A Chem. 2001, 173, 249–274. [Google Scholar] [CrossRef]
- Liu, Y.B.; Khemtong, C.; Hu, J. Synthesis and catalytic activity of a poly(N,N-dialkylcarbodiimide)/palladium nanoparticle composite: A case in the Suzuki coupling reaction using microwave and conventional heating. Chem. Commun. 2004, 4, 398–399. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.P.; Jutand, A.; Tian, Z.Q.; Amatore, C. Au-Pd core-shell nanoparticles catalyze Suzuki-Miyaura reactions in water through Pd leaching. Angew. Chem. Int. Ed. 2011, 50, 12184–12188. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Peng, Q.; Zhuang, Z.; He, W.; Li, Y. Evidence of an oxidative-addition-promoted Pd-leaching mechanism in the Suzuki reaction by using a Pd-nanostructure design. Chem. Eur. J. 2012, 18, 9813–9817. [Google Scholar] [CrossRef] [PubMed]
- Brazier, J.B.; Nguyen, B.N.; Adrio, L.A.; Barreiro, E.M.; Leong, W.P.; Newton, M.A.; Figueroa, S.J.A.; Hellgardt, K.; Hii, K.K.M. Catalysis in flow: Operando study of Pd catalyst speciation and leaching. Catal. Today 2014, 229, 95–103. [Google Scholar] [CrossRef]
- Gaikwad, A.V.; Holuigue, A.; Thathagar, M.B.; Ten Elshof, J.E.; Rothenberg, G. Ion- and atom-leaching mechanisms from palladium nanoparticles in cross-coupling reactions. Chem. A Eur. J. 2007, 13, 6908–6913. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.; Schmidt, M.; O’Dwyer, C.; Holmes, J.D.; McGlacken, G.P. The origin of shape sensitivity in palladium-catalyzed Suzuki-Miyaura cross coupling reactions. Angew. Chem. Int. Ed. 2014, 53, 4142–4145. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.; Schmidt, M.; Odwyer, C.; McGlacken, G.; Holmes, J.D. Enhanced catalytic activity of high-index faceted palladium nanoparticles in Suzuki-Miyaura coupling due to efficient leaching mechanism. ACS Catal. 2014, 4, 3105–3111. [Google Scholar] [CrossRef]
- Ellis, P.J.; Fairlamb, I.J.; Hackett, S.F.; Wilson, K.; Lee, A.F. Evidence for the surface-catalyzed Suzuki-Miyaura reaction over palladium nanoparticles: An operando xas study. Angew. Chem. Int. Ed. 2010, 49, 1820–1824. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Zhang, B.; Zhang, W.; Hong, S.Y.; Schlögl, R.; Su, D.S. The role of palladium dynamics in the surface catalysis of coupling reactions. Angew. Chem. Int. Ed. 2013, 52, 2114–2117. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.F.; Kurokhtina, A.A.; Larina, E.V. Simple kinetic method for distinguishing between homogeneous and heterogeneous mechanisms of catalysis, illustrated by the example of “ligand-free” Suzuki and heck reactions of aryl iodides and aryl bromides. Kinet. Catal. 2012, 53, 84–90. [Google Scholar] [CrossRef]
- Newton, M.A.; Brazier, J.B.; Barreiro, E.M.; Parry, S.; Emmerich, H.; Adrio, L.A.; Mulligan, C.J.; Hellgardt, K.; Hii, K.K.M. Operando xafs of supported Pd nanoparticles in flowing ethanol/water mixtures: Implications for catalysis. Green Chem. 2016, 18, 406–411. [Google Scholar] [CrossRef]
- Grieco, P.A. Organic Synthesis in Water; Blackie: London, UK, 1998. [Google Scholar]
- Genet, J.P.; Darses, S.; Michelet, W. Organometallic catalysts in synthetic organic chemistry: From reactions in aqueous media to gold catalysis. Pure Appl. Chem. 2008, 80, 831–844. [Google Scholar] [CrossRef]
- Li, C.J. Organic reactions in aqueous media with a focus on carbon-carbon bond formations: A decade update. Chem. Rev. 2005, 105, 3095–3165. [Google Scholar] [CrossRef] [PubMed]
- Lindström, U.M. Organic Reactions in Water: Principles, Strategies and Applications; Blackwell Pub.: Oxford, MI, USA, 2007. [Google Scholar]
- Ohtaka, A.; Kono, Y.; Teratani, T.; Fujii, S.; Matsuzawa, S.; Nakamura, Y.; Nomura, R. Polypyrrole-palladium nanocomposite-coated latex particles as a heterogeneous catalyst in water. Catal. Lett. 2011, 141, 1097–1103. [Google Scholar] [CrossRef]
- Fujii, S.; Matsuzawa, S.; Nakamura, Y.; Ohtaka, A.; Teratani, T.; Akamatsu, K.; Tsuruoka, T.; Nawafune, H. Synthesis and characterization of polypyrrole-palladium nanocomposite-coated latex particles and their use as a catalyst for Suzuki coupling reaction in aqueous media. Langmuir 2010, 26, 6230–6239. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.A. The Palladium-Catalysed Aerobic Oxidative Homocoupling Reaction of Arylboronic Acids in Aqueous Micellar Medium Kinetic and Mechanistic Studies; Cardiff University: Cardiff, UK, 2011. [Google Scholar]
- Bouleghlimat, A. Palladium Complexes, Nanoparticles and Their Use in Coupling Reactions of Arylboronic Acids in Aqueous Media; Cardiff University: Cardiff, UK, 2013. [Google Scholar]
- Saha, D.; Chattopadhyay, K.; Ranu, B.C. Aerobic ligand-free Suzuki coupling catalyzed by in situ-generated palladium nanoparticles in water. Tetrahedron Lett. 2009, 50, 1003–1006. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouleghlimat, A.; Othman, M.A.; Lagrave, L.V.; Matsuzawa, S.; Nakamura, Y.; Fujii, S.; Buurma, N.J. Halide-Enhanced Catalytic Activity of Palladium Nanoparticles Comes at the Expense of Catalyst Recovery. Catalysts 2017, 7, 280. https://doi.org/10.3390/catal7090280
Bouleghlimat A, Othman MA, Lagrave LV, Matsuzawa S, Nakamura Y, Fujii S, Buurma NJ. Halide-Enhanced Catalytic Activity of Palladium Nanoparticles Comes at the Expense of Catalyst Recovery. Catalysts. 2017; 7(9):280. https://doi.org/10.3390/catal7090280
Chicago/Turabian StyleBouleghlimat, Azzedine, Mazin A. Othman, Louis V. Lagrave, Soichiro Matsuzawa, Yoshinobu Nakamura, Syuji Fujii, and Niklaas J. Buurma. 2017. "Halide-Enhanced Catalytic Activity of Palladium Nanoparticles Comes at the Expense of Catalyst Recovery" Catalysts 7, no. 9: 280. https://doi.org/10.3390/catal7090280




