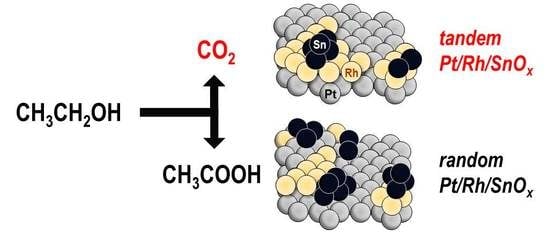
Ethanol Oxidation Reaction on Tandem Pt/Rh/SnOx Catalyst
Abstract
:1. Introduction
2. Results and Discussion
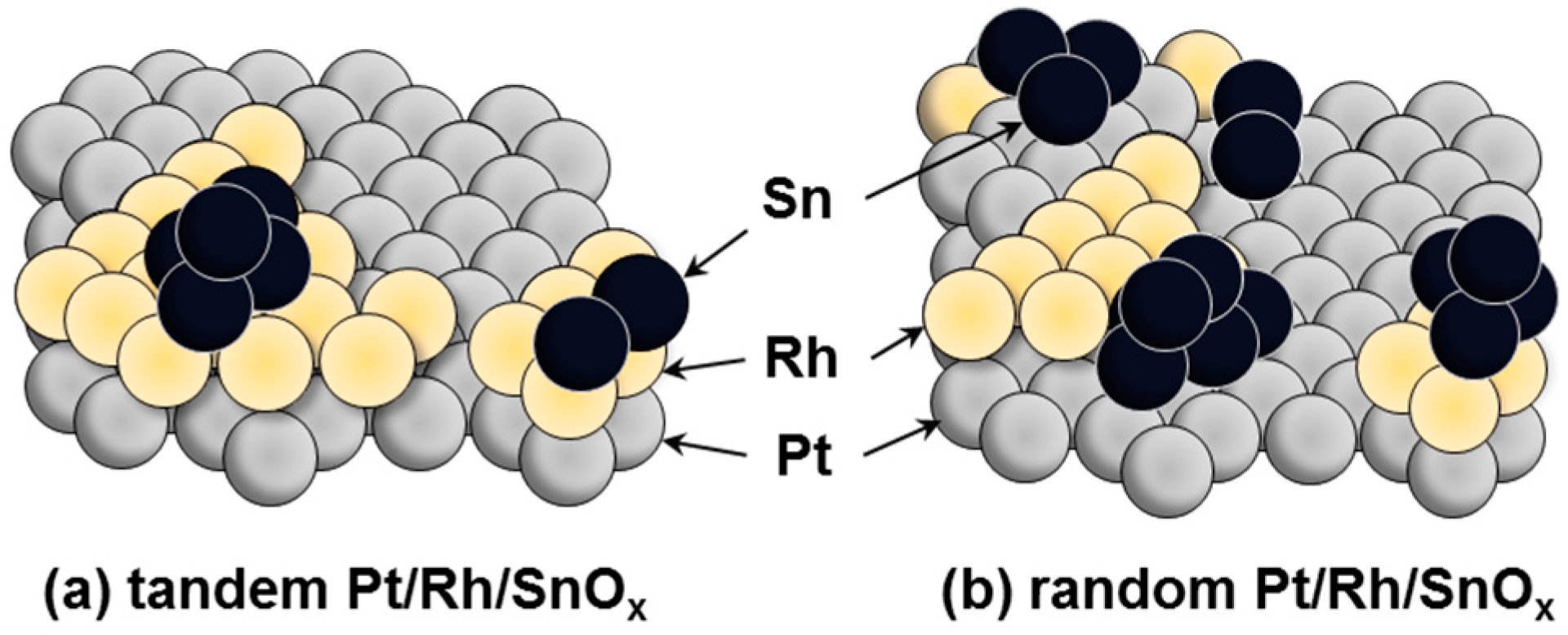
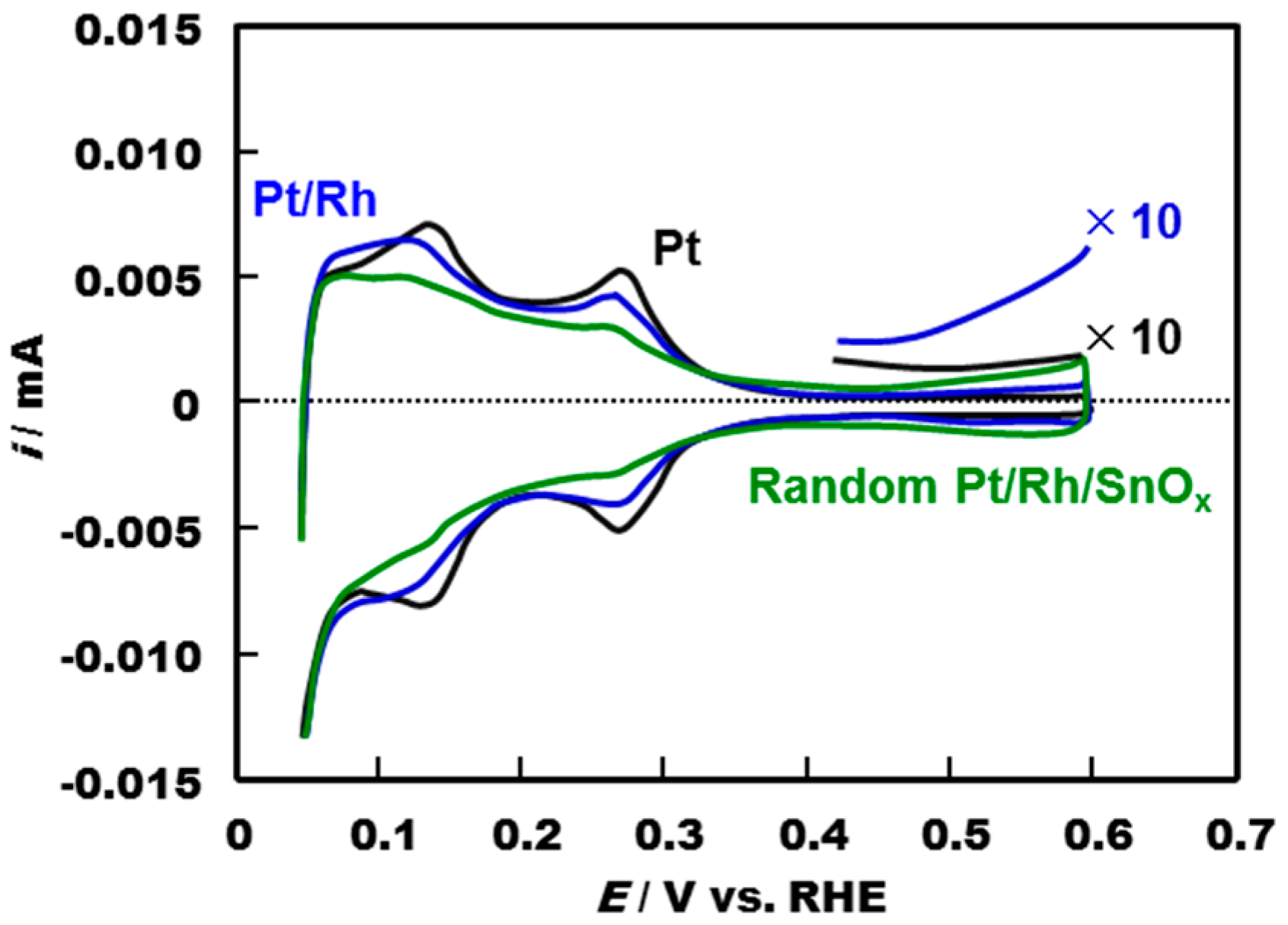
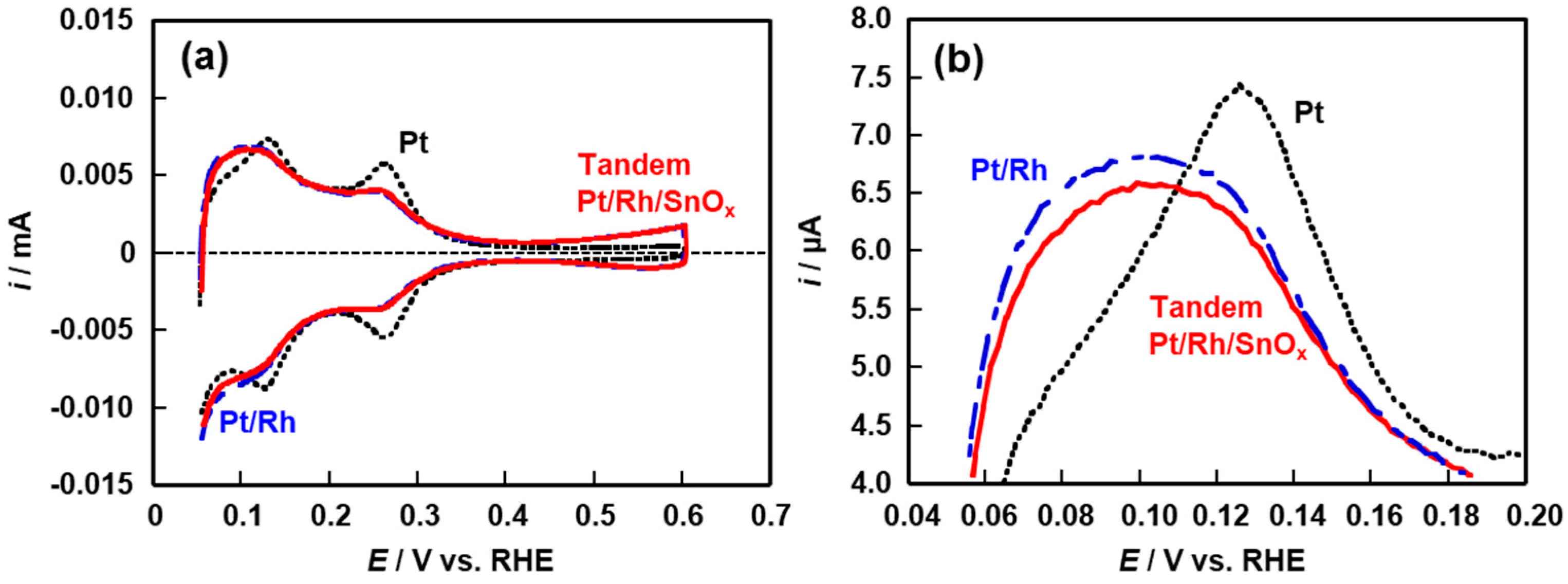
2.1. Electrochemical Properties of Pt/Rh and Pt/Rh/SnOx Catalysts
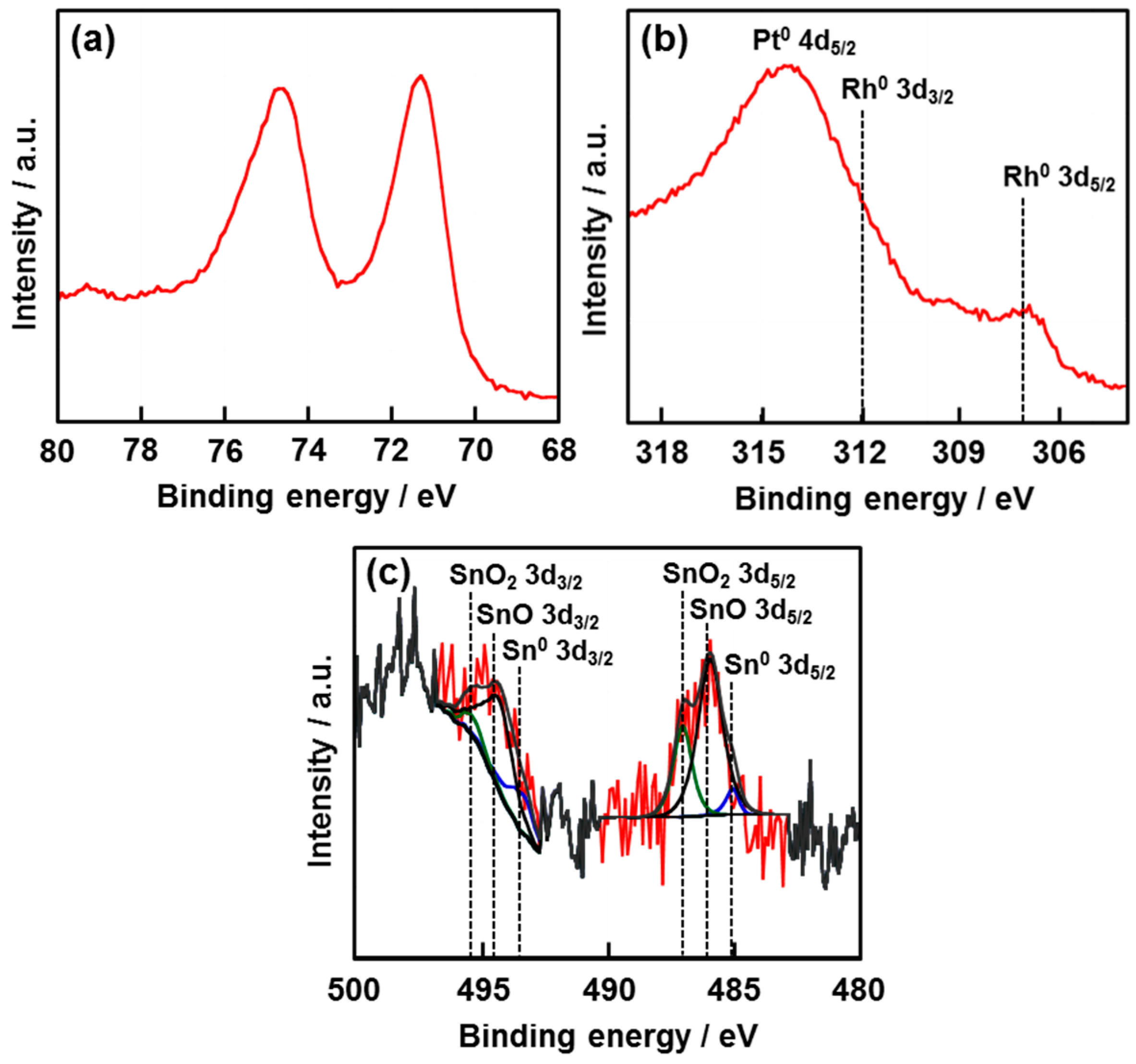
2.2. Electronic State of Each Element for Tandem Pt/Rh/SnOx Catalyst
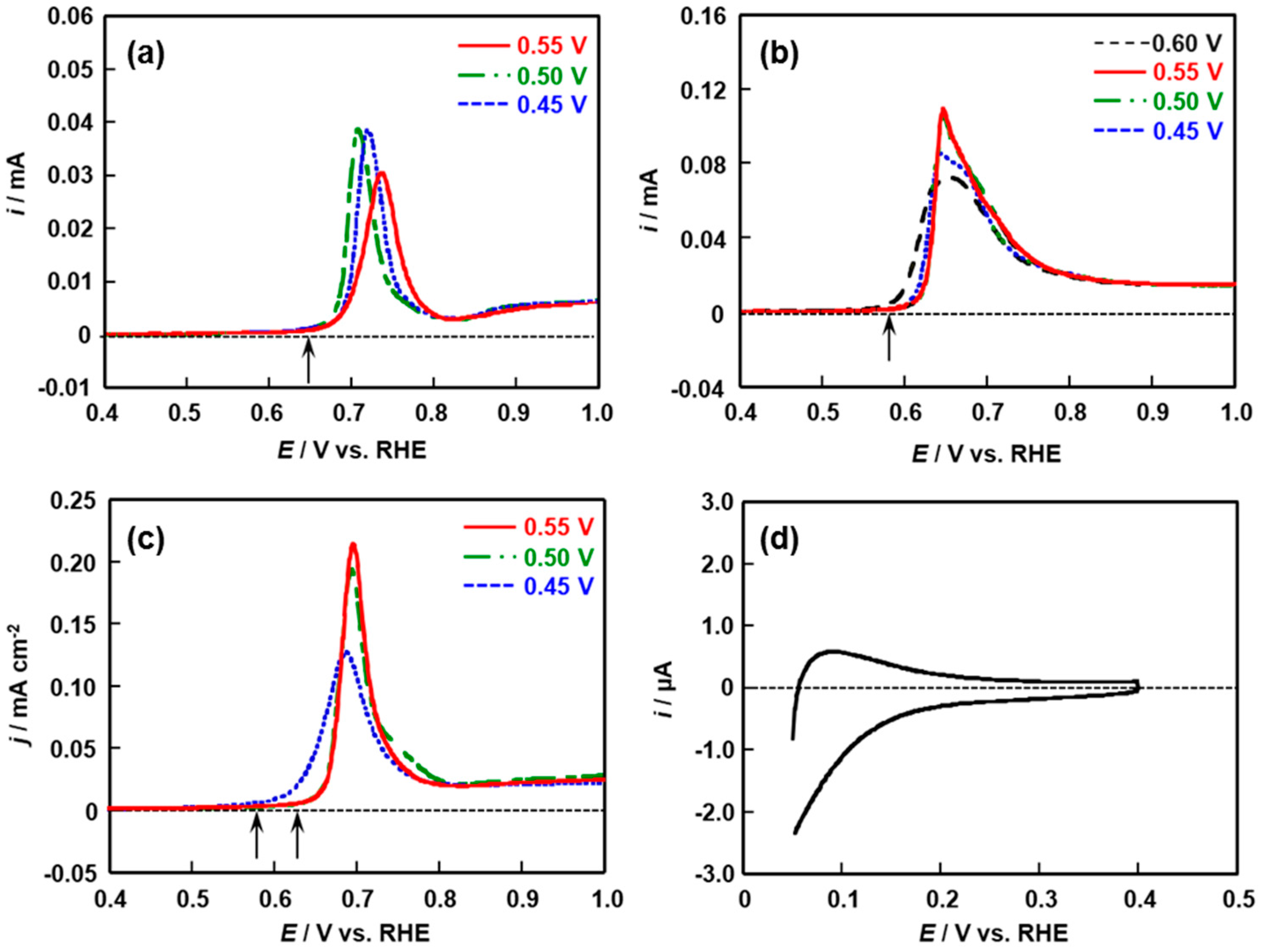
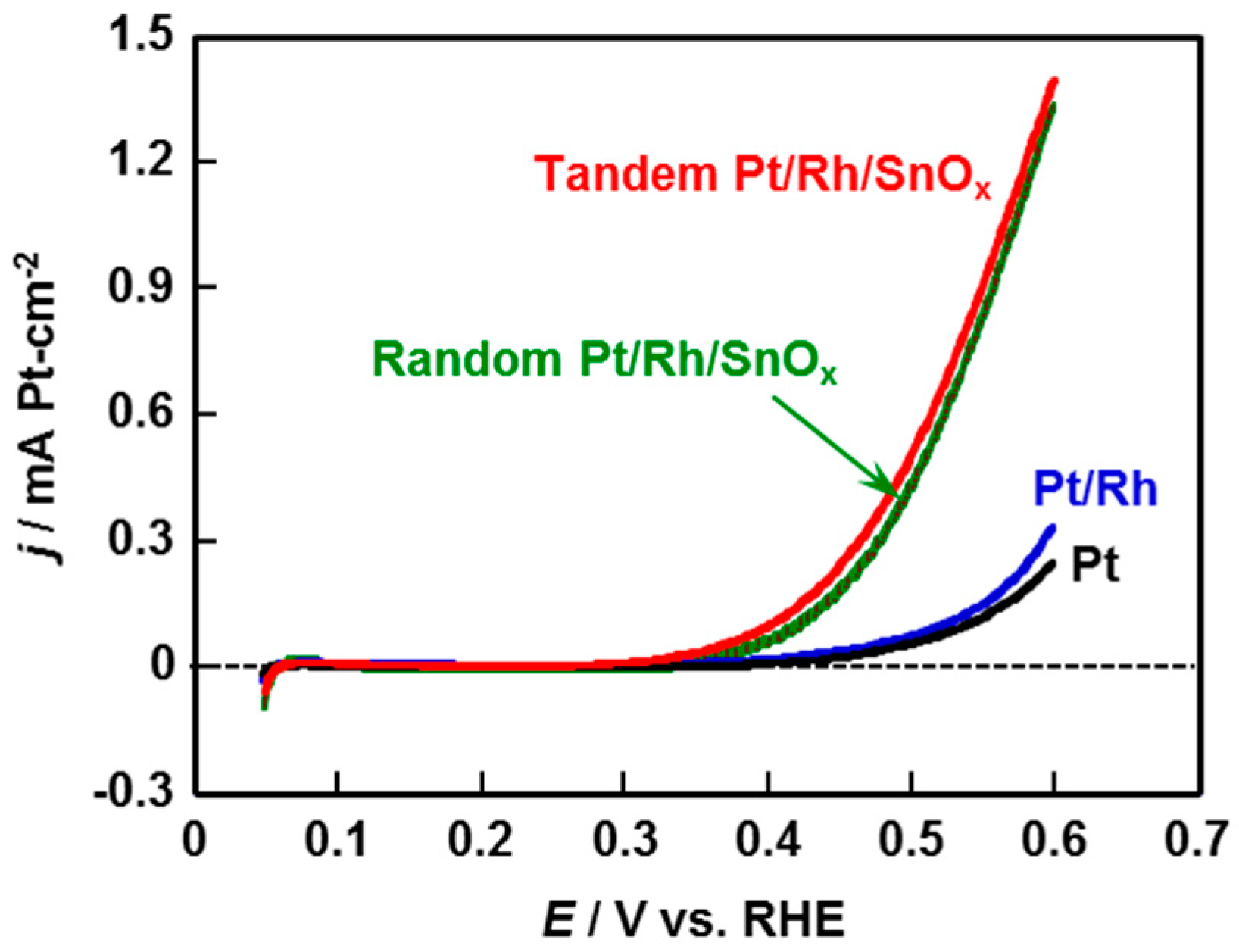
2.3. Ethanol Oxidation Reaction Activity and Product Distribution of Pt/Rh and Pt/Rh/SnOx Catalysts
2.4. Durability of Pt/Rh and Pt/Rh/SnOx Catalysts
3. Experimental
3.1. Preparation of Pt/Rh and Pt/Rh/SnOx Catalysts
3.2. Characterization of Pt/Rh and Pt/Rh/SnOx Catalysts
3.3. Electrochemical Measurements
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Antolini, A. Pt-Ni and Pt-M-Ni (M = Ru, Sn) Anode Catalysts for Low-Temperature Acidic Direct Alcohol Fuel Cells: A Review. Energies 2017, 10, 42. [Google Scholar] [CrossRef]
- Vigier, F.; Coutanceau, C.; Perrard, A.; Belgsir, E.M.; Lamy, C. Development of anode catalysts for a direct ethanol fuel cell. J. Appl. Electrochem. 2004, 34, 439–446. [Google Scholar] [CrossRef]
- Zhou, W.J.; Zhou, B.; Li, W.Z.; Zhou, Z.H.; Song, S.Q.; Sun, G.Q.; Xin, Q.; Douvartzides, S.; Goula, M.; Tsiakaras, P. Performance comparison of low-temperature direct alcohol fuel cells with different anode catalysts. J. Power Sources 2004, 126, 16–22. [Google Scholar] [CrossRef]
- Oliveira Neto, A.; Giz, M.J.; Perez, J.; Ticianelli, E.A.; Gonzalez, E.R. The Electro-oxidation of Ethanol on Pt-Ru and Pt-Mo Particles Supported on High-Surface-Area Carbon. J. Electrochem. Soc. 2002, 149, A272–A279. [Google Scholar] [CrossRef]
- Zhou, W.J.; Li, W.Z.; Song, S.Q.; Zhou, Z.H.; Jiang, L.H.; Sun, G.Q.; Xin, Q.; Poulianitis, K.; Kontou, S.; Tsiakaras, P. Bi- and tri-metallic Pt-based anode catalysts for direct ethanol fuel cells. J. Power Sources 2004, 131, 217–223. [Google Scholar] [CrossRef]
- Rodriguez Varela, F.J.; Savadogo, O. Catalytic Activity of Carbon-Supported Electrocatalysts for Direct Ethanol Fuel Cell Applications. J. Electrochem. Soc. 2008, 155, B618–B624. [Google Scholar] [CrossRef]
- Kepeniene, V.; Tamasaukaite-Tamasiunaite, L.; Jablonskiene, J.; Vaiciuniene, J.; Kondrotas, R.; Juskenas, R.; Norkus, E. Investigation of Graphene Supported Platinum-Cobalt Nanocomposites as Electrocatalysts for Ethanol Oxidation. J. Electrochem. Soc. 2014, 161, F1354–F1359. [Google Scholar] [CrossRef]
- Zhou, W.; Li, M.; Zhang, L.; Chan, S.H. Supported PtAu catalysts with different nano-structures for ethanol electrooxidation. Electrochim. Acta 2014, 123, 233–239. [Google Scholar] [CrossRef]
- Lamy, C.; Rousseau, S.; Belgsir, E.M.; Contanceau, C.; Leger, J.M. Recent progress in the direct ethanol fuel cell: Development of new platinum-tin electrocatalysts. Electrochim. Acta 2004, 49, 3901–3908. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, G.; Sun, S.; Liu, J.; Tang, S.; Li, H.; Zhou, B.; Xin, Q. Structure and chemical composition of supported Pt–Sn electrocatalysts for ethanol oxidation. Electrochim. Acta 2005, 50, 5384–5389. [Google Scholar] [CrossRef]
- Jinag, L.; Colmenares, L.; Jusys, Z.; Sun, G.Q.; Behm, R.J. Ethanol electrooxidation on novel carbon supported Pt/SnOx/C catalysts with varied Pt:Sn ratio. Electrochim. Acta 2007, 53, 377–389. [Google Scholar] [CrossRef]
- Ribeiro, J.; dos Anjos, D.M.; Kokoh, K.B.; Coutanceau, C.; Leger, J.-M.; Olivi, P.; de Andrade, A.R.; Tremiliosi-Filho, G. Carbon-supported ternary PtSnIr catalysts for direct ethanol fuel cell. Electrochim. Acta 2007, 52, 6997–7006. [Google Scholar] [CrossRef]
- Higuchi, E.; Miyata, K.; Takase, T.; Inoue, H. Ethanol oxidation reaction activity of highly dispersed Pt/SnO2 double nanoparticles on carbon black. J. Power Sources 2011, 196, 1730–1737. [Google Scholar] [CrossRef]
- De Souza, J.P.I.; Queiroz, S.L.; Bergamaski, K.; Gonzalez, E.R.; Nart, F.C. Electro-Oxidation of Ethanol on Pt, Rh, and PtRh Electrodes. A Study Using DEMS and in-situ FTIR Techniques. J. Phys. Chem. B 2002, 106, 9825–9830. [Google Scholar] [CrossRef]
- Lima, F.H.B.; Profeti, D.; Lizcano-Valbuena, W.H.; Ticianelli, E.A.; Gonzalez, E.R. Carbon-dispersed Pt-Rh nanoparticles for ethanol electro-oxidation. Effect of the crystallite size and of temperature. J. Electroanal. Chem. 2008, 617, 121–129. [Google Scholar] [CrossRef]
- Colmati, F.; Antolini, E.; Gonzalez, E.R. Preparation, structural characterization and activity for ethanol oxidation of carbon supported ternary Pt-Sn-Rh catalysts. J. Alloys Compd. 2008, 456, 264–270. [Google Scholar] [CrossRef]
- Kowal, A.; Li, M.; Shao, M.; Sasaki, K.; Vukmirovic, M.B.; Zhang, J.; Markovic, N.S.; Liu, P.; Frenkel, A.I.; Adzic, R.R. Ternary Pt/Rh/SnO2 electrocatalysts for oxidizing ethanol to CO2. Nat. Mater. 2009, 8, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kowal, A.; Sasaki, K.; Marinkovic, N.; Su, D.; Korach, E.; Liu, P.; Adzic, R.R. Ethanol oxidation on the ternary Pt-Rh-SnO2/C electrocatalysts with varied Pt:Rh:Sn ratios. Electrochim. Acta 2010, 55, 4331–4338. [Google Scholar] [CrossRef]
- Du, W.; Wang, Q.; LaScala, C.A.; Zhang, L.; Su, D.; Frenkel, A.I.; Mathur, V.K.; Teng, X. Ternary PtSnRh-SnO2 nanoclusters: Synthesis and electroactivity for ethanol oxidation fuel cell reaction. J. Mater. Chem. 2011, 21, 8887–8892. [Google Scholar] [CrossRef]
- Li, M.; Zhou, W.-P.; Marinkovic, N.S.; Sasaki, K.; Adzic, R.R. The role of rhodium and tin oxide in the platinum-based electrocatalysts for ethanol oxidation to CO2. Electrochim. Acta 2013, 104, 454–461. [Google Scholar] [CrossRef]
- Higuchi, E.; Takase, T.; Chiku, M.; Inoue, H. Preparation of ternary Pt/Rh/SnO2 anode catalysts for use in direct ethanol fuel cells and their electrocatalytic activity for ethanol oxidation reaction. J. Power Sources 2014, 263, 280–287. [Google Scholar] [CrossRef]
- De Souza, E.A.; Giz, M.J.; Camara, G.A.; Antolini, E.; Passos, R.R. Ethanol electro-oxidation on partially alloyed Pt–Sn–Rh/C catalysts. Electrochim. Acta 2014, 147, 483–489. [Google Scholar] [CrossRef]
- Erini, N.; Lopukrakpam, R.; Petkov, V.; Baranova, E.A.; Yang, R.; Teschner, D.; Huang, Y.; Brankovic, S.R.; Strasser, P. Ethanol Electro-Oxidation on Ternary Platinum-Rhodium-Tin Nanocatalysts: Insights in the Atomic 3D Structure of the Active Catalytic Phase. ACS Catal. 2014, 4, 1859–1867. [Google Scholar] [CrossRef]
- Solla-Gullón, J.; Rodríguez, P.; Herrero, E.; Aldaz, A.; Feliu, J.M. Surface characterization of platinum electrodes. Phys. Chem. Chem. Phys. 2008, 10, 1359–1373. [Google Scholar] [CrossRef] [PubMed]
- Jerkiewicz, G.; Borodzinski, J.J. Studies of formation of very thin oxide films on polycrystalline rhodium electrodes: Application of the Mott-Cabrera theory. Langmuir 1993, 9, 2202–2209. [Google Scholar] [CrossRef]
- Coméz, R.; Feliu, J.M. Rhodium adlayers on Pt(111) monocrystalline surfaces. Electrochemical behavior and electrocatalysis. Electrochim. Acta 1998, 44, 1191–1205. [Google Scholar]
- Vigier, F.; Coutanceau, C.; Hahn, F.; Belgsir, E.M.; Lamy, C. On the mechanism of ethanol electro-oxidation on Pt and PtSn catalysts: Electrochemical and in situ IR reflectance spectroscopy studies. J. Electroanal. Chem. 2004, 563, 81–89. [Google Scholar] [CrossRef]
- Leung, L.H.; Goodman, D.W. The oxidation of carbon monoxide on Rh(100) under steady state conditions: An FT-IR study. Catal. Lett. 1990, 5, 353–360. [Google Scholar] [CrossRef]
- Donadio, D.; Ghiringhelli, L.M.; Site, L.D. Autocatalytic and Cooperatively Stabilized Dissociation of Water on a Stepped Platinum Surface. J. Am. Chem. Soc. 2012, 134, 19217–19222. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, P.T.; Haze, A.; Chiku, M.; Higuchi, E.; Inoue, H. Ethanol Oxidation Reaction on Tandem Pt/Rh/SnOx Catalyst. Catalysts 2017, 7, 246. https://doi.org/10.3390/catal7090246
Mai PT, Haze A, Chiku M, Higuchi E, Inoue H. Ethanol Oxidation Reaction on Tandem Pt/Rh/SnOx Catalyst. Catalysts. 2017; 7(9):246. https://doi.org/10.3390/catal7090246
Chicago/Turabian StyleMai, Phuong Tu, Akinori Haze, Masanobu Chiku, Eiji Higuchi, and Hiroshi Inoue. 2017. "Ethanol Oxidation Reaction on Tandem Pt/Rh/SnOx Catalyst" Catalysts 7, no. 9: 246. https://doi.org/10.3390/catal7090246
APA StyleMai, P. T., Haze, A., Chiku, M., Higuchi, E., & Inoue, H. (2017). Ethanol Oxidation Reaction on Tandem Pt/Rh/SnOx Catalyst. Catalysts, 7(9), 246. https://doi.org/10.3390/catal7090246