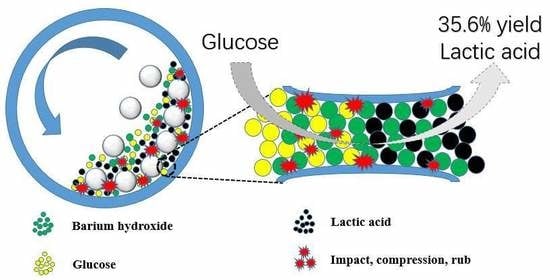
Mechanocatalytic Production of Lactic Acid from Glucose by Ball Milling
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Milling Duration
2.2. Effect of Sample/Ball Mass Ratio
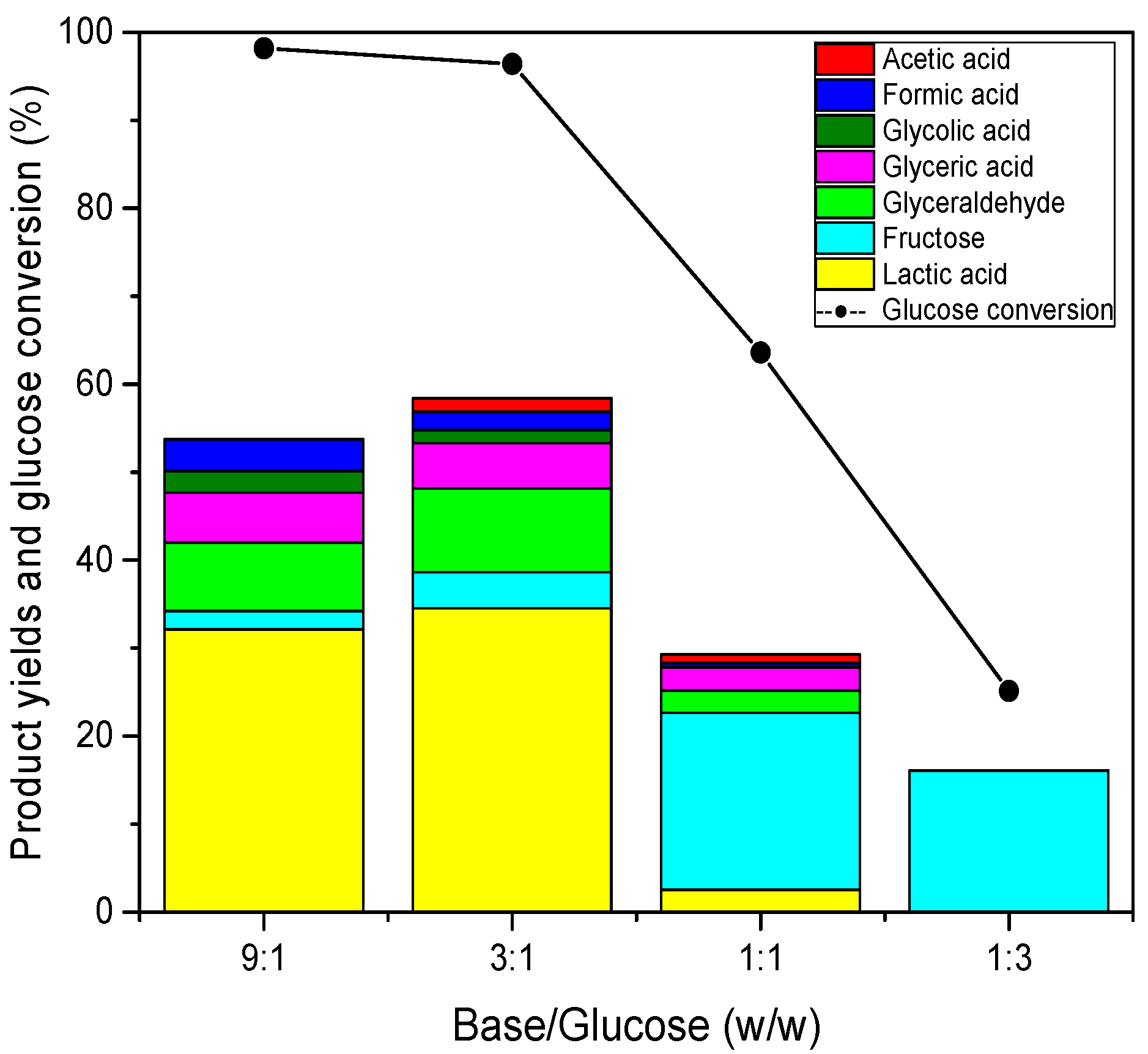
2.3. Effect of Base/Glucose Mass Ratio
2.4. Mechanocatalytic Conversion of Different Carbohydrates to Lactic Acid
3. Materials and Methods
3.1. Materials
3.2. Mechanocatalytic Process
3.3. Products Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barta, K.; Ford, P.C. Catalytic Conversion of Nonfood Woody Biomass Solids to Organic Liquids. Acc. Chem. Res. 2014, 47, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Vávrová, K.; Knápek, J.; Weger, J. Short-term Boosting of Biomass Energy Sources—Determination of Biomass Potential for Prevention of Regional Crisis Situations. Renew. Sustain. Energy Rev. 2017, 67, 426–436. [Google Scholar] [CrossRef]
- Qi, L.; Mui, Y.F.; Lo, S.W.; Lui, M.Y.; Akien, G.R.; Horváth, I.T. Catalytic Conversion of Fructose, Glucose, and Sucrose to 5-(Hydroxymethyl)furfural and Levulinic and Formic Acids in γ-Valerolactone As a Green Solvent. ACS Catal. 2014, 4, 1470–1477. [Google Scholar] [CrossRef]
- Yan, L.; Liu, N.; Yu, W.; Machida, H.; Qi, X. Production of 5-Hydroxymethylfurfural from Corn Stalk Catalyzed by Corn Stalk-derived Carbonaceous Solid Acid Catalyst. Bioresour. Technol. 2014, 173, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, X.; Zhang, J.; Guo, W.; Jin, F.; Yan, N. Transformation of Chitin and Waste Shrimp Shells into Acetic Acid and Pyrrole. ACS Sustain. Chem. Eng. 2016, 4, 3912–3920. [Google Scholar] [CrossRef]
- Mäkiarvela, P.; Simakova, I.L.; Salmi, T.; Murzin, D.Y. Production of Lactic Acid/Lactates from Biomass and Their Catalytic Transformations to Commodities. Chem. Rev. 2014, 114, 1909–1971. [Google Scholar] [CrossRef] [PubMed]
- Dusselier, M.; Wouwe, P.V.; Dewaele, A.; Makshina, E.; Sels, B.F. Lactic Acid as a Platform Chemical in the Biobased Economy: The Role of Chemocatalysis. Energy Environ. Sci. 2013, 6, 1415–1442. [Google Scholar] [CrossRef]
- Morales, M. Environmental and Economic Assessment of Lactic Acid Production from Glycerol using Cascade Bio- and Chemocatalysis. Energy Environ. Sci. 2015, 8, 558–567. [Google Scholar] [CrossRef]
- Yan, L.; Qi, X. Degradation of Cellulose to Organic Acids in its Homogeneous Alkaline Aqueous Solution. ACS Sustain. Chem. Eng. 2014, 2, 897–901. [Google Scholar] [CrossRef]
- Esposito, D. Chemical Conversion of Sugars to Lactic Acid by Alkaline Hydrothermal Processes. ChemSusChem 2013, 6, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Lux, S.; Siebenhofer, M. Synthesis of Lactic Acid from Dihydroxyacetone: Use of Alkaline-earth Metal Hydroxides. Catal. Sci. Technol. 2013, 3, 1380–1385. [Google Scholar] [CrossRef]
- Shen, Z.; Jin, F.; Zhang, Y.; Wu, B.; Kishita, A.; Tohji, K.; Kishida, H. Effect of Alkaline Catalysts on Hydrothermal Conversion of Glycerin into Lactic Acid. AIChE J. 2009, 56, 2727–2733. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friscic, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for New and Cleaner Synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Carrasquilloflores, R.; Käldström, M.; Schüth, F.; Dumesic, J.A.; Rinaldi, R. Mechanocatalytic Depolymerization of Dry (Ligno)cellulose As an Entry Process for High-Yield Production of Furfurals. ACS Catal. 2013, 3, 11167–11173. [Google Scholar]
- Hilgert, J.; Meine, N.; Rinaldi, R.; Schüth, F. Mechanocatalytic Depolymerization of Cellulose Combined with Hydrogenolysis as a Highly Efficient Pathway to Sugar Alcohols. Energy Environ. Sci. 2013, 6, 92–96. [Google Scholar] [CrossRef]
- Hick, S.M.; Griebel, C.; Restrepo, D.T.; Truitt, J.H.; Buker, E.J.; Bylda, C.; Blair, R.G. Mechanocatalysis for Biomass-derived Chemicals and Fuels. Green Chem. 2010, 12, 468–474. [Google Scholar] [CrossRef]
- Käldström, M. Deciphering ‘Water-soluble Lignocellulose’ Obtained by Mechanocatalysis: New Insights into the Chemical Processes Leading to Deep Depolymerization. Green Chem. 2014, 16, 3528–3538. [Google Scholar] [CrossRef]
- Kleine, T.; Buendia, J.; Bolm, C. Mechanochemical Degradation of Lignin and Wood by Solvent-free Grinding in a Reactive Medium. Green Chem. 2012, 15, 160–166. [Google Scholar] [CrossRef]
- Schneider, L.; Haverinen, J.; Jaakkola, M.; Lassi, U. Solid Acid-catalyzed Depolymerization of Barley Straw Driven by Ball Milling. Bioresour. Technol. 2016, 206, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Loustaucazalet, C.; Sambusiti, C.; Buche, P.; Solhy, A.; Bilal, E.; Larzek, M.; Barakat, A. Innovative Deconstruction of Biomass Induced by Dry Chemo-Mechanical Activation: Impact on Enzymatic Hydrolysis and Energy Efficiency. ACS Sustain. Chem. Eng. 2016, 4, 2689–2697. [Google Scholar] [CrossRef]
- Li, L.; Shen, F.; Smith, R.L.; Qi, X. Quantitative Chemocatalytic Production of Lactic Acid from Glucose under Anaerobic Conditions at Room Temperature. Green Chem. 2017, 19, 76–81. [Google Scholar] [CrossRef]
- Tang, Z.; Deng, W.; Wang, Y.; Zhu, E.; Wan, X.; Zhang, Q.; Wang, Y. Transformation of Cellulose and its Derived Carbohydrates into Formic and Lactic Acids Catalyzed by Vanadyl Cations. ChemSusChem 2014, 7, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G.; Sorrentino, A. Mechanical Milling as a Technology to Produce Dtructural and Functional Bio-nanocomposites. Green Chem. 2015, 17, 2610–2625. [Google Scholar] [CrossRef]
- Yang, X.; Yang, L.; Fan, W.; Lin, H. Effect of Redox Properties of LaCoO3 Perovskite Catalyst on Production of Lactic Acid From Cellulosic Biomass. Catal. Today 2016, 269, 56–64. [Google Scholar] [CrossRef]
- Nemoto, K.; Hirano, Y.; Hirata, K.I.; Takahashi, T.; Tsuneki, H.; Tominaga, K.I.; Sato, K. Cooperative In—Sn Catalyst System for Efficient Methyl Lactate Synthesis from Biomass-derived Sugars. Appl. Catal. B Environ. 2016, 183, 8–17. [Google Scholar] [CrossRef]
- Murillo, B. Conversion of Glucose to Lactic Acid Derivatives with Mesoporous Sn-MCM-41 and Microporous Titanosilicates. J. Chem. Technol. Biotechnol. 2014, 89, 1344–1350. [Google Scholar] [CrossRef]
- Jia, D.; Zhang, Z.; Yao, G.; Huo, Z.; Jin, F. Hydrothermal Conversion of Glucose into Lactic Acid with Sodium Silicate as a Base Catalyst. Catal. Today 2016, 263, 112–116. [Google Scholar]
- Huo, Z.; Fang, Y.; Ren, D.; Zhang, S.; Yao, G.; Zeng, X.; Jin, F. Selective Conversion of Glucose into Lactic Acid with Transition Metal Ions in Diluted Aqueous NaOH Solution. ACS Sustain. Chem. Eng. 2014, 2, 2765–2771. [Google Scholar] [CrossRef]
- Yang, L.; Yang, X.; Tian, E.; Vattipalli, V.; Fan, W.; Lin, H. Mechanistic Insights into the Production of Methyl Lactate by Catalytic Conversion of Carbohydrates on Mesoporous Zr-SBA-15. J. Catal. 2016, 333, 207–216. [Google Scholar] [CrossRef]
- Holm, M.S.; Saravanamurugan, S.; Taarning, E. Conversion of Sugars to Lactic Acid Derivatives using Heterogeneous Zeotype Catalysts. Science 2010, 328, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.S.; Pagántorres, Y.J.; Saravanamurugan, S.; Riisager, A.; Dumesic, J.A.; Taarning, E. Sn-Beta Catalysed Conversion of Hemicellulosic Sugars. Green Chem. 2012, 14, 702–706. [Google Scholar] [CrossRef]
- Santos, J.B.D. Fructose Conversion in the Presence of Sn(IV) Catalysts Exhibiting High Selectivity to Lactic Acid. RSC Adv. 2015, 5, 90952–90959. [Google Scholar] [CrossRef]



| Substrate | Catalyst | Condition | Solvent | Substrate Concentration (M) | Substrat Conversion (%) | Lactic Acid/Methyl Lactate Yield (mol %) | Reference |
|---|---|---|---|---|---|---|---|
| Glucose | Ba(OH)2 Solid 1.5 g | 500 r/min 4 h, ~40 °C | none | 0.5 g | 98.1 | 35.6 | This work |
| Fructose | Ba(OH)2 Solid 1.5 g | 500 r/min 4 h, ~40 °C | none | 0.5 g | 97.7 | 35.1 | This work |
| Glucose | Ba(OH)2 | 25 °C, 48 h | 25 °C, 48 h | 0.1 | 97.4 | 78.3 | [21] |
| Fructose | Ba(OH)2 | 25 °C, 48 h | 25 °C, 48 h | 0.1 | 98.7 | 83.5 | [21] |
| Glucose | LaCoO3 | 200 °C, 1 h | Water | 0.05 | N/A | 39.5 | [24] |
| Glucose | InCl3.4H2O | 200 °C,10 h | Methanol | 0.0025 | 97 | 52 | [25] |
| Glucose | Sn-MCM-41 (Si/Sn = 55) | 160 °C, 20 h | Methanol | 0.125 | 100 | 43 | [26] |
| Glucose | Na2SiO3 | 300 °C, 1 min | Water | 0.1 | N/A | 30 | [27] |
| Glucose | NaOH-NiCl2 | 300 °C, 1 min | Water | 0.097 | N/A | 25 | [28] |
| Fructose | Zr-SBA-15 | 240 °C, 6 h | Methanol | 0.056 | N/A | 44.1 | [29] |
| Fructose | Ti-Beta | 160 °C, 20 h | Methanol | 0.125 | 99 | 36 | [30] |
| Fructose | Sn-Beta | 160 °C, 16 h | Methanol | 0.125 | 98 | 54 | [31] |
| Fructose | (C4H9)2SnO | 190 °C, 0.5 h | Water | 0.044 | N/A | 63 | [32] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Yan, L.; Shen, F.; Qiu, M.; Qi, X. Mechanocatalytic Production of Lactic Acid from Glucose by Ball Milling. Catalysts 2017, 7, 170. https://doi.org/10.3390/catal7060170
Li L, Yan L, Shen F, Qiu M, Qi X. Mechanocatalytic Production of Lactic Acid from Glucose by Ball Milling. Catalysts. 2017; 7(6):170. https://doi.org/10.3390/catal7060170
Chicago/Turabian StyleLi, Luyang, Lulu Yan, Feng Shen, Mo Qiu, and Xinhua Qi. 2017. "Mechanocatalytic Production of Lactic Acid from Glucose by Ball Milling" Catalysts 7, no. 6: 170. https://doi.org/10.3390/catal7060170