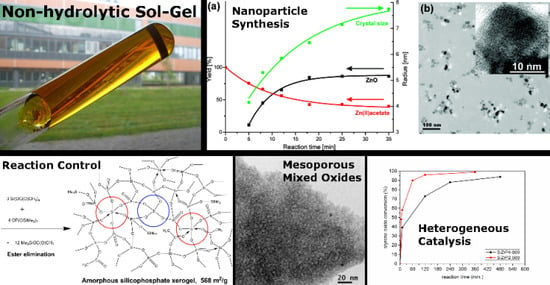
The Power of Non-Hydrolytic Sol-Gel Chemistry: A Review
Abstract
:1. Introduction
1.1. General Introduction
1.2. Overview of Known Reaction Pathways
2. Development of Condensation Routes
2.1. Mechanistic View into Alkyl Halide Elimination, Implications for Homogeneity
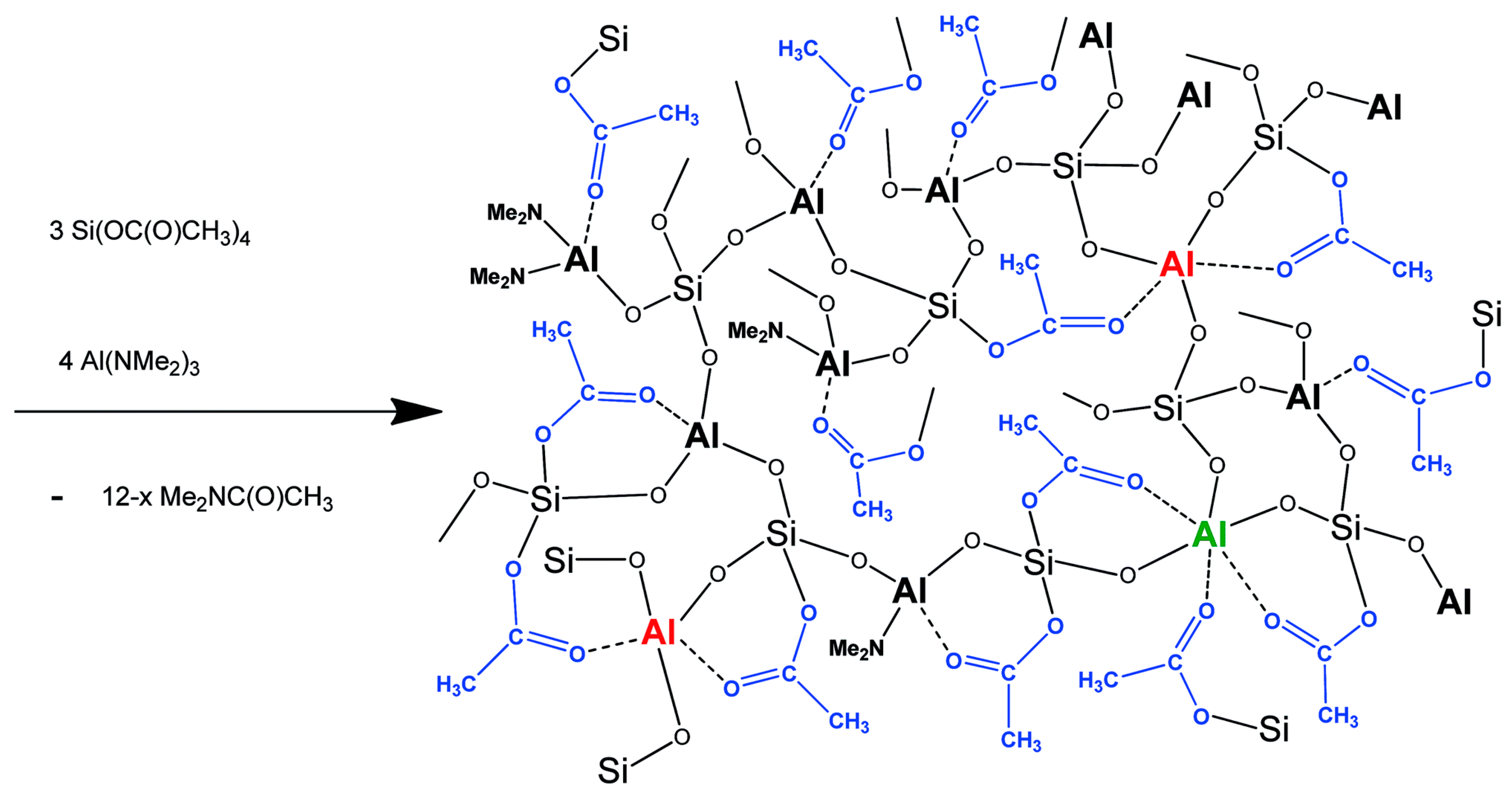
2.2. High Homogeneity of Mixed Metal Oxides in Acetamide Elimination
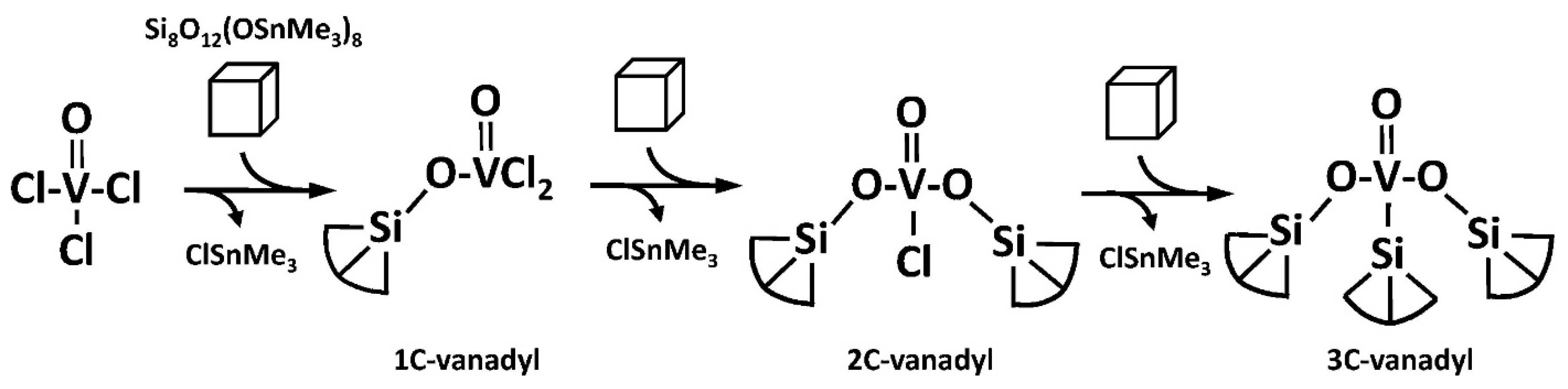
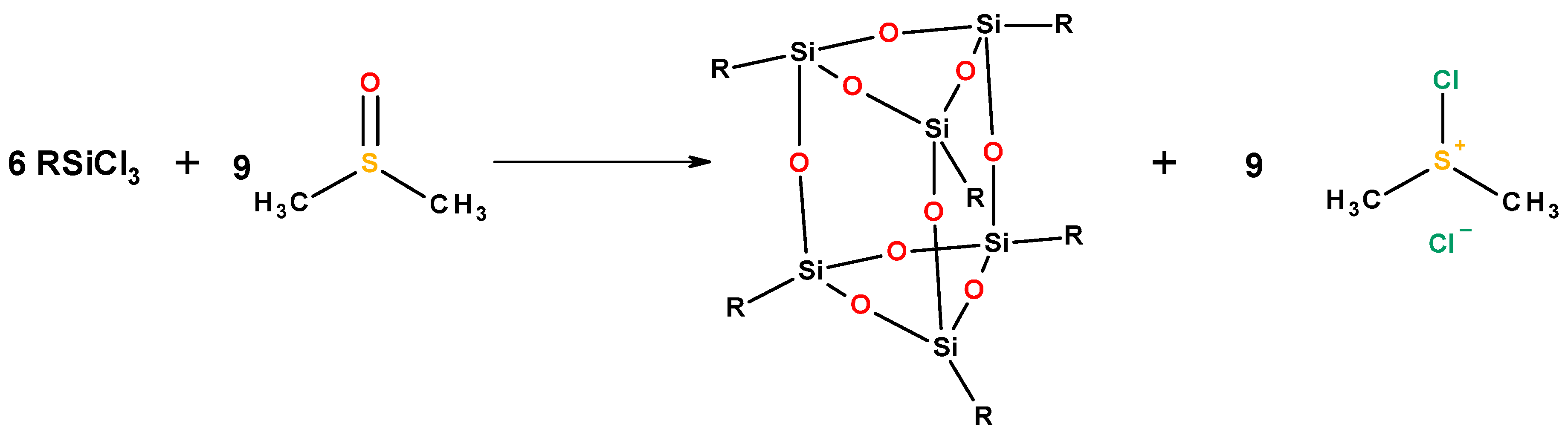
2.3. Building Block Approach to Single Site Metallosilicate Catalysts
2.4. New Materials by Ester Elimination: Silicophosphates and Their Hybrid Derivatives
2.5. Carboxylic Acids in NHSG
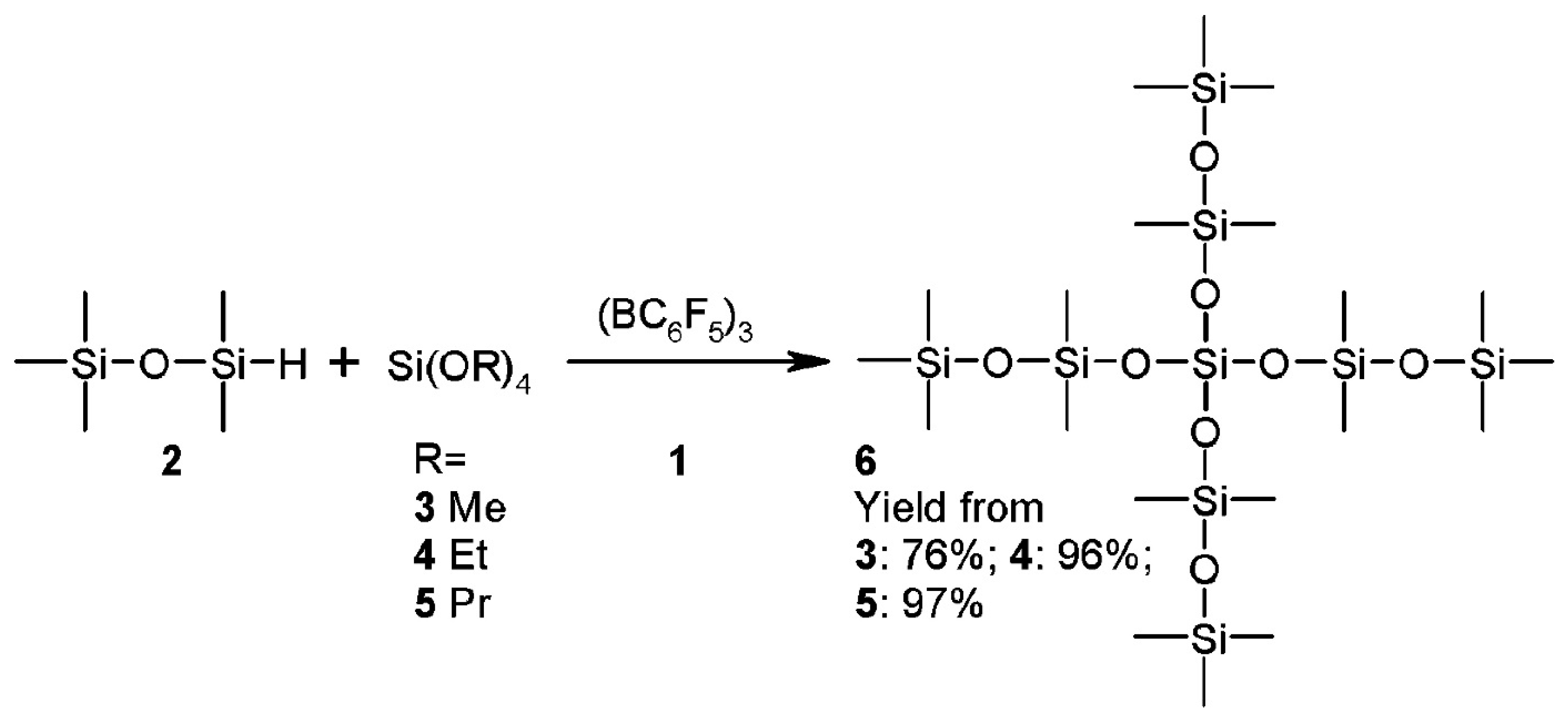
2.6. Siloxanes by NHSG Condensations, Piers-Rubinsztajn Reaction
2.7. Alcohols in NHSG
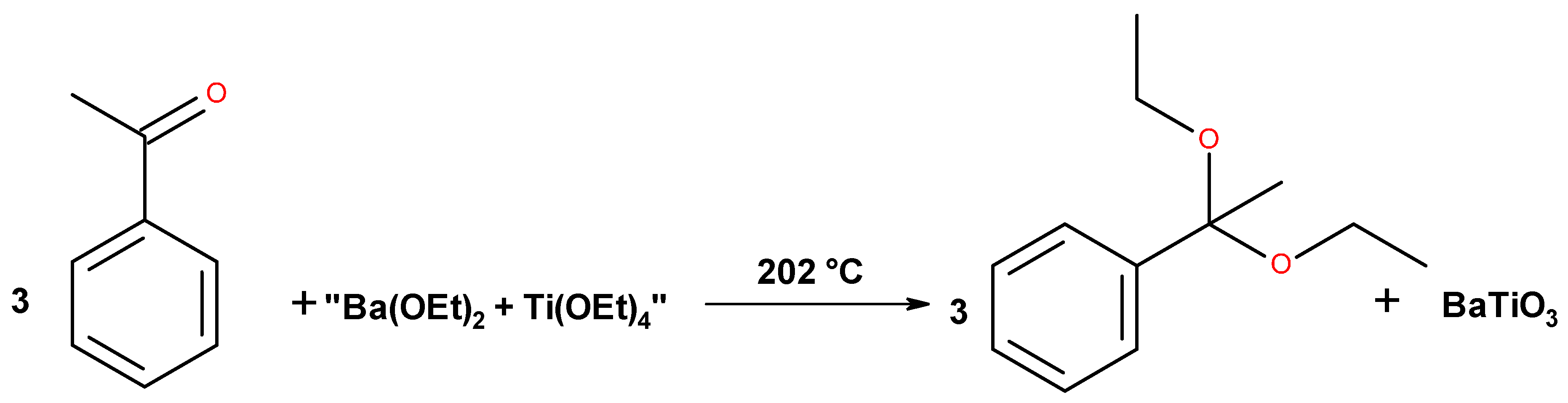
2.8. Benzyl Alcohol Route
2.9. Ether Elimination
2.10. Twin Polymerization
3. New Materials
3.1. Surface Area and Pore Size Control
3.1.1. Without Templates
3.1.2. With Templates
3.2. New Compositions
4. New Applications
4.1. Heterogeneous Catalysts
4.2. Luminiscent Materials
4.3. Electrodes in Li-Ion Batteries
4.4. Catalyst Supports
5. Overview and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Boston, MA, USA, 1990. [Google Scholar]
- Livage, J.; Henry, M.; Sanchez, C. Sol-gel chemistry of transition metal oxides. Prog. Solid State Chem. 1988, 18, 259–341. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of “sol-gel” chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Faustini, M.; Grosso, D.; Boissière, C.; Backov, R.; Sanchez, C. “Integrative sol-gel chemistry”: A nanofoundry for materials science. J. Sol-Gel Sci. Technol. 2014, 70, 216–226. [Google Scholar] [CrossRef]
- Debecker, D.P.; Mutin, P.H. Non-hydrolytic sol–gel routes to heterogeneous catalysts. Chem. Soc. Rev. 2012, 41, 3624–3650. [Google Scholar] [CrossRef] [PubMed]
- Flaig, S.; Akbarzadeh, J.; Dolcet, P.; Gross, S.; Peterlik, H.; Hüsing, N. Hierarchically Organized Silica-Titania Monoliths Prepared under Purely Aqueous Conditions. Chem. Eur. J. 2014, 20, 17409–17419. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liang, M.; Liu, J.; Li, S.; Xue, B.; Zhao, H. Effect of chelating agent acetylacetone on corrosion protection properties of silane-zirconium sol-gel coatings. Appl. Surf. Sci. 2016, 363, 229–239. [Google Scholar] [CrossRef]
- Hutter, R.; Dutoit, D.C.M.; Mallat, T.; Schneider, M.; Baiker, A. Novel Mesoporous Titania-Silica Aerogels Highly Active for the Selective Epoxidation of Cyclic Olefins. J. Chem. Soc. Chem. Commun. 1995, 163–164. [Google Scholar] [CrossRef]
- Gisler, A.; Müller, C.A.; Schneider, M.; Mallat, T.; Baiker, A. Titania-silica epoxidation catalysts modified by mono- and bidentate organic functional groups. Top. Catal. 2001, 15, 247–255. [Google Scholar] [CrossRef]
- Gerrard, W.; Griffey, P.F. A Novel Preparation of Boron Phosphate. Chem. Ind. 1959, 1959, 55. [Google Scholar]
- Lindquist, D.A.; Poindexter, S.M.; Rooke, S.S.; Stockdale, D.R.; Babb, K.; Smoot, A.L.; Young, W.E. Boron Phosphate and Aluminum Phosphate Aerogels. Proc. Ark. Acad. Sci. 1994, 48, 100–103. [Google Scholar]
- Goubeau, J.; Christe, K.O.; Teske, W.; Wilborn, W. Über die Silicophosphorsäure und ihre Derivate. Z. Anorg. Allg. Chem. 1963, 325, 26–42. [Google Scholar] [CrossRef]
- Debecker, D.P.; Hulea, V.; Mutin, P.H. Mesoporous mixed oxide catalysts via non-hydrolytic sol-gel: A review. Appl. Catal. A Gen. 2013, 451, 192–206. [Google Scholar] [CrossRef]
- Mutin, P.H.; Vioux, A. Nonhydrolytic processing of oxide-based materials: Simple routes to control homogeneity, morphology, and nanostructure. Chem. Mater. 2009, 21, 582–596. [Google Scholar] [CrossRef]
- Niederberger, M.; Garnweitner, G. Organic reaction pathways in the nonaqueous synthesis of metal oxide nanoparticles. Chem. Eur. J. 2006, 12, 7282–7302. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, M.; Garnweitner, G.; Buha, J.; Polleux, J.; Ba, J.; Pinna, N. Nonaqueous synthesis of metal oxide nanoparticles: Review and indium oxide as case study for the dependence of particle morphology on precursors and solvents. J. Sol-Gel Sci. Technol. 2006, 40, 259–266. [Google Scholar] [CrossRef]
- Djerdj, I.; Arčon, D.; Jagličić, Z.; Niederberger, M. Nonaqueous synthesis of metal oxide nanoparticles: Short review and doped titanium dioxide as case study for the preparation of transition metal-doped oxide nanoparticles. J. Solid State Chem. 2008, 181, 1571–1581. [Google Scholar] [CrossRef]
- Niederberger, M. Nonaqueous Sol-Gel Routes to Metal Oxide Nanoparticles. Acc. Chem. Res. 2007, 40, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Bilecka, I.; Niederberger, M. New developments in the nonaqueous and/or non-hydrolytic sol-gel synthesis of inorganic nanoparticles. Electrochim. Acta 2010, 55, 7717–7725. [Google Scholar] [CrossRef]
- Pinna, N.; Garnweitner, G.; Antonietti, M.; Niederberger, M. A general nonaqueous route to binary metal oxide nanocrystals involving a C–C bond cleavage. J. Am. Chem. Soc. 2005, 127, 5608–5612. [Google Scholar] [CrossRef] [PubMed]
- Garnweitner, G.; Niederberger, M. Nonaqueous and Surfactant-Free Synthesis Routes to Metal Oxide Nanoparticles. J. Am. Ceram. Soc. 2006, 89, 1801–1808. [Google Scholar] [CrossRef]
- Mutin, P.H.; Vioux, A. Recent advances in the synthesis of inorganic materials via non-hydrolytic condensation and related low-temperature routes. J. Mater. Chem. A 2013, 1, 11504–11512. [Google Scholar] [CrossRef]
- Hay, J.N.; Raval, H.M. Synthesis of Organic−Inorganic Hybrids via the Non-hydrolytic Sol-Gel Process. Chem. Mater. 2001, 13, 3396–3403. [Google Scholar] [CrossRef]
- Brook, M.A.; Grande, J.B.; Ganachaud, F. New Synthetic Strategies for Structured Silicones Using B(C6F5)3. In Advances in Polymer Science; Springer: Berlin, Germany, 2010; Volume 235, pp. 161–183. [Google Scholar]
- Thompson, D.B.; Brook, M.A. Rapid Assembly of Complex 3D Siloxane Architectures. J. Am. Chem. Soc. 2008, 130, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.; Yang, H.; Wenzel, S.; Grigoras, S.; Brook, M.A. Using “Anhydrous” Hydrolysis to Favor Formation of Hexamethylcyclotrisiloxane from Dimethyldichlorosilane. Organometallics 1998, 17, 556–564. [Google Scholar] [CrossRef]
- Wakabayashi, R.; Kawahara, K.; Kuroda, K. Nonhydrolytic Synthesis of Branched Alkoxysiloxane Oligomers Si[OSiH(OR)2]4 (R = Me, Et). Angew. Chem. Int. Ed. 2010, 49, 5273–5277. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, Y.; Shimojima, A.; Kuroda, K. Alkoxysilylated-Derivatives of Double-Four-Ring Silicate as Novel Building Blocks of Silica-Based Materials. Chem. Mater. 2008, 20, 1147–1153. [Google Scholar] [CrossRef]
- Bassindale, A.R.; MacKinnon, I.A.; Maesano, M.G.; Taylor, P.G. The preparation of hexasilsesquioxane (T6) cages by “Non Aqueous” Hydrolysis of trichlorosilanes. Chem. Commun. 2003, 12, 1382–1383. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Wakabayashi, R.; Tamai, M.; Kuroda, K. Synthesis of a multifunctional alkoxysiloxane oligomer. New J. Chem. 2014, 38, 5362–5368. [Google Scholar] [CrossRef]
- Clark, J.C.; Barnes, C.E. Reaction of the Si8O20(SnMe3)8 Building Block with Silyl Chlorides: A New Synthetic Methodology for Preparing Nanostructured Building Block Solids. Chem. Mater. 2007, 19, 3212–3218. [Google Scholar] [CrossRef]
- Lee, M.Y.; Jiao, J.; Mayes, R.; Hagaman, E.; Barnes, C.E. The targeted synthesis of single site vanadyl species on the surface and in the framework of silicate building block materials. Catal. Today 2011, 160, 153–164. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Atomic Layer Deposition Chemistry: Recent Developments and Future Challenges. Angew. Chem. Int. Ed. 2003, 42, 5548–5554. [Google Scholar] [CrossRef] [PubMed]
- Clavel, G.; Rauwel, E.; Willinger, M.-G.; Pinna, N. Non-aqueous sol–gel routes applied to atomic layer deposition of oxides. J. Mater. Chem. 2009, 19, 454–462. [Google Scholar] [CrossRef]
- Lourenço, M.A.O.; Silva, R.M.; Silva, R.F.; Pinna, N.; Pronier, S.; Pires, J.; Gomes, J.R.B.; Pinto, M.L.; Ferreira, P. Turning periodic mesoporous organosilicas selective to CO2/CH4 separation: deposition of aluminium oxide by atomic layer deposition. J. Mater. Chem. A 2015, 3, 22860–22867. [Google Scholar] [CrossRef]
- Arnal, P.; Corriu, R.J.P.; Leclercq, D.; Mutin, H.P.; Vioux, A. A Solution Chemistry Study of Nonhydrolytic Sol-Gel Routes to Titania. Chem. Mater. 1997, 9, 694–698. [Google Scholar] [CrossRef]
- Styskalik, A.; Skoda, D.; Pinkas, J.; Mathur, S. Non-hydrolytic synthesis of titanosilicate xerogels by acetamide elimination and their use as epoxidation catalysts. J. Sol-Gel Sci. Technol. 2012, 63, 463–472. [Google Scholar] [CrossRef]
- Styskalik, A.; Skoda, D.; Moravec, Z.; Abbott, J.G.; Barnes, C.E.; Pinkas, J. Synthesis of homogeneous silicophosphate xerogels by non-hydrolytic condensation reactions. Microporous Mesoporous Mater. 2014, 197, 204–212. [Google Scholar] [CrossRef]
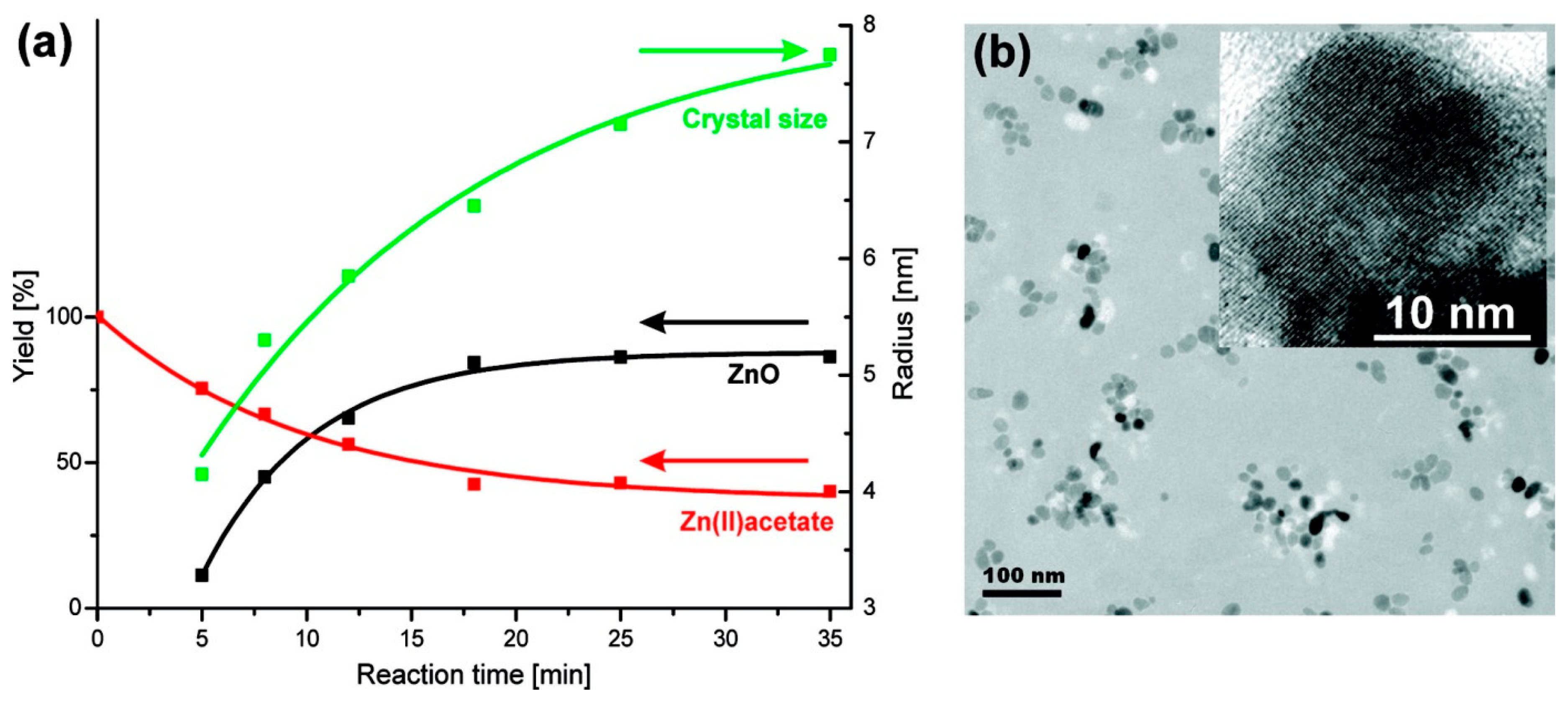
- Bilecka, I.; Elser, P.; Niederberger, M. Kinetic and thermodynamic aspects in the microwave-assisted synthesis of ZnO nanoparticles in benzyl alcohol. ACS Nano 2009, 3, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Kriesel, J.W.; Sander, M.S.; Tilley, T.D. Block Copolymer-Assisted Synthesis of Mesoporous, Multicomponent Oxides by Nonhydrolytic, Thermolytic Decomposition of Molecular Precursors in Nonpolar Media. Chem. Mater. 2001, 13, 3554–3563. [Google Scholar] [CrossRef]
- Bradley, D.C.; Thomas, I.M. Organosilyloxy-derivatives of metals. Part I. Alkylsilyloxy-derivatives of titanium, zirconium, niobium, and tantalum. J. Chem. Soc. 1959, 3404–3411. [Google Scholar] [CrossRef]
- Caruso, J.; Hampden-Smith, M.J. Ester Elimination: A General Solvent Dependent Non-Hydrolytic Route to Metal and Mixed-Metal Oxides. J. Sol-Gel Sci. Technol. 1997, 39, 35–39. [Google Scholar] [CrossRef]
- Caruso, J.; Hampden-Smith, M.J.; Duesler, E.N. Solvent Dependent Ester Elimination Reactions in the Preparation of Mixed-metal 0xo Clusters: The Synthesis of PbSn2(μ3-O)(OBut)4(OAc)4. J. Chem. Soc. Chem. Commun. 1995, 2, 1041–1042. [Google Scholar] [CrossRef]
- Jansen, M.; Guenther, E. Oxide Gels and Ceramics Prepared by a Nonhydrolytic Sol-Gel Process. Chem. Mater. 1995, 7, 2110–2114. [Google Scholar] [CrossRef]
- Iwasaki, M.; Yasumori, A.; Shibata, S.; Yamane, M. Preparation of high homogeneity BaO-TiO2-SiO2 gel. J. Sol-Gel Sci. Technol. 1994, 2, 387–391. [Google Scholar] [CrossRef]
- Styskalik, A.; Skoda, D.; Moravec, Z.; Babiak, M.; Barnes, C.E.; Pinkas, J. Control of micro/mesoporosity in non-hydrolytic hybrid silicophosphate xerogels. J. Mater. Chem. A 2015, 3, 7477–7487. [Google Scholar] [CrossRef]
- Skoda, D.; Styskalik, A.; Moravec, Z.; Bezdicka, P.; Barnes, C.E.; Pinkas, J. Mesoporous titanosilicates by templated non-hydrolytic sol–gel reactions. J. Sol-Gel Sci. Technol. 2015, 74, 810–822. [Google Scholar] [CrossRef]
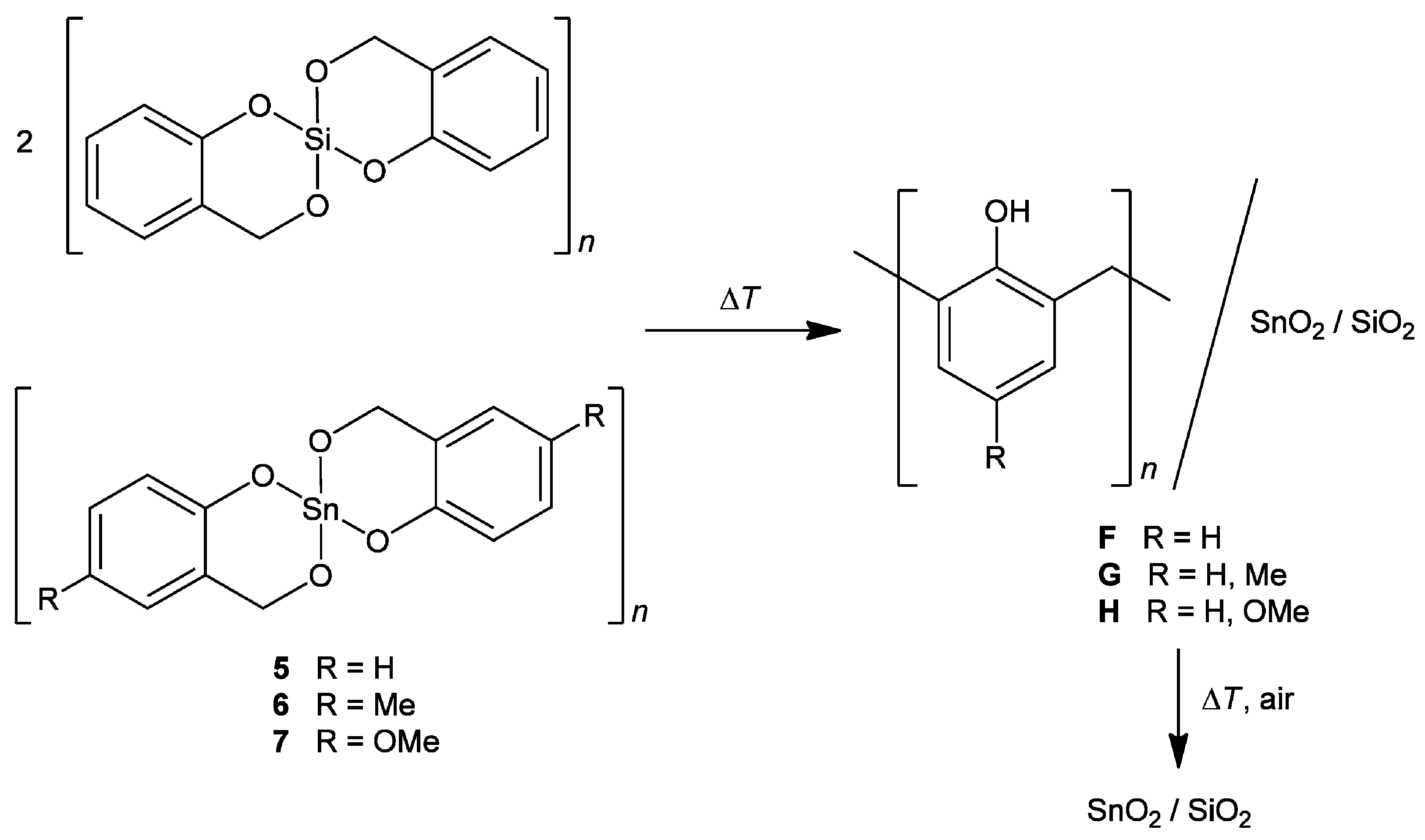
- Skoda, D.; Styskalik, A.; Moravec, Z.; Bezdicka, P.; Bursik, J.; Mutin, P.H.; Pinkas, J. Mesoporous SnO2-SiO2 and Sn-silica-carbon nanocomposites by novel non-hydrolytic templated sol–gel synthesis. RSC Adv. 2016, 6, 68739–68747. [Google Scholar] [CrossRef]
- Skoda, D.; Styskalik, A.; Moravec, Z.; Bezdicka, P.; Pinkas, J. Templated non-hydrolytic synthesis of mesoporous zirconium silicates and their catalytic properties. J. Mater. Sci. 2015, 50, 3371–3382. [Google Scholar] [CrossRef]
- Skoda, D.; Styskalik, A.; Moravec, Z.; Bezdicka, P.; Babiak, M.; Klementova, M.; Barnes, C.E.; Pinkas, J. Novel non-hydrolytic templated sol-gel synthesis of mesoporous aluminosilicates and their use as aminolysis catalysts. RSC Adv. 2016, 6, 24273–24284. [Google Scholar] [CrossRef]
- Chojnowski, J.; Rubinsztajn, S.; Cella, J.A.; Fortuniak, W.; Cypryk, M.; Kurjata, J.; Kaźmierski, K. Mechanism of the B(C6F5)3-Catalyzed Reaction of Silyl Hydrides with Alkoxysilanes. Kinetic and Spectroscopic Studies. Organometallics 2005, 24, 6077–6084. [Google Scholar] [CrossRef]
- Kurjata, J.; Fortuniak, W.; Rubinsztajn, S.; Chojnowski, J. B(C6F5)3 catalyzed dehydrocarbon polycondensation of PhSiH3 with (MeO)4Si as model polyfunctional comonomers in new route to hydrophobic silicone TQ resins. Eur. Polym. J. 2009, 45, 3372–3379. [Google Scholar] [CrossRef]
- Rubinsztajn, S. New Facile Process for Synthesis of Borosiloxane Resins. J. Inorg. Organomet. Polym. Mater. 2014, 24, 1092–1095. [Google Scholar] [CrossRef]
- Bradley, D.C.; Chakravarti, B.N.; Chatterjee, A.K.; Wardlaw, W.; Whitley, A. Niobium and tantalum mixed alkoxides. J. Chem. Soc. 1958, 99–101. [Google Scholar] [CrossRef]
- Pazik, R.; Tekoriute, R.; Håkansson, S.; Wiglusz, R.; Strek, W.; Seisenbaeva, G.A.; Gun’ko, Y.K.; Kessler, V.G. Precursor and solvent effects in the nonhydrolytic synthesis of complex oxide nanoparticles for bioimaging applications by the ether elimination (Bradley) reaction. Chem. Eur. J. 2009, 15, 6820–6826. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Lin, J.; Huang, W.; Feng, Z.; Li, D. Preparation of TeO2 based thin films by nonhydrolytic sol-gel process. Mater. Sci. Eng. B 2009, 164, 51–59. [Google Scholar] [CrossRef]
- Corriu, R.J.P.; Leclercq, D.; Lefèvre, P.; Mutin, P.H.; Vioux, A. Preparation of monolithic metal oxide gels by a non-hydrolytic sol-gel process. J. Mater. Chem. 1992, 2, 673–674. [Google Scholar] [CrossRef]
- Andrianainarivelo, M.; Corriu, R.J.P.; Leclercq, D.; Mutin, P.; Vioux, A. Nonhydrolytic sol-gel process: Aluminum titanate gels. Chem. Mater. 1997, 9, 1098–1102. [Google Scholar] [CrossRef]
- Wei, H.-Y.; Jiang, W.-H.; Lin, J.; Feng, G.; Feng, Z.-B. Comparative Research on the Synthesis of Aluminum Titanate Powders by Nonhydrolytic and Hydrolytic Sol-gel Method. J. Inorg. Mater. 2009, 24, 199–203. [Google Scholar] [CrossRef]
- Andrianainarivelo, M.; Corriu, R.J.P.; Leclercq, D.; Mutin, P.H.; Vioux, A. Non-hydrolytic sol-gel process: Zirconium titanate gels. J. Mater. Chem. 1997, 7, 279–284. [Google Scholar] [CrossRef]
- Young, L.; Alvarez, P.T.; Liu, H.; Lind, C. Extremely Low Temperature Crystallization in the A2M3O12 Family of Negative Thermal Expansion Materials. Eur. J. Inorg. Chem. 2016, 2016, 1251–1256. [Google Scholar] [CrossRef]
- Truitt, R.; Hermes, I.; Main, A.; Sendecki, A.; Lind, C. Low Temperature Synthesis and Characterization of AlScMo3O12. Materials 2015, 8, 700–716. [Google Scholar] [CrossRef]
- Gates, S.D.; Lind, C. Polymorphism in yttrium molybdate Y2Mo3O12. J. Solid State Chem. 2007, 180, 3510–3514. [Google Scholar] [CrossRef]
- Gates, S.D.; Colin, J.A.; Lind, C. Non-hydrolytic sol–gel synthesis, properties, and high-pressure behavior of gallium molybdate. J. Mater. Chem. 2006, 16, 4214–4219. [Google Scholar] [CrossRef]
- Acosta, S.; Corriu, R.; Leclercq, D.; Mutin, P.H.; Vioux, A. Novel Non-Hydrolytic Sol-Gel Route to Metal Oxides. J. Sol-Gel Sci. Technol. 1994, 2, 25–28. [Google Scholar] [CrossRef]
- Andrianainarivelo, M.; Corriu, R.; Leclercq, D.; Mutin, P.H.; Vioux, A. Mixed oxides SiO2–ZrO2 and SiO2–TiO2 by a non-hydrolytic sol-gel route. J. Mater. Chem. 1996, 6, 1665–1671. [Google Scholar] [CrossRef]
- Evans, D.L. Glass structure: The bridge between the molten and crystalline states. J. Non-Cryst. Solids 1982, 52, 115–128. [Google Scholar] [CrossRef]
- Crouzet, L.; Leclercq, D.; Mutin, P.H.; Vioux, A. Organic–inorganic materials by nonhydrolytic sol-gel processes: Organosilsesquioxane-metal oxide hybrids. J. Sol-Gel Sci. Technol. 2003, 26, 335–338. [Google Scholar] [CrossRef]
- Lorret, O.; Lafond, V.; Mutin, P.H.; Vioux, A. One-Step Synthesis of Mesoporous Hybrid Titania−Silica Xerogels for the Epoxidation of Alkenes. Chem. Mater. 2006, 18, 4707–4709. [Google Scholar] [CrossRef]
- Arnal, P.; Corriu, R.J.P.; Leclercq, D.; Mutin, P.H.; Vioux, A. Preparation of anatase, brookite and rutile at low temperature by non-hydrolytic sol-gel methods. J. Mater. Chem. 1996, 6, 1925–1932. [Google Scholar] [CrossRef]
- Joo, J.; Yu, T.; Kim, Y.W.; Park, H.M.; Wu, F.; Zhang, J.Z.; Hyeon, T. Multigram Scale Synthesis and Characterization of Monodisperse Tetragonal Zirconia Nanocrystals. J. Am. Chem. Soc. 2003, 125, 6553–6557. [Google Scholar] [CrossRef] [PubMed]
- Depner, S.W.; Kort, K.R.; Jaye, C.; Fischer, D.A.; Banerjee, S. Nonhydrolytic Synthesis and Electronic Structure of Ligand-Capped CeO2−δ and CeOCl Nanocrystals. J. Phys. Chem. C 2009, 113, 14126–14134. [Google Scholar] [CrossRef]
- Depner, S.W.; Cultrara, N.D.; Farley, K.E.; Qin, Y.; Banerjee, S. Ferroelastic Domain Organization and Precursor Control of Size in Solution-Grown Hafnium Dioxide Nanorods. ACS Nano 2014, 8, 4678–4688. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Fabbri, J.; Robinson, R.D.; Zhu, Y.; Herman, I.P.; Steigerwald, M.L.; Brus, L.E. Solid-Solution Nanoparticles: Use of a Nonhydrolytic Sol-Gel Synthesis To Prepare HfO2 and HfxZr1−xO2 Nanocrystals. Chem. Mater. 2004, 16, 1336–1342. [Google Scholar] [CrossRef]
- Trentler, T.J.; Denler, T.E.; Bertone, J.F.; Agrawal, A.; Colvin, V.L. Synthesis of TiO2 Nanocrystals by Nonhydrolytic Solution-Based Reactions. J. Am. Chem. Soc. 1999, 121, 1613–1614. [Google Scholar] [CrossRef]
- Goto, Y.; Omata, T.; Otsuka-Yao-Matsuo, S. Extremely Suppressed Grain Growth of Y2O3-Stabilized Zirconia Nanocrystals Synthesized by the Nonhydrolytic Sol-Gel Technique. J. Electrochem. Soc. 2009, 156, K4–K9. [Google Scholar] [CrossRef]
- Bourget, L.; Mutin, P.H.; Vioux, A.; Frances, J.M. Nonhydrolytic Synthesis and Structural Study of Methoxyl-Terminated Polysiloxane D/Q Resins. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 2415–2425. [Google Scholar] [CrossRef]
- Lafond, V.; Mutin, P.H.; Vioux, A. Control of the Texture of Titania-Silica Mixed Oxides Prepared by Nonhydrolytic Sol-Gel. Chem. Mater. 2004, 16, 5380–5386. [Google Scholar] [CrossRef]
- Acosta, S.; Corriu, R.J.P.; Leclercq, D.; Lefèvre, P.; Mutin, P.H.; Vioux, A. Preparation of Alumina gels by a non-hydrolytic sol-gel processing method. J. Non-Cryst. Solids 1994, 170, 234–242. [Google Scholar] [CrossRef]
- Shoyama, M.; Matsumoto, N.; Hashimoto, T.; Nasu, H.; Kamiya, K. Sol-gel synthesis of zircon-effect of addition of lithium ions. J. Mater. Sci. 1998, 33, 4821–4828. [Google Scholar] [CrossRef]
- Veytizou, C.; Quinson, J.-F.; Douy, A. Sol-gel synthesis via an aqueous semi-alkoxide route and characterization of zircon powders. J. Mater. Chem. 2000, 10, 365–370. [Google Scholar] [CrossRef]
- Parcianello, G.; Bernardo, E.; Colombo, P. Low temperature synthesis of zircon from silicone resins and oxide nano-sized particles. J. Eur. Ceram. Soc. 2012, 32, 2819–2824. [Google Scholar] [CrossRef]
- Sanchez, C.; Livage, J.; Henry, M.; Babonneau, F. Chemical modification of alkoxide precursors. J. Non-Cryst. Solids 1988, 100, 65–76. [Google Scholar] [CrossRef]
- Ghosh, N.N.; Clark, J.C.; Eldridge, G.T.; Barnes, C.E. Building block syntheses of site-isolated vanadyl groups in silicate oxides. Chem. Commun. 2004, 856–857. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.E.; Sharp, K.; Albert, A.A.; Peretich, M.E.; Fulvio, P. Metal-silicate catalysts: Single site, mesoporous systems without templates. J. Nanosci. Lett. 2015, 4, 107. [Google Scholar]
- Caruso, J.; Hampden-Smith, M.J.; Rheingold, A.L.; Yap, G. Ester Elimination Versus Ligand Exchange: The Role of the Solvent in Tin-Oxo Cluster-building Reactions. J. Chem. Soc. Chem. Commun. 1995, 49, 157–158. [Google Scholar] [CrossRef]
- Caruso, J.; Roger, C.; Schwertfeger, F.; Hampden-Smith, M.J.; Rheingold, A.L.; Yap, G. Solvent-Dependent Ester Elimination and Ligand Exchange Reactions between Trimethylsilyl Acetate and Tin(IV) Tetra-tert-Butoxide. Inorg. Chem. 1995, 34, 449–453. [Google Scholar] [CrossRef]
- Fujiwara, M.; Wessel, H.; Park, H.S.; Roesky, H.W. A sol-gel method using tetraethoxysilane and acetic anhydride: Immobilization of cubic μ-oxo Si-Ti complex in a silica matrix. Chem. Mater. 2002, 14, 4975–4981. [Google Scholar] [CrossRef]
- Beckett, M.A.; Rugen-Hankey, M.P.; Varma, K.S.; Varma, K.S. Trimethoxyboroxine as an “oxygen-transfer” reagent: A non-aqueous “sol-gel” route to alkali-free borosilicate glass. Chem. Commun. 2000, 37, 1499–1500. [Google Scholar] [CrossRef]
- Beckett, M.A.; Rugen-Hankey, M.P.; Varma, K.S. Formation of borosilicate glasses from silicon alkoxides and metaborate esters in dry non-aqueous solvents. J. Sol-Gel Sci. Technol. 2006, 39, 95–101. [Google Scholar] [CrossRef]
- Beckett, M.A.; Rugen-Hankey, M.P.; Timmis, J.L.; Varma, K.S.; Sanchez, C.; Tallon, J.; Whyman, R. Synthesis of aluminium borate-boron oxide and binary titanium-boron and zirconium-boron oxides from metal alkoxides and (MeO)3B3O3 in non-aqueous solvents. Dalton Trans. 2008, 23, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.C.; Taylor, C.J.; Roberts, J.; Campbell, S.A.; Tiner, M.; Hegde, R.; Hobbs, C.; Gladfelter, W.L. Observation of Precursor Control over Film Stoichiometry during the Chemical Vapor Deposition of Amorphous TixSi1-xO2 Films. Chem. Mater. 2000, 12, 2822–2824. [Google Scholar] [CrossRef]
- Styskalik, A.; Skoda, D.; Moravec, Z.; Barnes, C.E.; Pinkas, J. Surface reactivity of non-hydrolytic silicophosphate xerogels: A simple method to create Brønsted or Lewis acid sites on porous supports. New J. Chem. 2016, 40, 3705–3715. [Google Scholar] [CrossRef]
- Styskalik, A.; Skoda, D.; Moravec, Z.; Roupcova, P.; Barnes, C.E.; Pinkas, J. Non-aqueous template-assisted synthesis of mesoporous nanocrystalline silicon orthophosphate. RSC Adv. 2015, 5, 73670–73676. [Google Scholar] [CrossRef]
- Sharp, K.G. A two-component, non-aqueous route to silica gel. J. Sol-Gel Sci. Technol. 1994, 2, 35–41. [Google Scholar] [CrossRef]
- Kirszensztejn, P.; Kawałko, A.; Tolińska, A.; Przekop, R. Synthesis of SiO2–SnO2 gels in water free conditions. J. Porous Mater. 2010, 18, 241–249. [Google Scholar] [CrossRef]
- Martyła, A.; Olejnik, B.; Kirszensztejn, P.; Przekop, R. Influence of the method of synthesis on hydrogen adsorption properties of mesoporous binary B2O3/Al2O3 gel systems. Int. J. Hydrog. Energy 2011, 36, 8358–8364. [Google Scholar] [CrossRef]
- Przekop, R.; Kirszensztejn, P. Porous xerogel systems B2O3–Al2O3 obtained by the sol-gel method. J. Non-Cryst. Solids 2014, 402, 128–134. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.J.; Li, Z.Y.; Liu, H.T.; He, P.; Li, J.H. Preparation of porous aminopropylsilsesquioxane by a nonhydrolytic sol-gel method in ionic liquid solvent. Langmuir 2005, 21, 1618–1622. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Singh, R.K.; Chandra, S. Crystallization kinetics behavior of ionic liquid [EMIM][BF4] confined in mesoporous silica matrices. RSC Adv. 2014, 4, 22277–22287. [Google Scholar] [CrossRef]
- Verma, Y.L.; Gupta, A.K.; Singh, R.K.; Chandra, S. Preparation and characterisation of ionic liquid confined hybrid porous silica derived from ultrasonic assisted non-hydrolytic sol-gel process. Microporous Mesoporous Mater. 2014, 195, 143–153. [Google Scholar] [CrossRef]
- Viau, L.; Néouze, M.-A.; Biolley, C.; Volland, S.; Brevet, D.; Gaveau, P.; Dieudonné, P.; Galarneau, A.; Vioux, A. Ionic Liquid Mediated Sol-Gel Synthesis in the Presence of Water or Formic Acid: Which Synthesis for Which Material? Chem. Mater. 2012, 24, 3128–3134. [Google Scholar] [CrossRef]
- Néouze, M.-A.; Le Bideau, J.; Gaveau, P.; Bellayer, S.; Vioux, A. Ionogels, New Materials Arising from the Confinement of Ionic Liquids within Silica-Derived Networks. Chem. Mater. 2006, 18, 3931–3936. [Google Scholar] [CrossRef]
- Boday, D.J.; Tolbert, S.; Keller, M.W.; Li, Z.; Wertz, J.T.; Muriithi, B.; Loy, D.A. Non-hydrolytic formation of silica and polysilsesquioxane particles from alkoxysilane monomers with formic acid in toluene/tetrahydrofuran solutions. J. Nanopart. Res. 2014, 16, 2313. [Google Scholar] [CrossRef]
- Park, C.-S.; Mahadik, D.B.; Park, H.-H. Enhancement of the O2 gas sensing properties of mesoporous Sr0.9La0.1TiO3 films by increasing the pore connectivity. RSC Adv. 2015, 5, 66384–66390. [Google Scholar] [CrossRef]
- Bau, J.A.; Li, P.; Marenco, A.J.; Trudel, S.; Olsen, B.C.; Luber, E.J.; Buriak, J.M. Nickel/iron oxide nanocrystals with a nonequilibrium phase: Controlling size, shape, and composition. Chem. Mater. 2014, 26, 4796–4804. [Google Scholar] [CrossRef]
- Joo, J.; Kwon, S.G.; Yu, T.; Cho, M.; Lee, J.; Yoon, J. Large-Scale Synthesis of TiO2 Nanorods via Nonhydrolytic Sol-Gel Ester Elimination Reaction and Their Application to Photocatalytic Inactivation of E. coli. J. Phys. Chem. 2005, 109, 15297–15302. [Google Scholar] [CrossRef] [PubMed]
- Sliem, M.A.; Schmidt, D.A.; Bétard, A.; Kalidindi, S.B.; Gross, S.; Havenith, M.; Devi, A.; Fischer, R.A. Surfactant-induced nonhydrolytic synthesis of phase-pure ZrO2 nanoparticles from metal-organic and oxocluster precursors. Chem. Mater. 2012, 24, 4274–4282. [Google Scholar] [CrossRef]
- Kejik, M.; Moravec, Z.; Barnes, C.E.; Pinkas, J. Hybrid microporous and mesoporous organosilicate covalent polymers with high porosity. Microporous Mesoporous Mater. 2017, 240, 205–215. [Google Scholar] [CrossRef]
- Wakabayashi, R.; Kuroda, K. Siloxane-bond formation promoted by lewis acids: A nonhydrolytic sol-gel process and the piers-rubinsztajn reaction. ChemPlusChem 2013, 78, 764–774. [Google Scholar] [CrossRef]
- Bourget, L.; Leclercq, D.; Vioux, A. Catalyzed nonhydrolytic sol-gel route to organosilsesquioxane gels. J. Sol-Gel Sci. Technol. 1999, 14, 137–147. [Google Scholar] [CrossRef]
- Hay, J.N.; Porter, D.; Raval, H.M. A versatile route to organically-modified silicas and porous silicas via the non-hydrolytic sol-gel process. J. Mater. Chem. 2000, 10, 1811–1818. [Google Scholar] [CrossRef]
- Apperley, D.; Hay, J.N.; Raval, H.M. Silica-dimethylsiloxane hybrids-non-hydrolytic sol-gel synthesis and characterization by NMR spectroscopy. Chem. Mater. 2002, 14, 983–988. [Google Scholar] [CrossRef]
- Hay, J.; Raval, H.; Porter, D. A non-hydrolytic route to organically-modified silica. Chem. Commun. 1999, 1, 81–82. [Google Scholar] [CrossRef]
- Wakabayashi, R.; Tamai, M.; Kawahara, K.; Tachibana, H.; Imamura, Y.; Nakai, H.; Kuroda, K. Direct alkoxysilylation of alkoxysilanes for the synthesis of explicit alkoxysiloxane oligomers. J. Organomet. Chem. 2012, 716, 26–31. [Google Scholar] [CrossRef]
- Saito, S.; Yamasue, N.; Wada, H.; Shimojima, A.; Kuroda, K. Cubic Siloxanes with Both Si−H and Si−OtBu Groups for Site-Selective Siloxane Bond Formation. Chem. Eur. J. 2016, 22, 13857–13864. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, K.; Hagiwara, Y.; Kuroda, K. Dendritic, Nanosized Building Block for Siloxane-Based Materials: A Spherosilicate Dendrimer. Chem. Eur. J. 2011, 17, 13188–13196. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Shimojima, A.; Kawahara, K.; Wakabayashi, R.; Tamura, Y.; Asakura, Y.; Kitahara, M. Utilization of Alkoxysilyl Groups for the Creation of Structurally Controlled Siloxane-Based Nanomaterials. Chem. Mater. 2014, 26, 211–220. [Google Scholar] [CrossRef]
- Arkhireeva, A.; Hay, J.N.; Manzano, M. Preparation of silsesquioxane particles via a nonhydrolytic sol-gel route. Chem. Mater. 2005, 17, 875–880. [Google Scholar] [CrossRef]
- Jaumann, M.; Rebrov, E.A.; Kazakova, V.V.; Muzafarov, A.M.; Goedel, W.A.; Möller, M. Hyperbranched Polyalkoxysiloxanes via AB3-Type Monomers. Macromol. Chem. Phys. 2003, 204, 1014–1026. [Google Scholar] [CrossRef]
- Zhu, X.; Jaumann, M.; Peter, K.; Möller, M.; Melian, C.; Adams-Buda, A.; Demco, D.E.; Blümich, B. One-pot synthesis of hyperbranched polyethoxysiloxanes. Macromolecules 2006, 39, 1701–1708. [Google Scholar] [CrossRef]
- McNamee, C.E.; Jaumann, M.; Möller, M.; Ding, A.; Hemeltjen, S.; Ebert, S.; Baumann, W.; Goedel, W.A. Formation of a Freely Suspended Membrane via a Combination of Interfacial Reaction and Wetting. Langmuir 2005, 21, 10475–10480. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, X.; Tsarkova, L.; Pich, A.; Möller, M. All-Silica Colloidosomes with a Particle-Bilayer Shell. ACS Nano 2011, 5, 3937–3942. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Agrawal, G.; Tsarkova, L.; Zhu, X.; Möller, M. Self-Templating Amphiphilic Polymer Precursors for Fabricating Mesostructured Silica Particles: A Water-Based Facile and Universal Method. Adv. Mater. 2013, 25, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; An, J.; Su, Y.; Zhang, J.; Wang, Z.; Cheng, T.; Liu, G. Amphiphilic Hyperbranched Polyethoxysiloxane: A Self-Templating Assembled Platform to Fabricate Functionalized Mesostructured Silicas for Aqueous Enantioselective Reactions. ACS Catal. 2016, 6, 6229–6235. [Google Scholar] [CrossRef]
- Grande, J.B.; Urlich, T.; Dickie, T.; Brook, M.A. Silicone dendrons and dendrimers from orthogonal SiH coupling reactions. Polym. Chem. 2014, 5, 6728–6739. [Google Scholar] [CrossRef]
- Rubinsztajn, S.; Cella, J.A. A new polycondensation process for the preparation of polysiloxane copolymers. Macromolecules 2005, 38, 1061–1063. [Google Scholar] [CrossRef]
- Grande, J.B.; Fawcett, A.S.; McLaughlin, A.J.; Gonzaga, F.; Bender, T.P.; Brook, M.A. Anhydrous formation of foamed silicone elastomers using the Piers-Rubinsztajn reaction. Polymer 2012, 53, 3135–3142. [Google Scholar] [CrossRef]
- Chojnowski, J.; Rubinsztajn, S.; Fortuniak, W.; Kurjata, J. Synthesis of Highly Branched Alkoxysiloxane-Dimethylsiloxane Copolymers by Nonhydrolytic Dehydrocarbon Polycondensation Catalyzed by Tris(pentafluorophenyl)borane. Macromolecules 2008, 41, 7352–7358. [Google Scholar] [CrossRef]
- Pan, D.; Yi, E.; Doan, P.H.; Furgal, J.C.; Schwartz, M.; Clark, S.; Goodson Iii, T.; Laine, R.M. Microporous inorganic/organic hybrids via oxysilylation of a cubic symmetry nanobuilding block [(HMe2SiOSiO1.5)8] with RxSi(OEt)4−x. J. Ceram. Soc. Jpn. 2015, 123, 756–763. [Google Scholar] [CrossRef]
- Nassar, E.J.; Dos Santos Pereira, P.F.; De Oliveira Nassor, E.C.; Ávila, L.R.; Ciuffi, K.J.; Calefi, P.S. Nonhydrolytic sol-gel synthesis and characterization of YAG. J. Mater. Sci. 2007, 42, 2244–2249. [Google Scholar] [CrossRef]
- Pereira, P.F.S.; Matos, M.G.; Ferreira, C.M.A.; De Faria, E.H.; Calefi, P.S.; Rocha, L.A.; Ciuffi, K.J.; Nassar, E.J. Aluminate matrix doped with Tm3+/Tb3+/Eu3+ obtained by non-hydrolytic sol-gel route: White light emission. J. Lumin. 2014, 146, 394–397. [Google Scholar] [CrossRef]
- Matos, M.G.; De Faria, E.H.; Rocha, L.A.; Calefi, P.S.; Ciuffi, K.J.; Nassar, E.J.; Sarmento, V.H.V. Synthesis and photoluminescent properties of yttrium vanadate phosphor prepared by the non-hydrolytic sol-gel process. J. Lumin. 2014, 147, 190–195. [Google Scholar] [CrossRef]
- Matos, M.G.; Pereira, P.F.S.; Calefi, P.S.; Ciuffi, K.J.; Nassar, E.J. Preparation of a GdCaAl3O7 matrix by the non-hydrolytic sol-gel route. J. Lumin. 2009, 129, 1120–1124. [Google Scholar] [CrossRef]
- Mokhothu, T.H.; Luyt, A.S.; Messori, M. Preparation and characterization of EPDM/silica nanocomposites prepared through non-hydrolytic sol-gel method in the absence and presence of a coupling agent. Express Polym. Lett. 2014, 8, 809–822. [Google Scholar] [CrossRef]
- Nassar, E.J.; Avila, L.R.; Pereira, P.F.S.; Mello, C.; De Lima, O.J.; Ciuffi, K.J.; Carlos, L.D. Eu(III) incorporation in sol-gel aluminum-yttrium matrix by non-hydrolytic route. J. Lumin. 2005, 111, 159–166. [Google Scholar] [CrossRef]
- Orsini, G.; Tricoli, V. Facile nonhydrolytic sol-gel route to mesoporous mixed-conducting tungsten oxide. J. Mater. Chem. 2011, 21, 14530–14542. [Google Scholar] [CrossRef]
- Pereira, P.F.S.; Matos, M.G.; Ávila, L.R.; Nassor, E.C.O.; Cestari, A.; Ciuffi, K.J.; Calefi, P.S.; Nassar, E.J. Red, green and blue (RGB) emission doped Y3Al5O12 (YAG) phosphors prepared by non-hydrolytic sol-gel route. J. Lumin. 2010, 130, 488–493. [Google Scholar] [CrossRef]
- Silva, G.M.; De Faria, E.H.; Nassar, E.J.; Ciuffi, K.J.; Calefi, P.S. Synthesis of Indium Tin Oxide Nanoparticles by a Nonhydrolytic Sol-Gel Method. Quim. Nova 2012, 35, 473–476. [Google Scholar] [CrossRef]
- Yoo, Y.B.; Park, J.H.; Song, K.M.; Lee, S.J.; Baik, H.K. Non-hydrolytic ester-elimination reaction and its application in solution-processed zinc tin oxide thin film transistors. J. Sol-Gel Sci. Technol. 2012, 64, 257–263. [Google Scholar] [CrossRef]
- Hamden, Z.; Bouattour, S.; Ferraria, A.M.; Ferreira, D.P.; Vieira Ferreira, L.F.; Botelho do Rego, A.M.; Boufi, S. In situ generation of TiO2 nanoparticles using chitosan as a template and their photocatalytic activity. J. Photochem. Photobiol. A Chem. 2016, 321, 211–222. [Google Scholar] [CrossRef]
- Choi, K.M.; Wakabayashi, R.; Tatsumi, T.; Yokoi, T.; Kuroda, K. Usefulness of alkoxyltitanosiloxane for the preparation of mesoporous silica containing a large amount of isolated titanium. J. Colloid Interface Sci. 2011, 359, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Bassindale, A.R.; Gentle, T. Derivatisation of octasilsesquioxane with alcohols and silanols. J. Organomet. Chem. 1996, 521, 391–393. [Google Scholar] [CrossRef]
- Wada, Y.; Iyoki, K.; Sugawara-Narutaki, A.; Okubo, T.; Shimojima, A. Diol-linked microporous networks of cubic siloxane cages. Chem. Eur. J. 2013, 19, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Moravec, Z.; Sluka, R.; Necas, M.; Jancik, V.; Pinkas, J. A structurally diverse series of aluminum chloride alkoxides [ClxAl(μ-OR)y]n (R = nBu, cHex, Ph, 2,4-tBu2C6H3). Inorg. Chem. 2009, 48, 8106–8114. [Google Scholar] [CrossRef] [PubMed]
- Kitschke, P.; Schulze, S.; Hietschold, M.; Mehring, M. Synthesis of germanium dioxide nanoparticles in benzyl alcohol—A comparison. Main Group Met. Chem. 2013, 36, 209–214. [Google Scholar] [CrossRef]
- Niederberger, M.; Garnweitner, G.; Pinna, N.; Antonietti, M. Nonaqueous and Halide-Free Route to Crystalline BaTiO3, SrTiO3, and (Ba,Sr)TiO3 Nanoparticles via a Mechanism Involving C−C Bond Formation. J. Am. Chem. Soc. 2004, 126, 9120–9126. [Google Scholar] [CrossRef] [PubMed]
- Pinna, N.; Antonietti, M.; Niederberger, M. A novel nonaqueous route to V2O3 and Nb2O5 nanocrystals. Colloids Surf. A 2004, 250, 211–213. [Google Scholar] [CrossRef]
- Pinna, N.; Garnweitner, G.; Beato, P.; Niederberger, M.; Antonietti, M. Synthesis of Yttria-Based Crystalline and Lamellar Nanostructures and their Formation Mechanism. Small 2004, 1, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Bilecka, I.; Luo, L.; Djerdj, I.; Rossell, M.D.; Jagodič, M.; Jagličić, Z.; Masubuchi, Y.; Kikkawa, S.; Niederberger, M. Microwave-Assisted Nonaqueous Sol-Gel Chemistry for Highly Concentrated ZnO-Based Magnetic Semiconductor Nanocrystals. J. Phys. Chem. C 2011, 115, 1484–1495. [Google Scholar] [CrossRef]
- Luo, L.; Bozyigit, D.; Wood, V.; Niederberger, M. High-quality transparent electrodes spin-cast from preformed antimony-doped tin oxide nanocrystals for thin film optoelectronics. Chem. Mater. 2013, 25, 4901–4907. [Google Scholar] [CrossRef]
- Bilecka, I.; Hintennach, A.; Djerdj, I.; Novák, P.; Niederberger, M. Efficient microwave-assisted synthesis of LiFePO4 mesocrystals with high cycling stability. J. Mater. Chem. 2009, 19, 5125–5128. [Google Scholar] [CrossRef]
- Bilecka, I.; Hintennach, A.; Rossell, M.D.; Xie, D.; Novák, P.; Niederberger, M. Microwave-assisted solution synthesis of doped LiFePO4 with high specific charge and outstanding cycling performance. J. Mater. Chem. 2011, 21, 5881–5890. [Google Scholar] [CrossRef]
- Morselli, D.; Niederberger, M.; Bilecka, I.; Bondioli, F. Double role of polyethylene glycol in the microwaves-assisted non-hydrolytic synthesis of nanometric TiO2: Oxygen source and stabilizing agent. J. Nanopart. Res. 2014, 16, 4519–4521. [Google Scholar] [CrossRef]
- Ito, D.; Yokoyama, S.; Zaikova, T.; Masuko, K.; Hutchison, J.E. Synthesis of ligand-stabilized metal oxide nanocrystals and epitaxial core/shell nanocrystals via a lower-temperature esterification process. ACS Nano 2014, 8, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.C.; Chakravarti, B.N.; Wardlaw, W. Structural chemistry of the alkoxides. Part VIII. Isomeric butoxides and pentyloxides of niobium. J. Chem. Soc. 1956, 4439–4442. [Google Scholar] [CrossRef]
- Turova, N.Y.; Kessler, V.G.; Kucheiko, S.I. Molybdenum and tungsten (VI) bimetallic alkoxides. Decomposition accompanied by dialkylether elimination. Polyhedron 1991, 10, 2617–2628. [Google Scholar] [CrossRef]
- Senouci, A.; Yaakoub, M.; Huguenard, C.; Henry, M. Molecular templating using titanium(iv)(oxo)alkoxides and titanium(iv)(oxo)aryloxides. J. Mater. Chem. 2004, 14, 3215–3230. [Google Scholar] [CrossRef]
- Kessler, V.G.; Turova, N.Y.; Panov, A.N.; Starikova, Z.A.; Yanovsky, A.I.; Struchkov, Y.T.; Benlian, D. Synthesis, crystal and molecular structure of a new heterometallic oxo-2-methoxyethoxide, BaMo2O5(OC2H4OMe)4(HOC2H4OMe). Polyhedron 1998, 17, 4189–4193. [Google Scholar] [CrossRef]
- Laye, R.H.; McInnes, E.J.L. Solvothermal Synthesis of Paramagnetic Molecular Clusters. Eur. J. Inorg. Chem. 2004, 2004, 2811–2818. [Google Scholar] [CrossRef]
- Pazik, R.; Seisenbaeva, G.A.; Gohil, S.; Wiglusz, R.; Kȩpiński, L.; Strek, W.; Kessler, V.G. Simple and efficient synthesis of a Nd:LaAlO3 NIR nanophosphor from rare earth alkoxo-monoaluminates Ln2Al2(OiPr)12(iPrOH)2 single source precursors by Bradley reaction. Inorg. Chem. 2010, 49, 2684–2691. [Google Scholar] [CrossRef] [PubMed]
- Abtmeyer, S.; Pązik, R.; Wiglusz, R.J.; Małecka, M.; Seisenbaeva, G.A.; Kessler, V.G. Lanthanum Molybdate Nanoparticles from the Bradley Reaction: Factors Influencing Their Composition, Structure, and Functional Characteristics as Potential Matrixes for Luminescent Phosphors. Inorg. Chem. 2014, 53, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Kessler, V.G.; Nikitin, K.V.; Belokon, A.I. A new argument in favor of the ether elimination mechanism: Formation of acetals on action of molybdenum alkoxides on carbonyl compounds. Polyhedron 1998, 17, 2309–2311. [Google Scholar] [CrossRef]
- Pązik, R.; Piasecka, E.; Małecka, M.; Kessler, V.G.; Idzikowski, B.; Śniadecki, Z.; Wiglusz, R.J. Facile non-hydrolytic synthesis of highly water dispersible, surfactant free nanoparticles of synthetic MFe2O4 (M–Mn2+, Fe2+, Co2+, Ni2+) ferrite spinel by a modified Bradley reaction. RSC Adv. 2013, 3, 12230–12243. [Google Scholar] [CrossRef]
- Leonhardt, C.; Brumm, S.; Seifert, A.; Cox, G.; Lange, A.; Rüffer, T.; Schaarschmidt, D.; Lang, H.; Jöhrmann, N.; Hietschold, M.; et al. Tin Oxide Nanoparticles and SnO2/SiO2 Hybrid Materials by Twin Polymerization Using Tin(IV) Alkoxides. ChemPlusChem 2013, 78, 1400–1412. [Google Scholar] [CrossRef]
- Böttger-Hiller, F.; Lungwitz, R.; Seifert, A.; Hietschold, M.; Schlesinger, M.; Mehring, M.; Spange, S. Nanoscale Tungsten Trioxide Synthesized by In Situ Twin Polymerization. Angew. Chem. Int. Ed. 2009, 48, 8878–8881. [Google Scholar] [CrossRef] [PubMed]
- Spange, S.; Grund, S. Nanostructured Organic-Inorganic Composite Materials by Twin Polymerization of Hybrid Monomers. Adv. Mater. 2009, 21, 2111–2116. [Google Scholar] [CrossRef]
- Spange, S.; Kempe, P.; Seifert, A.; Auer, A.A.; Ecorchard, P.; Lang, H.; Falke, M.; Hietschold, M.; Pohlers, A.; Hoyer, W.; et al. Nanocomposites with Structure Domains of 0.5 to 3 nm by Polymerization of Silicon Spiro Compounds. Angew. Chem. Int. Ed. 2009, 48, 8254–8258. [Google Scholar] [CrossRef] [PubMed]
- Mehner, A.; Rüffer, T.; Lang, H.; Pohlers, A.; Hoyer, W.; Spange, S. Synthesis of Nanosized TiO2 by Cationic Polymerization of (µ4-oxido)-hexakis(µ-furfuryloxo)-octakis(furfuryloxo)-tetra-titanium. Adv. Mater. 2008, 20, 4113–4117. [Google Scholar] [CrossRef]
- Crespy, D.; Landfester, K. Making dry fertile: A practical tour of non-aqueous emulsions and miniemulsions, their preparation and some applications. Soft Matter 2011, 7, 11054–11064. [Google Scholar] [CrossRef]
- Bagshaw, S.A.; Prouzet, E.; Pinnavaia, T.J. Templating of Mesoporous Molecular Sieves by Nonionic Polyethylene Oxide Surfactants. Science 1995, 269, 1242–1244. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhao, D.; Margolese, D.I.; Chmelka, B.F.; Stucky, G.D. Block Copolymer Templating Syntheses of Mesoporous Metal Oxides with Large Ordering Lengths and Semicrystalline Framework. Chem. Mater. 1999, 11, 2813–2826. [Google Scholar] [CrossRef]
- Dombrowski, J.P.; Johnson, G.R.; Bell, A.T.; Tilley, T.D. Ga[OSi(OtBu)3]3·THF, a thermolytic molecular precursor for high surface area gallium-containing silica materials of controlled dispersion and stoichiometry. Dalton Trans. 2016, 45, 11025–11034. [Google Scholar] [CrossRef] [PubMed]
- Brutchey, R.L.; Lugmair, C.G.; Schebaum, L.O.; Tilley, T.D. Thermolytic conversion of a bis(alkoxy)tris(siloxy)tantalum(V) single-source molecular precursor to catalytic tantala-silica materials. J. Catal. 2005, 229, 72–81. [Google Scholar] [CrossRef]
- Skoda, D. Synthesis of Metallosilicate Materials by Non-Hydrolytic Methods. Ph.D. Thesis, Masarykova Univerzita, Brno, Czech Republic, 2016. [Google Scholar]
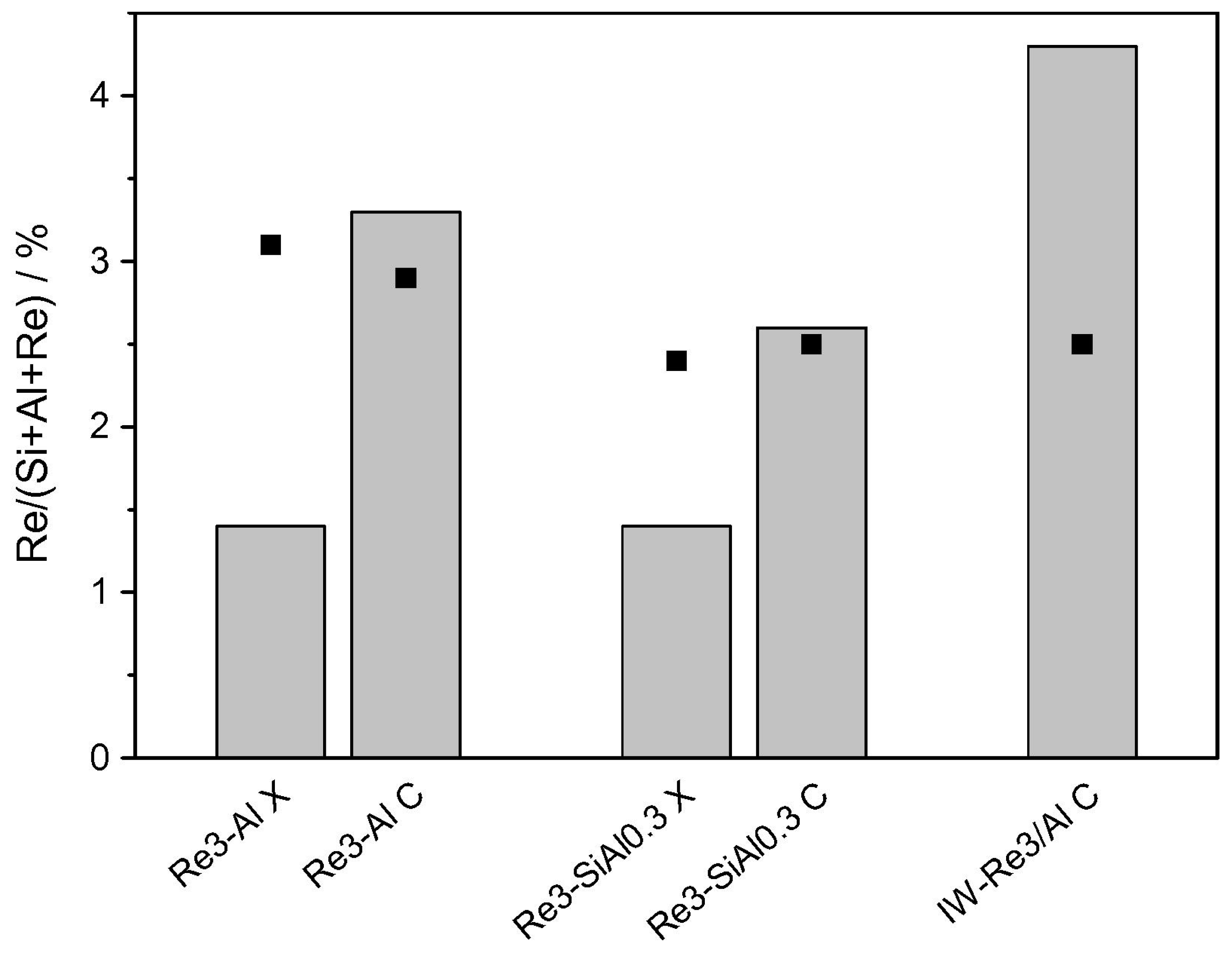
- Bouchmella, K.; Hubert Mutin, P.; Stoyanova, M.; Poleunis, C.; Eloy, P.; Rodemerck, U.; Gaigneaux, E.M.; Debecker, D.P. Olefin metathesis with mesoporous rhenium–silicium–aluminum mixed oxides obtained via a one-step non-hydrolytic sol-gel route. J. Catal. 2013, 301, 233–241. [Google Scholar] [CrossRef]
- Bouchmella, K.; Stoyanova, M.; Rodemerck, U.; Debecker, D.P.; Hubert Mutin, P. Avoiding rhenium loss in non-hydrolytic synthesis of highly active Re-Si-Al olefin metathesis catalysts. Catal. Commun. 2015, 58, 183–186. [Google Scholar] [CrossRef]
- Debecker, D.P.; Bouchmella, K.; Delaigle, R.; Eloy, P.; Poleunis, C.; Bertrand, P.; Gaigneaux, E.M.; Mutin, P.H. One-step non-hydrolytic sol-gel preparation of efficient V2O5-TiO2 catalysts for VOC total oxidation. Appl. Catal. B 2010, 94, 38–45. [Google Scholar] [CrossRef]
- Debecker, D.P.; Bouchmella, K.; Stoyanova, M.; Rodemerck, U.; Gaigneaux, E.M.; Hubert Mutin, P. A non-hydrolytic sol-gel route to highly active MoO3–SiO2–Al2O3 metathesis catalysts. Catal. Sci. Technol. 2012, 2, 1157–1164. [Google Scholar] [CrossRef]
- Debecker, D.P.; Delaigle, R.; Bouchmella, K.; Eloy, P.; Gaigneaux, E.M.; Mutin, P.H. Total oxidation of benzene and chlorobenzene with MoO3- and WO3-promoted V2O5/TiO2 catalysts prepared by a nonhydrolytic sol-gel route. Catal. Today 2010, 157, 125–130. [Google Scholar] [CrossRef]
- Maksasithorn, S.; Praserthdam, P.; Suriye, K.; Devillers, M.; Debecker, D.P. WO3-based catalysts prepared by non-hydrolytic sol-gel for the production of propene by cross-metathesis of ethene and 2-butene. Appl. Catal. A Gen. 2014, 488, 200–207. [Google Scholar] [CrossRef]
- Caetano, B.L.; Rocha, L.A.; Molina, E.; Rocha, Z.N.; Ricci, G.; Calefi, P.S.; de Lima, O.J.; Mello, C.; Nassar, E.J.; Ciuffi, K.J. Cobalt aluminum silicate complexes prepared by the non-hydrolytic sol-gel route and their catalytic activity in hydrocarbon oxidation. Appl. Catal. A Gen. 2006, 311, 122–134. [Google Scholar] [CrossRef]
- Ricci, G.P.; Rocha, Z.N.; Nakagaki, S.; Castro, K.A.D.F.; Crotti, A.E.M.; Calefi, P.S.; Nassar, E.J.; Ciuffi, K.J. Iron-alumina materials prepared by the non-hydrolytic sol-gel route: Synthesis, characterization and application in hydrocarbons oxidation using hydrogen peroxide as oxidant. Appl. Catal. A Gen. 2010, 389, 147–154. [Google Scholar] [CrossRef]
- Moggi, P.; Predieri, G.; Cauzzi, D.; Devillers, M.; Ruiz, P.; Morselli, S.; Ligabue, O. Sol-gel preparation of pure and silica-dispersed vanadium and niobium catalysts active in oxidative dehydrogenation of propane. In Scientific Bases for the Preparation of Heterogeneous Catalysts; Elsevier: Boston, MA, USA, 2000; Volume 143, pp. 149–157. [Google Scholar]
- Matos, M.G.; Calefi, P.S.; Ciuffi, K.J.; Nassar, E.J. Synthesis and luminescent properties of gadolinium aluminates phosphors. Inorg. Chim. Acta 2011, 375, 63–69. [Google Scholar] [CrossRef]
- Hofmann, M.; Rainer, M.; Schulze, S.; Hietschold, M.; Mehring, M. Nonaqueous Synthesis of a Bismuth Vanadate Photocatalyst By Using Microwave Heating: Photooxidation versus Photosensitized Decomposition in. ChemCatChem 2015, 7, 1357–1365. [Google Scholar] [CrossRef]
- Baiz, T.I.; Gindhart, A.M.; Kraemer, S.K.; Lind, C. Synthesis of MgHf(WO4)3 and MgZr(WO4)3 using a non-hydrolytic sol-gel method. J. Sol-Gel Sci. Technol. 2008, 47, 128–130. [Google Scholar] [CrossRef]
- Lind, C.; Gates, S.D.; Pedoussaut, N.M.; Baiz, T.I. Novel Materials through Non-Hydrolytic Sol-Gel Processing: Negative Thermal Expansion Oxides and Beyond. Materials 2010, 3, 2567–2587. [Google Scholar] [CrossRef]
- Zhou, X.; Heinrich, C.P.; Kluenker, M.; Dolique, S.; Mull, D.L.; Lind, C. Non-hydrolytic sol-gel synthesis of tantalum sulfides. J. Sol-Gel Sci. Technol. 2014, 69, 596–604. [Google Scholar] [CrossRef]
- Zhou, X.; Soldat, A.C.; Lind, C. Phase selective synthesis of copper sulfides by non-hydrolytic sol-gel methods. RSC Adv. 2014, 4, 717–726. [Google Scholar] [CrossRef]
- Leidich, S.; Buechele, D.; Lauenstein, R.; Kluenker, M.; Lind, C. “Non-hydrolytic” sol-gel synthesis of molybdenum sulfides. J. Solid State Chem. 2016, 242, 175–181. [Google Scholar] [CrossRef]
- Ludi, B.; Olliges-Stadler, I.; Rossell, M.D.; Niederberger, M. Extension of the benzyl alcohol route to metal sulfides: “Nonhydrolytic” thio sol-gel synthesis of ZnS and SnS2. Chem. Commun. 2011, 47, 5280–5282. [Google Scholar] [CrossRef] [PubMed]
- Kemnitz, E.; Noack, J. The non-aqueous fluorolytic sol-gel synthesis of nanoscaled metal fluorides. Dalton Trans. 2015, 44, 19411–19431. [Google Scholar] [CrossRef] [PubMed]
- Livage, J.; Barboux, P.; Vandenborre, M.T.; Schmutz, C.; Taulelle, F. Sol-gel synthesis of phosphates. J. Non-Cryst. Solids 1992, 147–148, 18–23. [Google Scholar] [CrossRef]
- Willinger, M.-G.; Clavel, G.; Di, W.; Pinna, N. A general soft-chemistry route to metal phosphate nanocrystals. J. Ind. Eng. Chem. 2009, 15, 883–887. [Google Scholar] [CrossRef]
- Jähnigen, S.; Brendler, E.; Böhme, U.; Kroke, E. Synthesis of silicophosphates containing SiO6-octahedra under ambient conditions-reactions of anhydrous H3PO4 with alkoxysilanes. Chem. Commun. 2012, 48, 7675–7677. [Google Scholar] [CrossRef] [PubMed]
- Jähnigen, S.; Brendler, E.; Böhme, U.; Heide, G.; Kroke, E. Silicophosphates containing SiO6 octahedra-anhydrous synthesis under ambient conditions. New J. Chem. 2014, 38, 744–751. [Google Scholar] [CrossRef]
- Tokuda, Y.; Tanaka, Y.; Takahashi, M.; Ihara, R.; Yoko, T. Silicophosphate/Silicophosphite Hybrid Materials Prepared by Solventless Ethanol Condensation. J. Ceram. Soc. Jpn. 2009, 117, 842–846. [Google Scholar] [CrossRef]
- Niida, H.; Tokuda, Y.; Takahashi, M.; Uchino, T.; Yoko, T. Structure of organic–inorganic hybrid low-melting glasses from 29Si NMR and ab initio molecular orbital calculations. J. Non-Cryst. Solids 2002, 311, 145–153. [Google Scholar] [CrossRef]
- Mizuno, M.; Takahashi, M.; Tokuda, Y.; Yoko, T. Organic−Inorganic Hybrid Material of Phenyl-Modified Polysilicophosphate Prepared through Nonaqueous Acid-Base Reaction. Chem. Mater. 2006, 18, 2075–2080. [Google Scholar] [CrossRef]
- Niida, H.; Takahashi, M.; Uchino, T.; Yoko, T. Preparation and structure of organic–inorganic hybrid precursors for new type low-melting glasses. J. Non-Cryst. Solids 2002, 306, 292–299. [Google Scholar] [CrossRef]
- Mizuno, M.; Takahashi, M.; Tokuda, Y.; Yoko, T. Substituent effect on the formation of organically-modified silicate-phosphate alternating copolymer through nonaqueous acid–base reaction. J. Sol-Gel Sci. Technol. 2007, 44, 47–52. [Google Scholar] [CrossRef]
- Pinkas, J.; Wessel, H.; Yang, Y.; Montero, M.L.; Noltemeyer, M.; Fröba, M.; Roesky, H.W. Reactions of Phosphoric Acid Triesters with Aluminum and Gallium Amides. Inorg. Chem. 1998, 37, 2450–2457. [Google Scholar] [CrossRef]
- Pinkas, J.; Chakraborty, D.; Yang, Y.; Murugavel, R.; Noltemeyer, M.; Roesky, H.W. Reactions of Trialkyl Phosphates with Trialkyls of Aluminum and Gallium: New Route to Alumino- and Gallophosphate Compounds via Dealkylsilylation. Organometallics 1999, 18, 523–528. [Google Scholar] [CrossRef]
- Di Vona, M.L.; Traversa, E.; Licoccia, S. Nonhydrolytic Synthesis of NASICON of Composition Na3Zr2Si2PO12: A Spectroscopic Study. Chem. Mater. 2001, 13, 141–144. [Google Scholar] [CrossRef]
- Corriu, R.J. P.; Leclercq, D.; Mutin, P.H.; Sarlin, L.; Vioux, A. Nonhydrolytic sol-gel routes to layered metal(IV) and silicon phosphonates. J. Mater. Chem. 1998, 8, 1827–1833. [Google Scholar] [CrossRef]
- Grader, G.S.; Shter, G.E.; De Hazan, Y. Novel ceramic foams from crystals of AlCl3(Pri2O) complex. J. Mater. Res. 1999, 14, 1485–1494. [Google Scholar] [CrossRef]
- Grader, G.; Shter, G.; Dehazan, Y. Method of Producing Ceramic Foams. EP1093448 A1, 17 March 1999. [Google Scholar]
- Kaper, H.; Bouchmella, K.; Mutin, P.H.; Goettmann, F. High-Surface-Area SiO2-ZrO2 Mixed Oxides as Catalysts for the Friedel-Crafts-Type Alkylation of Arenes with Alcohols and Tandem Cyclopropanation Reactions. ChemCatChem 2012, 4, 1813–1818. [Google Scholar] [CrossRef]
- Lafond, V.; Mutin, P.H.; Vioux, A. Non-hydrolytic sol-gel routes based on alkyl halide elimination: Toward better mixed oxide catalysts and new supports: Application to the preparation of a SiO2–TiO2 epoxidation catalyst. J. Mol. Catal. A Chem. 2002, 182–183, 81–88. [Google Scholar] [CrossRef]
- Cojocariu, A.M.; Mutin, P.H.; Dumitriu, E.; Aboulaich, A.; Vioux, A.; Fajula, F.; Hulea, V. Non-hydrolytic SiO2–TiO2 mesoporous xerogels—Efficient catalysts for the mild oxidation of sulfur organic compounds with hydrogen peroxide. Catal. Today 2010, 157, 270–274. [Google Scholar] [CrossRef]
- Cojocariu, A.M.; Mutin, P.H.; Dumitriu, E.; Fajula, F.; Vioux, A.; Hulea, V. Mild oxidation of bulky organic compounds with hydrogen peroxide over mesoporous TiO2-SiO2 xerogels prepared by non-hydrolytic sol-gel. Appl. Catal. B 2010, 97, 407–413. [Google Scholar] [CrossRef]
- Debecker, D.P.; Bouchmella, K.; Poleunis, C.; Eloy, P.; Bertrand, P.; Gaigneaux, E.M.; Mutin, P.H. Design of SiO2−Al2O3−MoO3 Metathesis Catalysts by Nonhydrolytic Sol-Gel. Chem. Mater. 2009, 21, 2817–2824. [Google Scholar] [CrossRef]
- Debecker, D.P.; Stoyanova, M.; Colbeau-Justin, F.; Rodemerck, U.; Boissière, C.; Gaigneaux, E.M.; Sanchez, C. One-pot aerosol route to MoO3–SiO2–Al2O3 catalysts with ordered super microporosity and high olefin metathesis activity. Angew. Chem. Int. Ed. 2012, 51, 2129–2131. [Google Scholar] [CrossRef] [PubMed]
- Albrbar, A.J.; Djokić, V.; Bjelajac, A.; Kovač, J.; Ćirković, J.; Mitrić, M.; Janaćković, D.; Petrović, R. Visible-light active mesoporous, nanocrystalline N,S-doped and co-doped titania photocatalysts synthesized by non-hydrolytic sol-gel route. Ceram. Int. 2016, 42, 16718–16728. [Google Scholar] [CrossRef]
- Escamilla-Pérez, A.M.; Louvain, N.; Kaschowitz, M.; Freunberger, S.; Fontaine, O.; Boury, B.; Brun, N.; Mutin, P.H. Lithium insertion properties of mesoporous nanocrystalline TiO2 and TiO2–V2O5 microspheres prepared by non-hydrolytic sol-gel. J. Sol-Gel Sci. Technol. 2016, 79, 270–278. [Google Scholar] [CrossRef]
- Yu, S.-H.; Pucci, A.; Herntrich, T.; Willinger, M.-G.; Baek, S.-H.; Sung, Y.-E.; Pinna, N. Surfactant-free nonaqueous synthesis of lithium titanium oxide (LTO) nanostructures for lithium ion battery applications. J. Mater. Chem. 2011, 21, 806–810. [Google Scholar] [CrossRef]
- Baek, S.; Yu, S.-H.; Park, S.-K.; Pucci, A.; Marichy, C.; Lee, D.-C.; Sung, Y.-E.; Piao, Y.; Pinna, N. A one-pot microwave-assisted non-aqueous sol-gel approach to metal oxide/graphene nanocomposites for Li-ion batteries. RSC Adv. 2011, 1, 1687–1690. [Google Scholar] [CrossRef]
- Yu, S.H.; Conte, D.E.; Baek, S.; Lee, D.C.; Park, S.K.; Lee, K.J.; Piao, Y.; Sung, Y.E.; Pinna, N. Structure-properties relationship in iron oxide-reduced graphene oxide nanostructures for Li-ion batteries. Adv. Funct. Mater. 2013, 23, 4293–4305. [Google Scholar] [CrossRef]
- Caresani, J.R.; Lattuada, R.M.; Radtke, C.; Dos Santos, J.H.Z. Attempts made to heterogenize MAO via encapsulation within silica through a non-hydrolytic sol-gel process. Powder Technol. 2014, 252, 56–64. [Google Scholar] [CrossRef]
- Fisch, A.G.; Cardozo, N.S.M.; Secchi, A.R.; Stedile, F.C.; da Silveira, N.P.; dos Santos, J.H.Z. Investigation of silica particle structure containing metallocene immobilized by a sol-gel method. J. Non-Cryst. Solids 2008, 354, 3973–3979. [Google Scholar] [CrossRef]
- Capeletti, L.B.; Dos Santos, J.H.Z.; Moncada, E.; Da Rocha, Z.N.; Pepe, I.M. Encapsulated alizarin red species: The role of the sol-gel route on the interaction with silica matrix. Powder Technol. 2013, 237, 117–124. [Google Scholar] [CrossRef]
- Coles, M.P.; Lugmair, C.G.; Terry, K.W.; Tilley, T.D. Titania-Silica Materials from the Molecular Precursor Ti[OSi(OtBu)3]4: Selective Epoxidation Catalysts. Chem. Mater. 2000, 12, 122–131. [Google Scholar] [CrossRef]
- Hutter, R.; Mallat, T.; Baiker, A. Titania Silica Mixed Oxides II. Catalytic Behavior in Olefin Epoxidation. J. Catal. 1995, 153, 177–189. [Google Scholar] [CrossRef]
- Bertinchamps, F.; Grégoire, C.; Gaigneaux, E.M. Systematic investigation of supported transition metal oxide based formulations for the catalytic oxidative elimination of (chloro)-aromatics. Appl. Catal. B 2006, 66, 10–22. [Google Scholar] [CrossRef]
- Green, W.H.; Le, K.P.; Grey, J.; Au, T.T.; Sailor, M.J. White Phosphors from a Silicate-Carboxylate Sol-Gel Precursor That Lack Metal Activator Ions. Science 1997, 276, 1826–1828. [Google Scholar] [CrossRef]
- Xi, W.; Scott, T.F.; Kloxin, C.J.; Bowman, C.N. Click chemistry in materials science. Adv. Funct. Mater. 2014, 24, 2572–2590. [Google Scholar] [CrossRef]
- Cattoën, X.; Noureddine, A.; Croissant, J.; Moitra, N.; Bürglová, K.; Hodačová, J.; De Los Cobos, O.; Lejeune, M.; Rossignol, F.; Toulemon, D.; et al. Click approaches in sol-gel chemistry. J. Sol-Gel Sci. Technol. 2014, 70, 245–253. [Google Scholar] [CrossRef]
- Bürglová, K.; Noureddine, A.; Hodačová, J.; Toquer, G.; Cattoën, X.; Wongchiman, M. A general method for preparing bridged organosilanes with pendant functional groups and functional mesoporous organosilicas. Chem. Eur. J. 2014, 20, 10371–10382. [Google Scholar] [CrossRef] [PubMed]






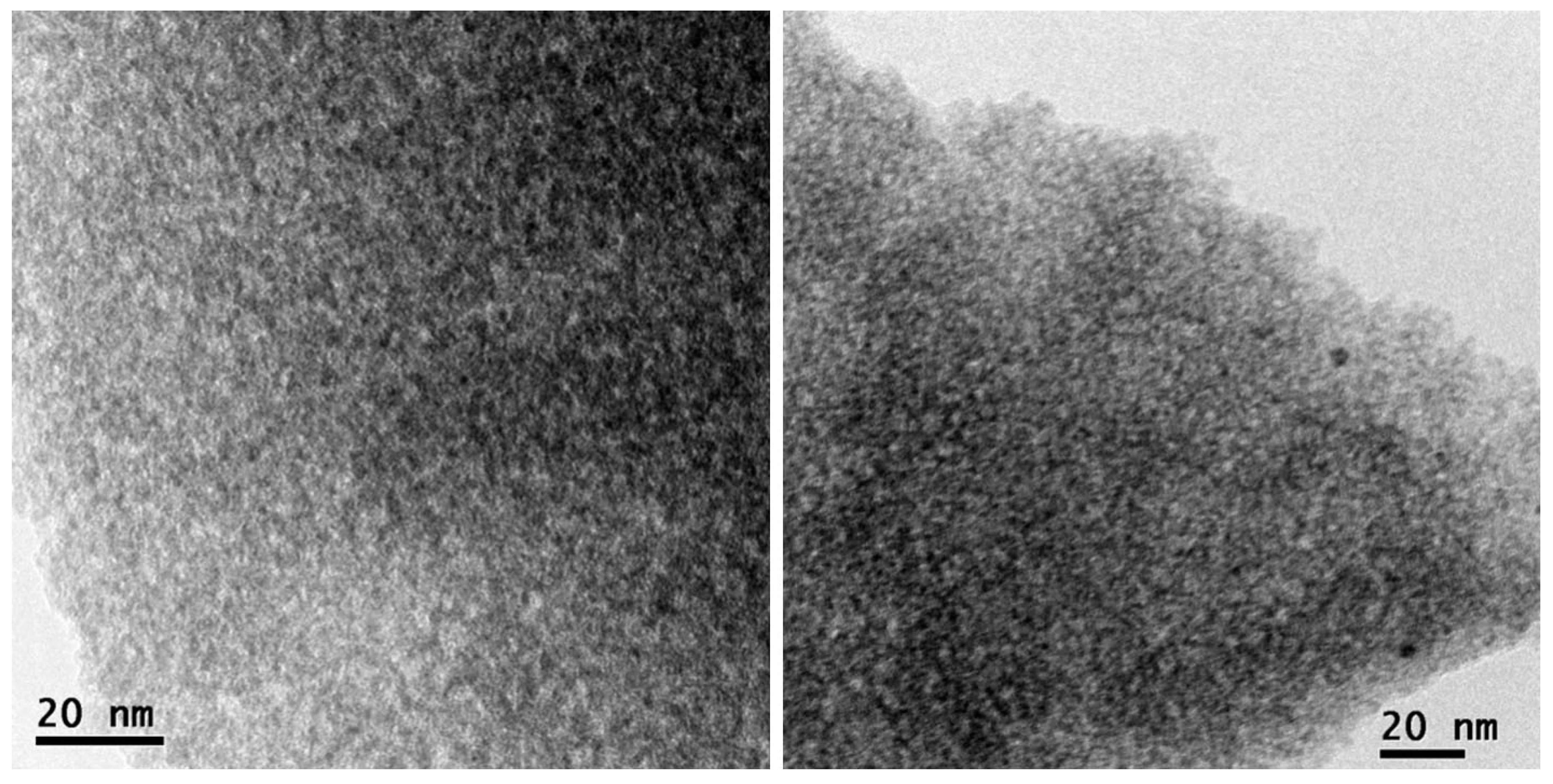

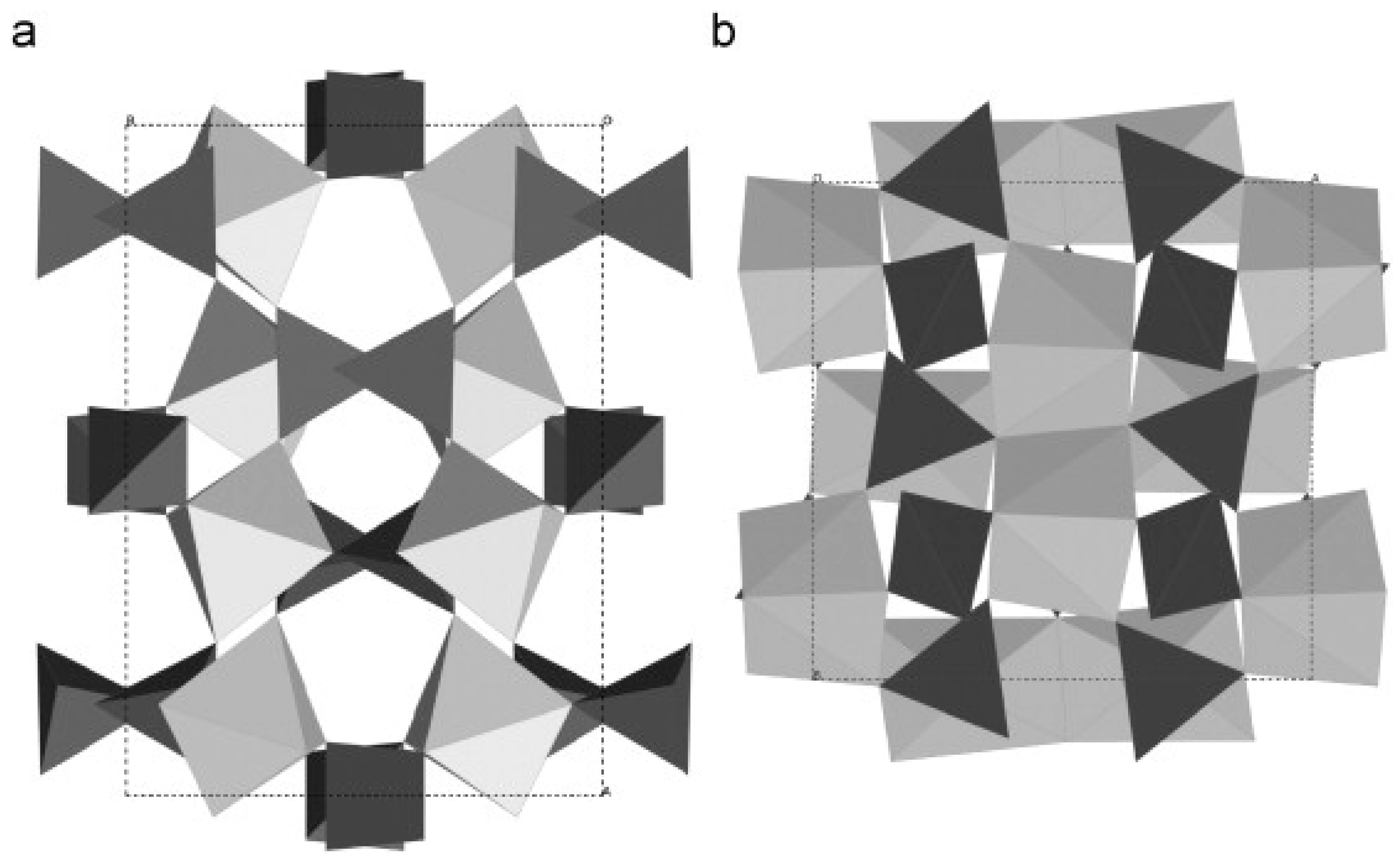

| Mixed Oxide | Non-Hydrolytic Synthetic Route | Application | References |
|---|---|---|---|
| Al-Si | acetamide elimination | aminolysis catalyst | [51] |
| Zr-Si | acetamide elimination, alkyl halide elimination | aminolysis, MPV, Friedel-Crafts catalysts | [50,210] |
| Sn-Si | acetamide elimination, twin polymerization | aminolysis catalyst | [49,166] |
| Ti-Si | acetamide elimination, alkyl halide elimination | mild oxidation, epoxidation catalysts | [38,48,211,212,213] |
| Fe-Al | alkyl halide elimination (ether route) | mild oxidation catalyst | [184] |
| Co-Al-Si | alkyl halide elimination (ether route) | mild oxidation catalysts | [183] |
| Re-Al-Si | alkyl halide elimination (ether route) | olefin metathesis catalyst | [177,178] |
| Mo-Al-Si | alkyl halide elimination (ether route) | olefin metathesis catalyst | [180,214,215] |
| Mo-Ti-V | alkyl halide elimination (ether route) | total oxidation catalysts | [179,181] |
| W-Ti-V | alkyl halide elimination (ether route) | total oxidation catalysts | [179,181] |
| Nb-V-Si | alkyl halide elimination | oxidative dehydrogenation catalyst | [185] |
| Ga-Si | thermal decompositon of Ga[OSi(OtBu)3]3·THF | Lewis acid catalyst | [174] |
| Si-P | ester elimination | Brønsted acid catalyst | [95] |
| BiVO4 | microwave assisted twin polymerization | photocatalyst | [187] |
| TiO2 | ether elimination (chitosan templated) | photocatalyst | [142] |
| TiO2 | alkyl halide elimination (alkoxide route) | photocatalyst, S, N doped | [216] |
| Y-V | alkyl halide elimination | luminescent materials, Ln3+ doped | [134] |
| Y-Al | alkyl halide elimination | luminescent materials, Ln3+ doped | [132,133,137,139] |
| Gd-Ca-Al | alkyl halide elimination | luminescent materials, Ln3+ doped | [135] |
| Gd-Al | alkyl halide elimination | luminescent materials, Ln3+ doped | [186] |
| Ln-P | benzyl alcohol route, microwave assisted | luminescent materials, Tb3+ doped | [196] |
| Ti-V | alkyl halide elimination (ether route) | Li-ion batteries | [217] |
| Li4Ti5O12 | benzyl alcohol route | Li-ion batteries | [218] |
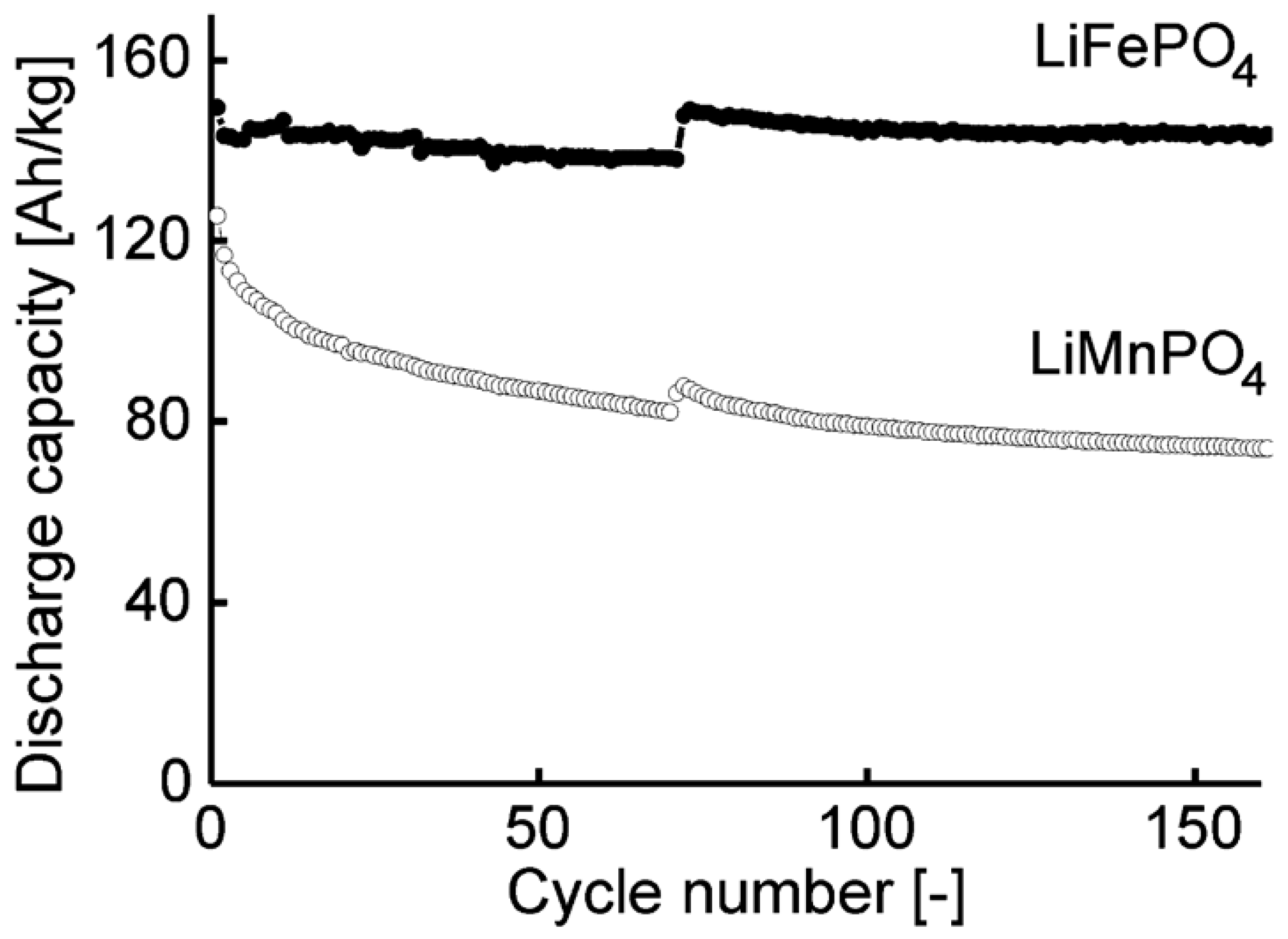
| LiFePO4 | benzyl alcohol route, microwave assisted | Li-ion batteries | [153,154] |
| LiMnPO4 | benzyl alcohol route, microwave assisted | Li-ion batteries | [153] |
| SnO2/GO | benzyl alcohol route, microwave assisted | Li-ion batteries | [219] |
| Fe3O4/GO | benzyl alcohol route, microwave assisted | Li-ion batteries | [220] |
| ZnO | benzyl alcohol route, microwave assisted | magnetic semiconductors, doped | [151] |
| SnO2 | benzyl alcohol route, microwave assisted | optoelectonic devices, Sb doped | [152] |
| Si-P | ester elimination | catalyst support | [94] |
| SiO2 | alkyl halide elimination (alkoxide route) | catalyst support | [221,222,223] |
| Catalyst | Ti [wt %] | Ti in Catalyst [mmol] | Cyclohexene [mmol] | CHP [mmol] | T [°C] | SABET [m2·g−1] | Epoxide Yield [%] | TON a |
|---|---|---|---|---|---|---|---|---|
| SiTi1 [48] | 12.48 | 0.13 | 25 | 5 | 65 | 12 | 69 | 26 |
| 9SiTi [48] | 3.72 | 0.03 | 25 | 5 | 65 | - | 77 | 126 |
| SiTiP2-500 [48] | 27.43 | 0.28 | 25 | 5 | 65 | 325 | 65 | 12 |
| SiTiP2-500N [48] | 22.01 | 0.23 | 25 | 5 | 65 | 453 | 80 | 16 |
| 9SiTiP-500 [48] | 8.09 | 0.09 | 25 | 5 | 65 | 442 | 96 | 52 |
| 9SiTiP-500N [48] | 6.64 | 0.03 | 25 | 5 | 65 | 615 | 96 | 158 |
| SiO2.TiO2 (NHSG) [211] | 7 | 0.0731 | 25 | 5 | 65 | 780 | 91 | 62 |
| Shell [211] | 9 | 0.0094 | 25 | 5 | 65 | 200 | 68 | 360 |
| SiO2.TiO2 xerogel [224] | 15 | 0.157 | 25 | 5 | 65 | 552 | 38 | 12 |
| SiO2.TiO2 aerogel [224] | 15 | 0.157 | 25 | 5 | 65 | 677 | 49 | 16 |
| 10LT HSG aerogel [225] | 6 | 0.125 | 77 | 13.4 | 60 | 683 | 87 | 93 |
| 20LT HSG aerogel [225] | 12 | 0.251 | 77 | 13.4 | 60 | 674 | 93 | 50 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Styskalik, A.; Skoda, D.; Barnes, C.E.; Pinkas, J. The Power of Non-Hydrolytic Sol-Gel Chemistry: A Review. Catalysts 2017, 7, 168. https://doi.org/10.3390/catal7060168
Styskalik A, Skoda D, Barnes CE, Pinkas J. The Power of Non-Hydrolytic Sol-Gel Chemistry: A Review. Catalysts. 2017; 7(6):168. https://doi.org/10.3390/catal7060168
Chicago/Turabian StyleStyskalik, Ales, David Skoda, Craig E. Barnes, and Jiri Pinkas. 2017. "The Power of Non-Hydrolytic Sol-Gel Chemistry: A Review" Catalysts 7, no. 6: 168. https://doi.org/10.3390/catal7060168
APA StyleStyskalik, A., Skoda, D., Barnes, C. E., & Pinkas, J. (2017). The Power of Non-Hydrolytic Sol-Gel Chemistry: A Review. Catalysts, 7(6), 168. https://doi.org/10.3390/catal7060168