Ion-Exchange of Cu2+ Promoted Layered Perovskite K2La2Ti3O10 for Photocatalytic Degradation Chlorobenzene under Simulated Solar Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
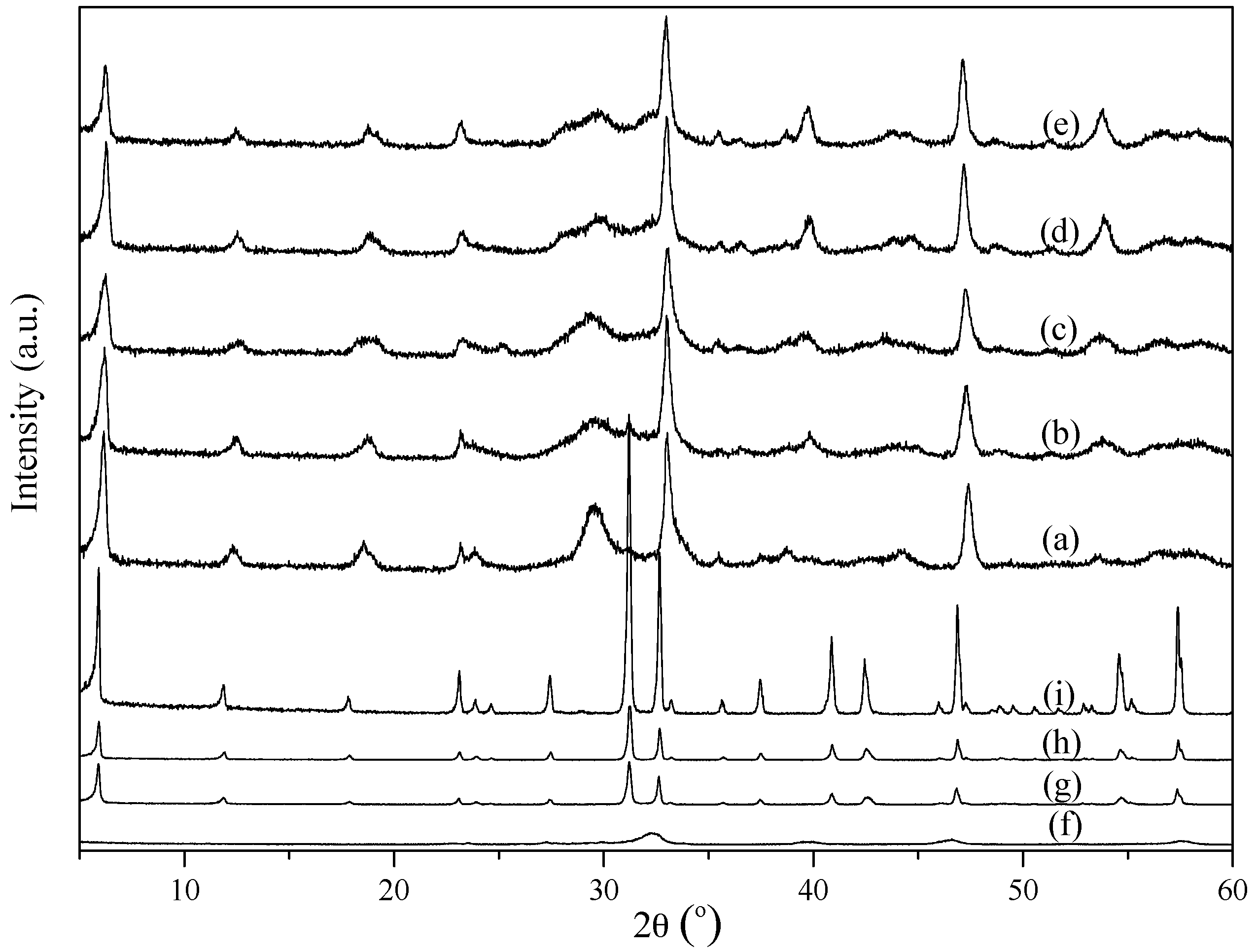
2.1. Characterization of the Powders
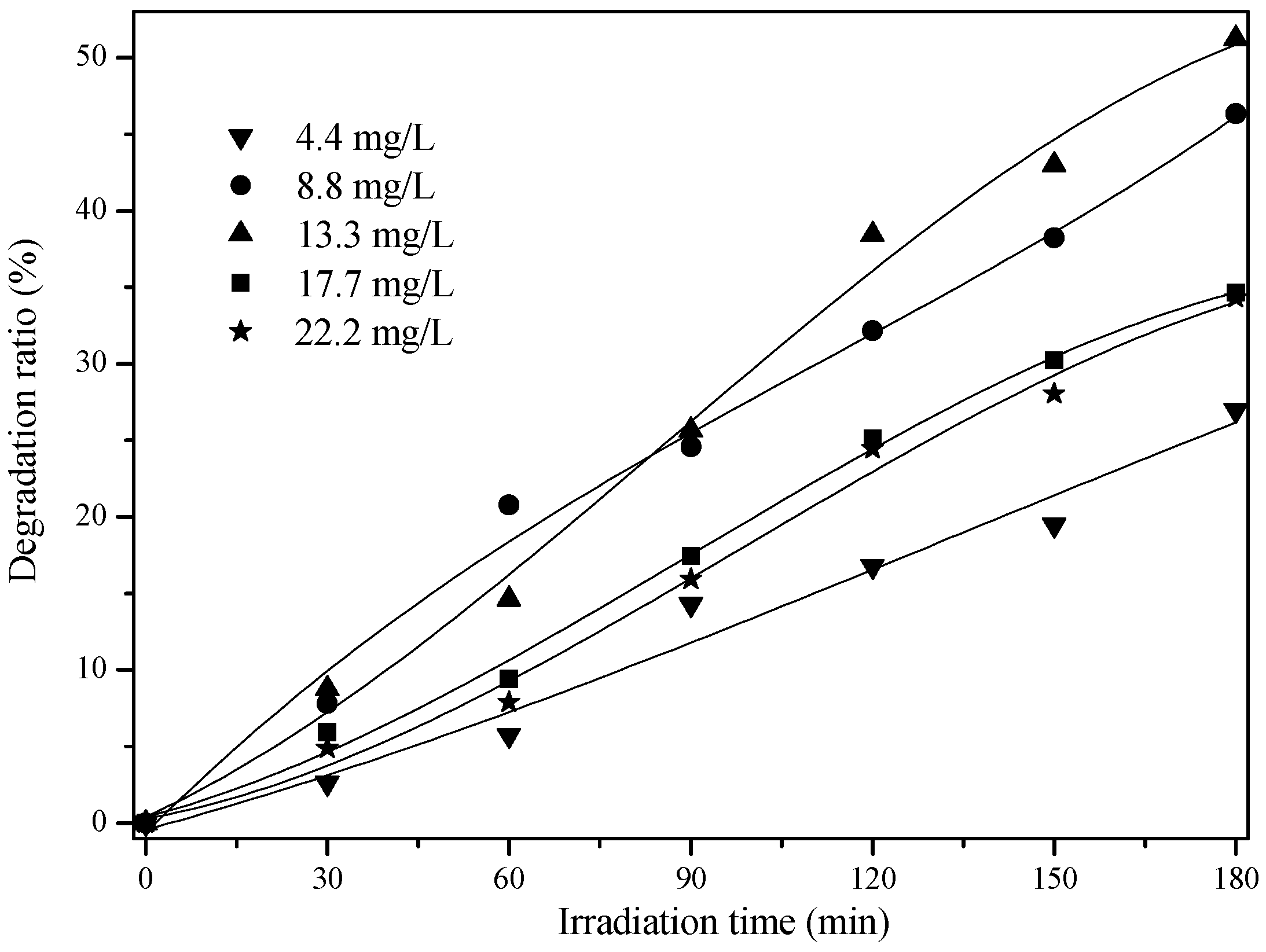
2.2. Effect of Initial Concentration on the Photocatalytic Activity
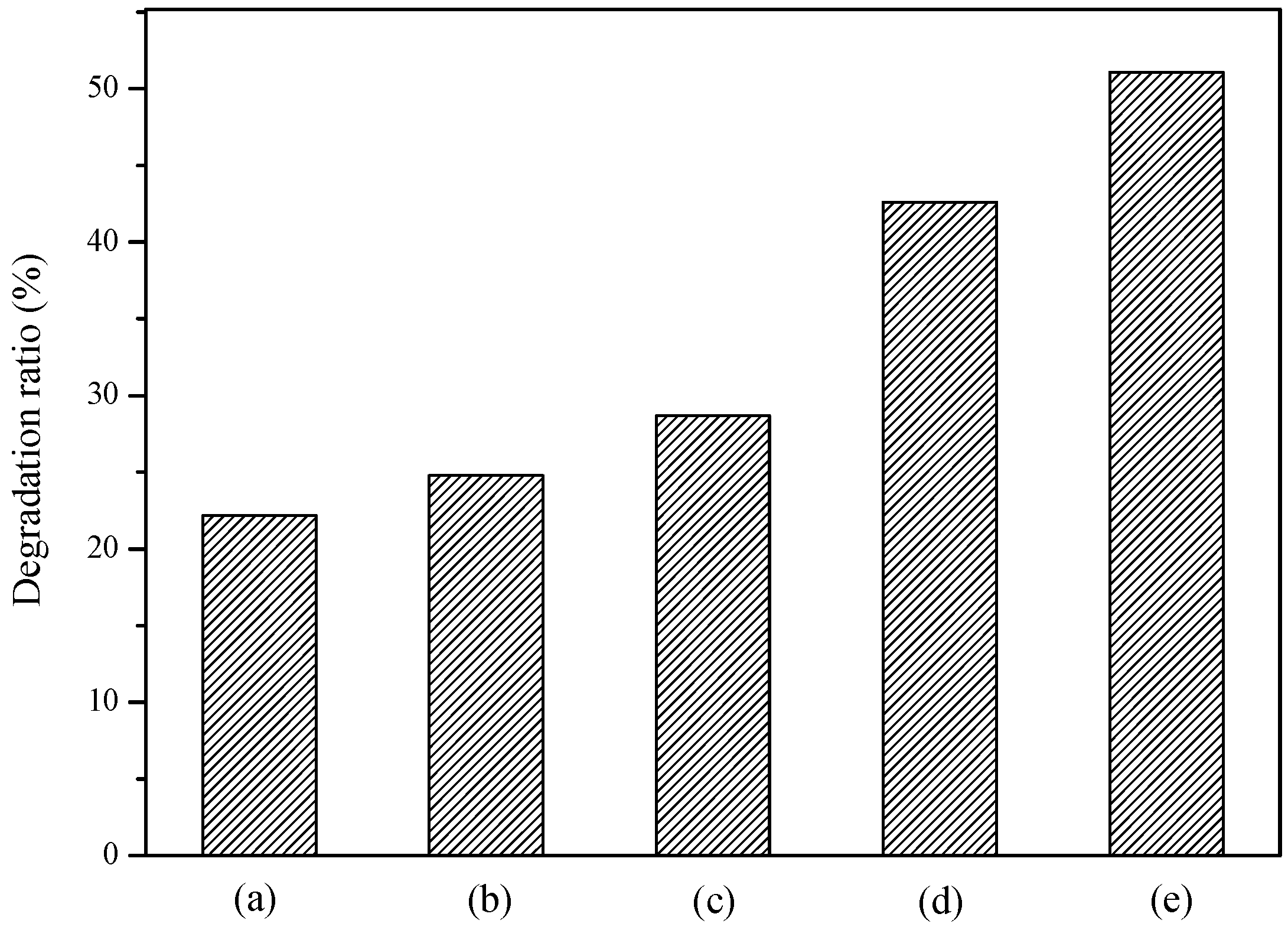
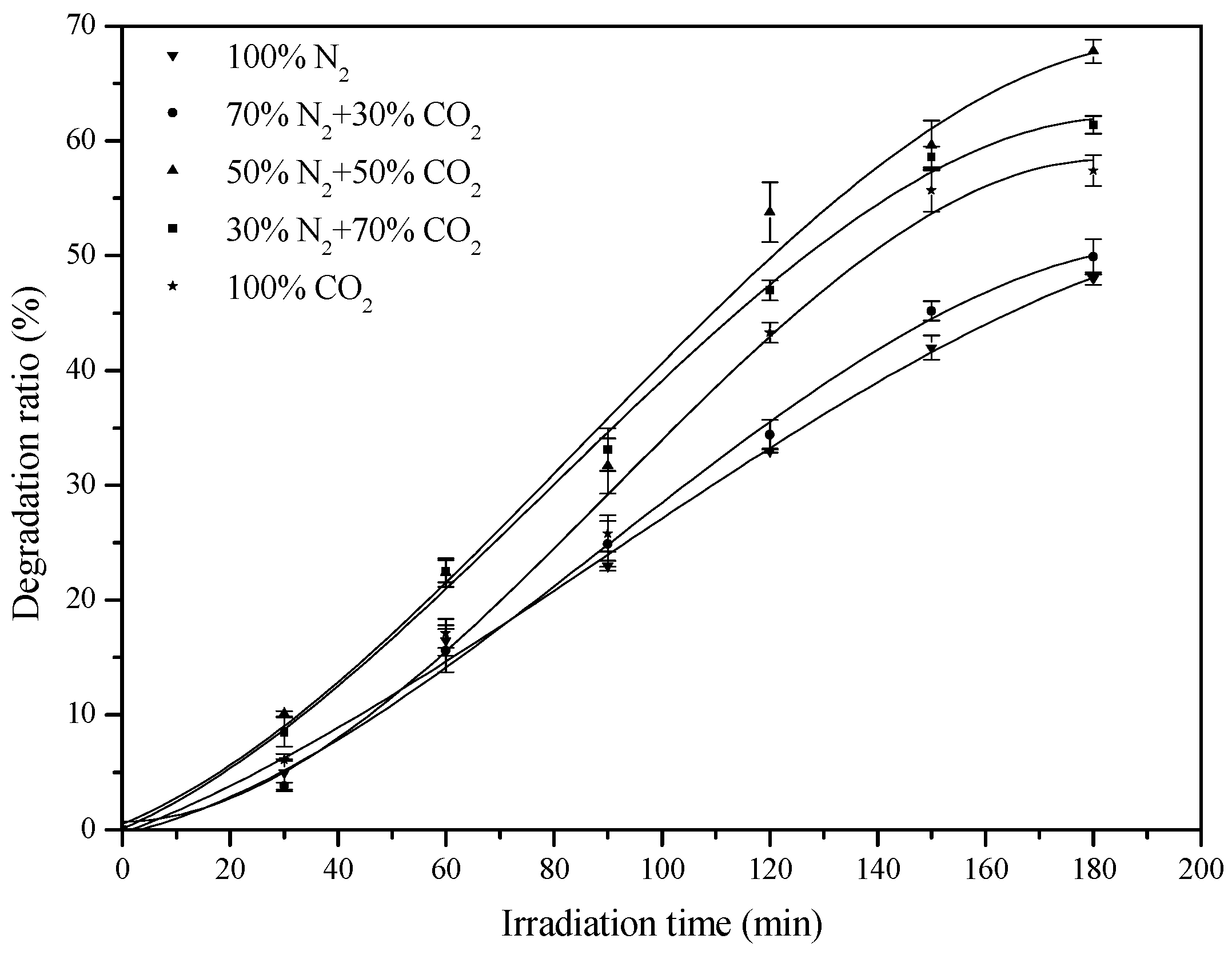
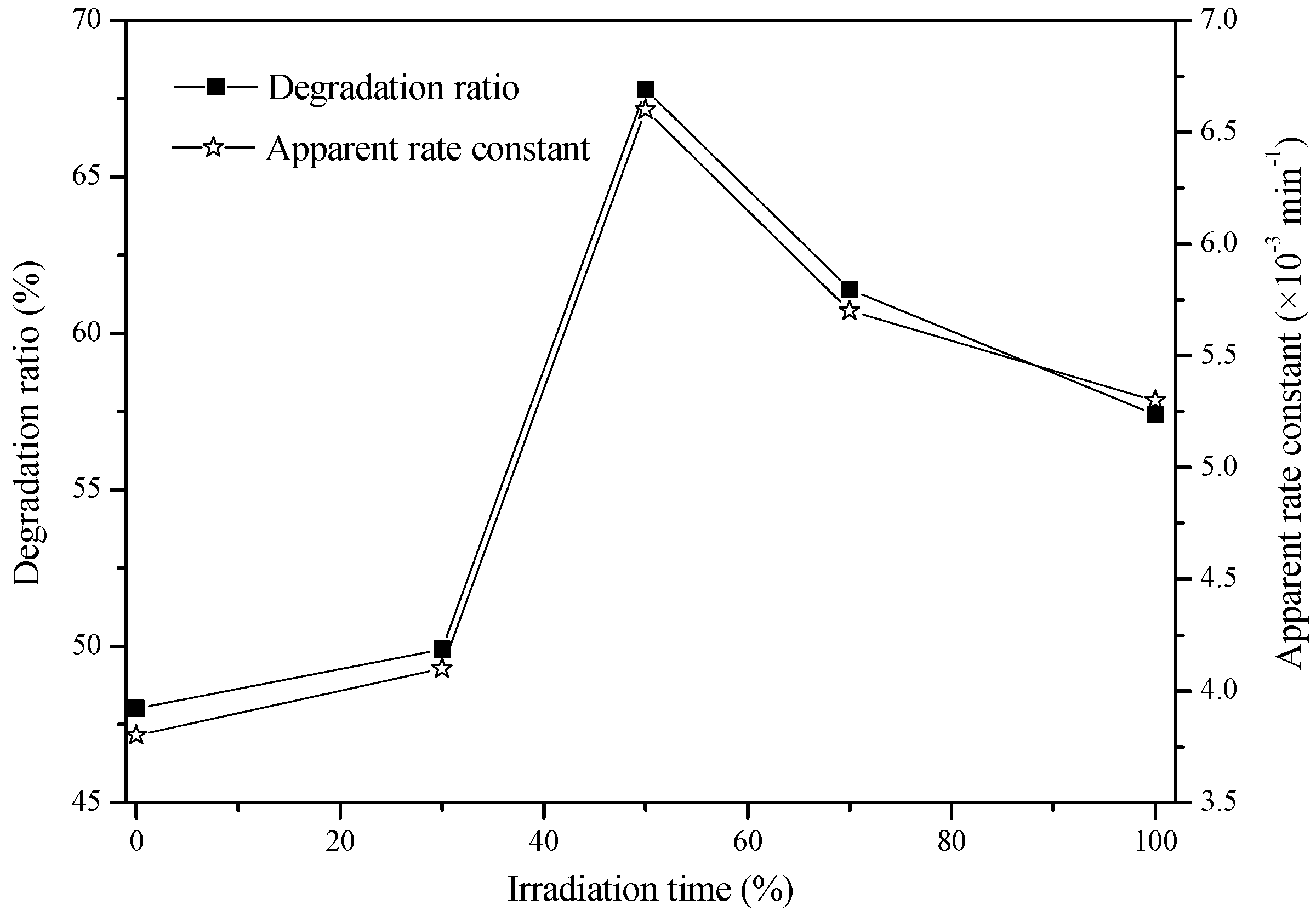
2.3. Effect of CO2 Concentration on the Photocatalytic Activity
3. Experimental
3.1. Preparation of Photocatalysts
3.2. Characterization of Photocatalysts
3.3. Photocatalytic Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pang, D.; Dong, M.; Ma, X.; Ouyang, F. Photocatalytic decomposition of gas-phase chlorobenzene with transition metal-doped KLaTi2O6 under visible light irradiation. Environ. Eng. Sci. 2014, 31, 1–8. [Google Scholar] [CrossRef]
- Cheng, Z.; Gu, Z.; Chen, J.; Yu, J.; Zhou, L. Synthesis, characterization, and photocatalytic activity of porous La-N-co-doped TiO2 nanotubes for gaseous chlorobenzene oxidation. J. Environ. Sci. China 2016, 46, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Mao, Y.; Jiang, L.; Chen, J. Performance of chlorobenzene removal in a nonthermal plasma catalysis reactor and evaluation of its byproducts. Chem. Eng. J. 2015, 279, 463–471. [Google Scholar] [CrossRef]
- Kumar, V.; Govind; Uma, S. Investigation of cation (Sn2+) and anion (N3−) substitution in favor of visible light photocatalytic activity in the layered perovskite K2La2Ti3O10. J. Hazard. Mater. 2011, 189, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.; Lai, M.; Pan, C. Synthesis and visible-Light-Derived photocatalysis of titania nanosphere stacking layers prepared by chemical vapor deposition. J. Chem. Technol. Biotechnol. 2010, 85, 1168–1174. [Google Scholar] [CrossRef]
- Huang, Y.; Wei, Y.; Cheng, S.; Fan, L.; Li, Y.; Lin, J.; Wu, J. Photocatalytic property of nitrogen-Doped layered perovskite K2La2Ti3O10. Sol. Energy Mater. Sol. C 2010, 94, 761–766. [Google Scholar] [CrossRef]
- Liu, L.; Guo, D.; Cui, W.; Hu, J.; Liang, Y. Photocatalytic hydrogen evolution from the splitting of water over Cd11−x ZnxS/K2La2Ti3O10 composites under visible light irradiation. J. Wuhan Univ. Technol. 2015, 30, 928–934. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic materials as catalysts for photochemical splitting of water. Chem. Mater. 2008, 20, 35–54. [Google Scholar] [CrossRef]
- Abe, R.; Shinmei, K.; Koumura, N.; Hara, K.; Ohtani, B. Visible-Light-Induced water splitting based on two-Step photoexcitation between dye-Sensitized layered niobate and tungsten oxide photocatalysts in the presence of a triiodide/iodide shuttle redox mediator. J. Am. Chem. Soc. 2013, 135, 16872–16884. [Google Scholar] [CrossRef] [PubMed]
- Uma, S.; Singh, J.; Thakral, V. Facile room temperature ion-Exchange synthesis of Sn2+ incorporated pyrochlore-type oxides and their photocatalytic activities. Inorg. Chem. 2009, 48, 11624–11630. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yeh, Y.; Shah, S.I.; Huang, C.P. Concurrent photoelectrochemical reduction of CO2 and oxidation of methyl orange using nitrogen-Doped TiO2. Appl. Catal. B Environ. 2012, 123–124, 414–423. [Google Scholar] [CrossRef]
- Ulagappan, N.; Frei, H. Mechanistic study of CO2 photoreduction in Ti silicalite molecular sieve by FT-IR spectroscopy. J. Phys. Chem. A 2000, 104, 7834–7839. [Google Scholar] [CrossRef]
- Toda, K.; Watanabe, J.; Sato, M. Crystal structure determination of ion-Exchangeable layered perovskite compounds, K2La2Ti3010 and Li2La2Ti5010. Mater. Res. Bull. 1996, 31, 1427–1435. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Chen, Q.-Y.; Yin, Z.-L.; Li, J. Study on the photocatalytic activity of K2La2Ti3O10 doped with zinc(Zn). Appl. Surf. Sci. 2009, 255, 8419–8424. [Google Scholar]
- Yavuz, O.; Altunkaynak, Y.; Guzel, F. Removal of copper, nickel, cobalt and manganese from aqueous solution by kaolinite. Water Res. 2003, 37, 948–952. [Google Scholar] [CrossRef]
- Dean, J.A. Lang’s Handbook of Chemistry; McGraw-Hill, Inc.: New York, NY, USA, 1991; p. 319. [Google Scholar]
- Kitano, M.; Hara, M. Heterogeneous photocatalytic cleavage of water. J. Mater. Chem. 2010, 20, 627–641. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Zhang, M.; Yuan, Q.; Dong, B. Ultrathin hexagonal SnS2 nanosheets coupled with g-C3N4 nanosheets as 2D/2D heterojunction photocatalysts toward high photocatalytic activity. Appl. Catal. B Environ. 2015, 163, 298–305. [Google Scholar] [CrossRef]
- Pang, D.; Qiu, L.; Zhu, R.; Ouyang, F. Silica supported SO42−/TiO2 for photocatalytic decomposition of acrylonitrile under simulant solar light irradiation. Chem. Eng. J. 2015, 270, 590–596. [Google Scholar] [CrossRef]
- Sakthivel, S.; Neppolian, B.; Shankar, M.V.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cell 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Zhang, L.; Sawell, S.; Moralejo, C.; Anderson, W. Heterogeneous photocatalytic decomposition of gas-Phase chlorobenzene. Appl. Catal. B Environ. 2007, 71, 135–142. [Google Scholar] [CrossRef]
- Pang, D.; Wang, Y.; Ma, X.; Ouyang, F. Fluorine promoted and silica supported TiO2 for photocatalytic decomposition of acrylonitrile under simulant solar light irradiation. Chem. Eng. J. 2014, 258, 43–50. [Google Scholar] [CrossRef]
- Dilmeghani, M.; Zahir, K. Kinetics and mechanism of chlorobenzene degradation in aqueous samples using advanced oxidation processes. J. Environ. Qual. 2001, 30, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Tseng, D.; Juang, L.; Huang, H. Effect of oxygen and hydrogen peroxide on the photocatalytic degradation of monochlorobenzene in aqueous suspension. Int. J. Photoenergy 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, Q.; Wang, Y. Semiconductor-Based nanocomposites for photocatalytic H2 production and CO2 conversion. Phys. Chem. Chem. Phys. 2013, 15, 2632–2649. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, N.M.; Vijayan, B.K.; Poluektov, O.G.; Rajh, T.; Gray, K.A.; He, H.; Zapol, P. Role of water and carbonates in photocatalytic transformation of CO2 to CH4 on titania. J. Am. Chem. Soc. 2011, 133, 3964–3971. [Google Scholar] [CrossRef] [PubMed]

| Samples | Grain Size (nm) a | Specific Surface Area (m2/g) | Degradation Ratio (%) |
|---|---|---|---|
| Original K2La2Ti3O10 at 950 °C | 61.8 | 2.0 | 34.2 |
| Cu2+/K2La2Ti3O10 at 300 °C | 13.0 | 5.8 | 46.0 |
| Cu2+/K2La2Ti3O10 at 400 °C | 15.5 | 5.0 | 48.0 |
| Cu2+/K2La2Ti3O10 at 500 °C | 13.0 | 4.6 | 51.1 |
| Cu2+/K2La2Ti3O10 at 600 °C | 13.8 | 3.4 | 45.0 |
| Cu2+/K2La2Ti3O10 at 700 °C | 20.3 | 2.7 | 44.0 |
| Irradiation Time (min) | Degradation Ratio (%) | Apparent Rate Constant (min−1) | R2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | 150 | 180 | |||
| 100% N2 | 0 | 5 | 16.5 | 23.0 | 33.0 | 42.0 | 48.0 | 0.0038 | 0.9882 |
| 70% N2 + 30% CO2 | 0 | 3.8 | 15.6 | 24.9 | 34.4 | 45.2 | 49.9 | 0.0041 | 0.9836 |
| 50% N2 + 50% CO2 | 0 | 10.1 | 22.4 | 31.7 | 53.8 | 59.6 | 67.8 | 0.0066 | 0.9714 |
| 30% N2 + 70% CO2 | 0 | 8.5 | 22.5 | 33.1 | 47.0 | 58.6 | 61.4 | 0.0057 | 0.9823 |
| 100% CO2 | 0 | 6.1 | 17.1 | 25.8 | 43.3 | 55.7 | 57.4 | 0.0053 | 0.9601 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, D.; Gao, J.; Ouyang, F.; Zhu, R.; Xie, C. Ion-Exchange of Cu2+ Promoted Layered Perovskite K2La2Ti3O10 for Photocatalytic Degradation Chlorobenzene under Simulated Solar Light Irradiation. Catalysts 2017, 7, 126. https://doi.org/10.3390/catal7050126
Pang D, Gao J, Ouyang F, Zhu R, Xie C. Ion-Exchange of Cu2+ Promoted Layered Perovskite K2La2Ti3O10 for Photocatalytic Degradation Chlorobenzene under Simulated Solar Light Irradiation. Catalysts. 2017; 7(5):126. https://doi.org/10.3390/catal7050126
Chicago/Turabian StylePang, Dandan, Jie Gao, Feng Ouyang, Rongshu Zhu, and Charlene Xie. 2017. "Ion-Exchange of Cu2+ Promoted Layered Perovskite K2La2Ti3O10 for Photocatalytic Degradation Chlorobenzene under Simulated Solar Light Irradiation" Catalysts 7, no. 5: 126. https://doi.org/10.3390/catal7050126





