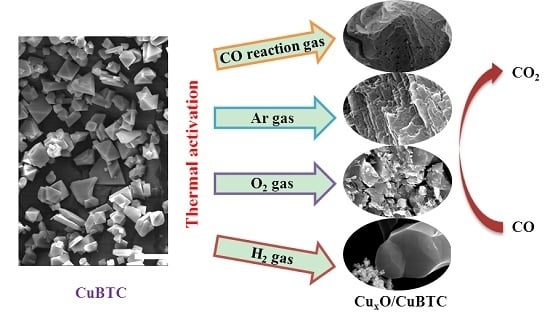
Thermal Activation of CuBTC MOF for CO Oxidation: The Effect of Activation Atmosphere
Abstract
:1. Introduction
2. Results and Discussion
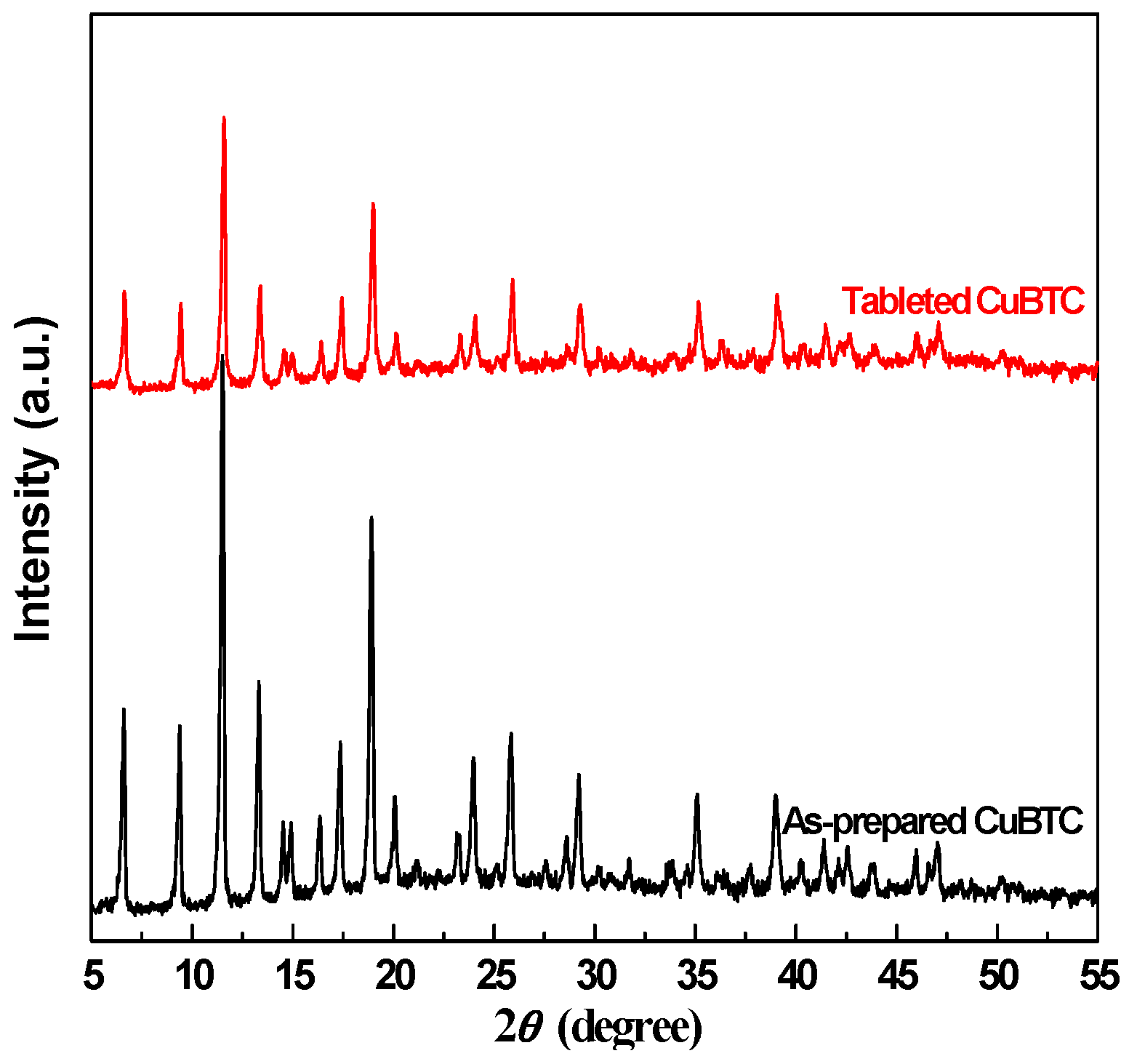
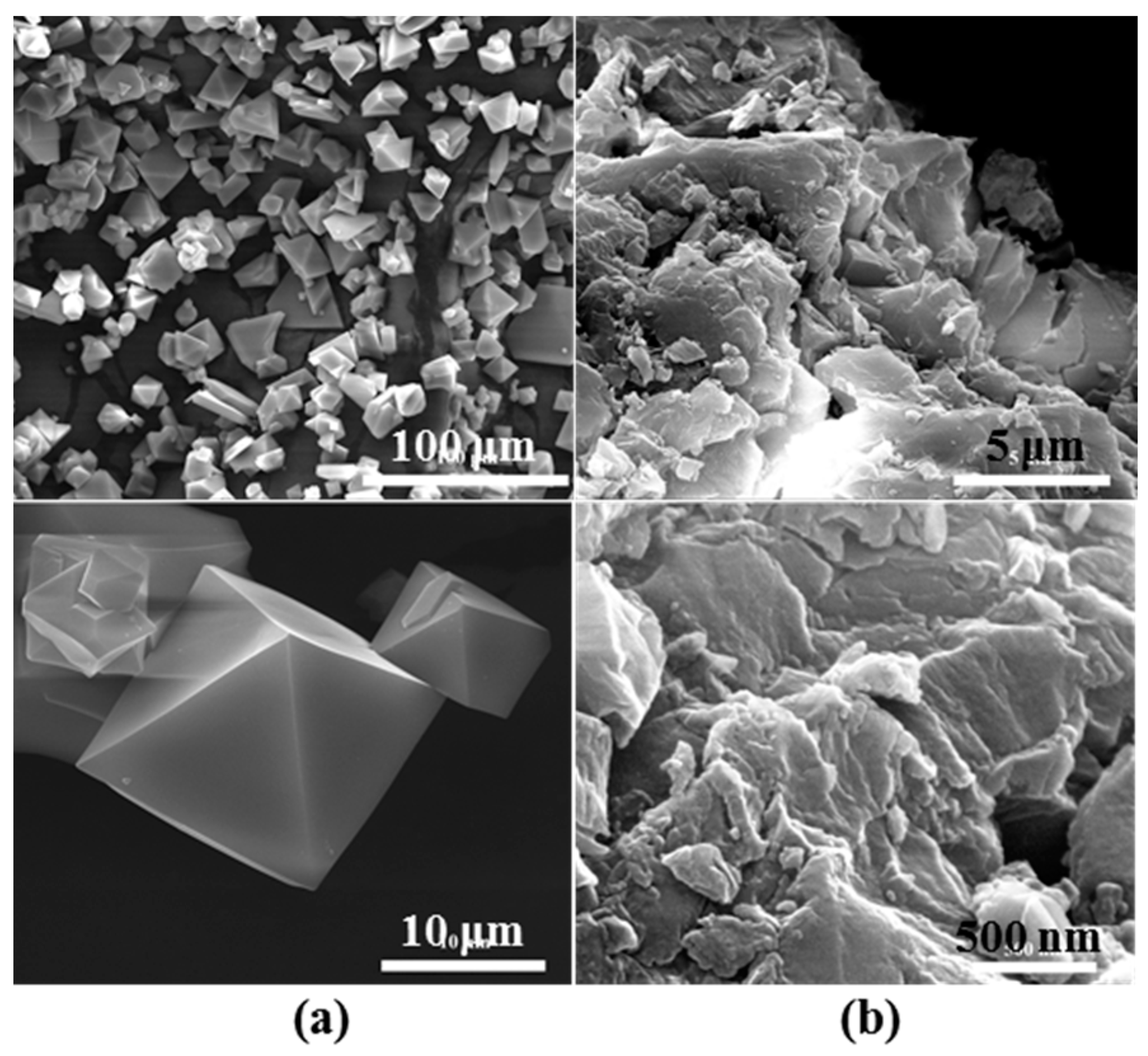
2.1. Crystalline Phase Structure of CuBTC and the Effects of Tableting
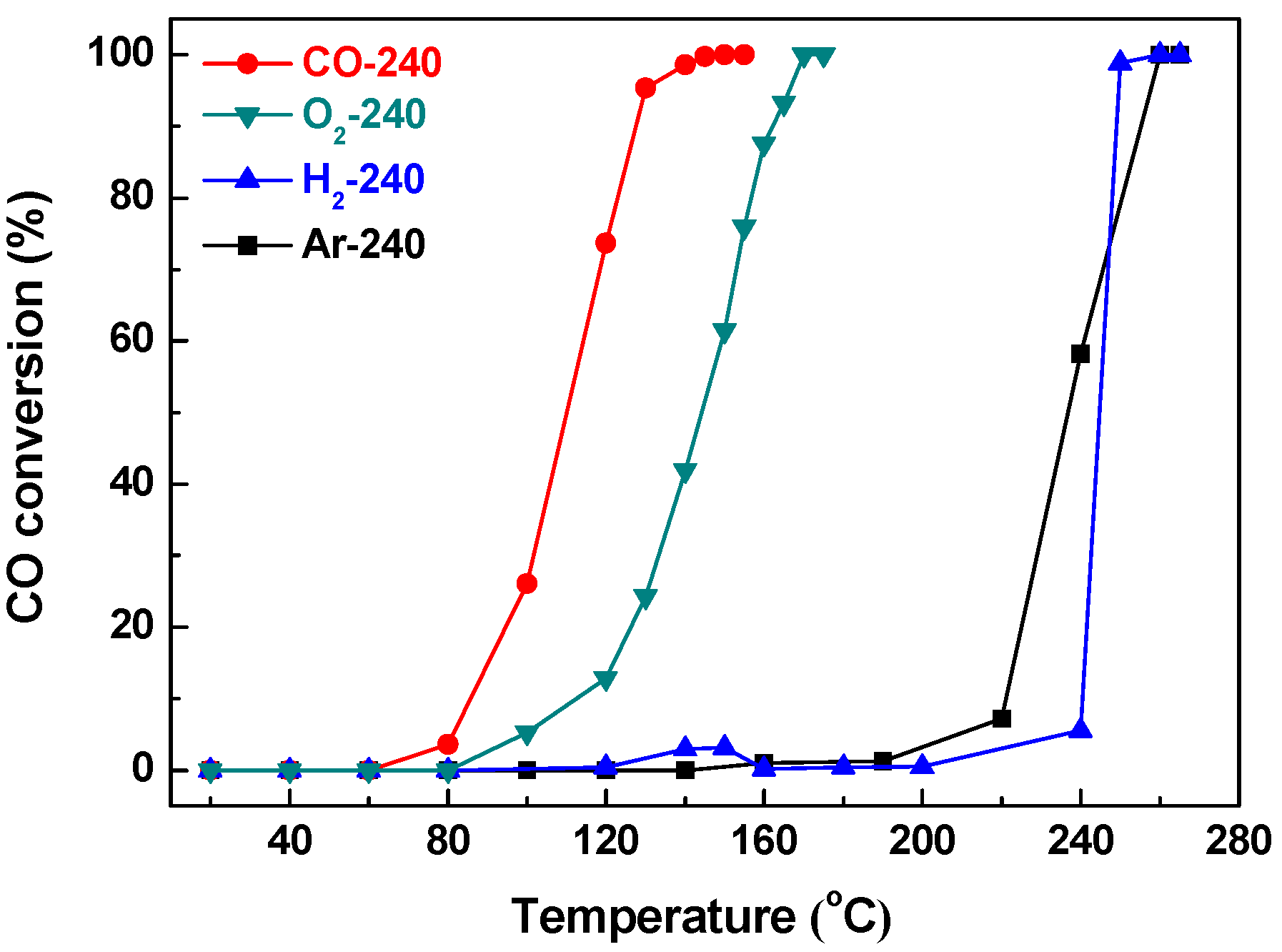
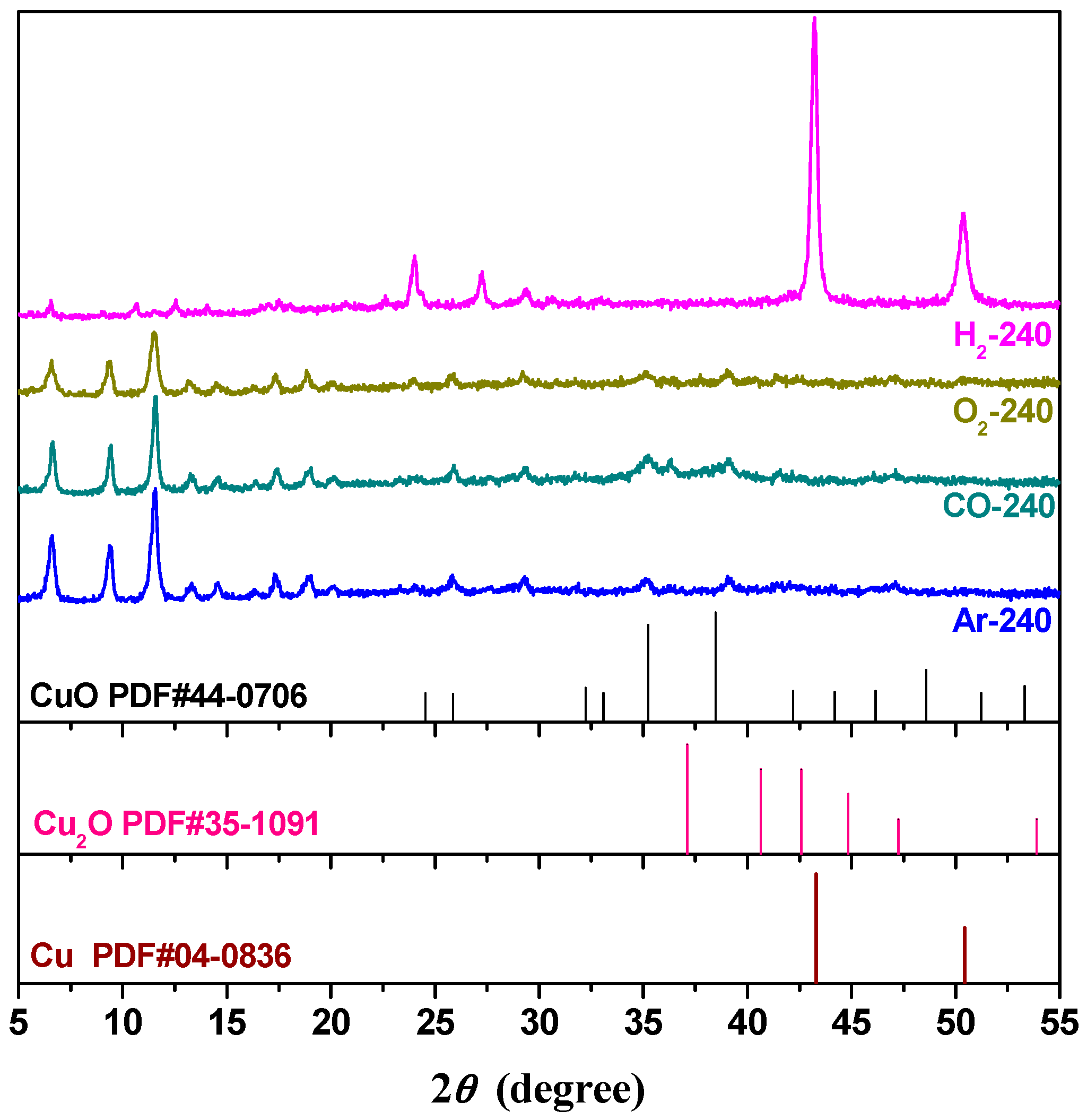
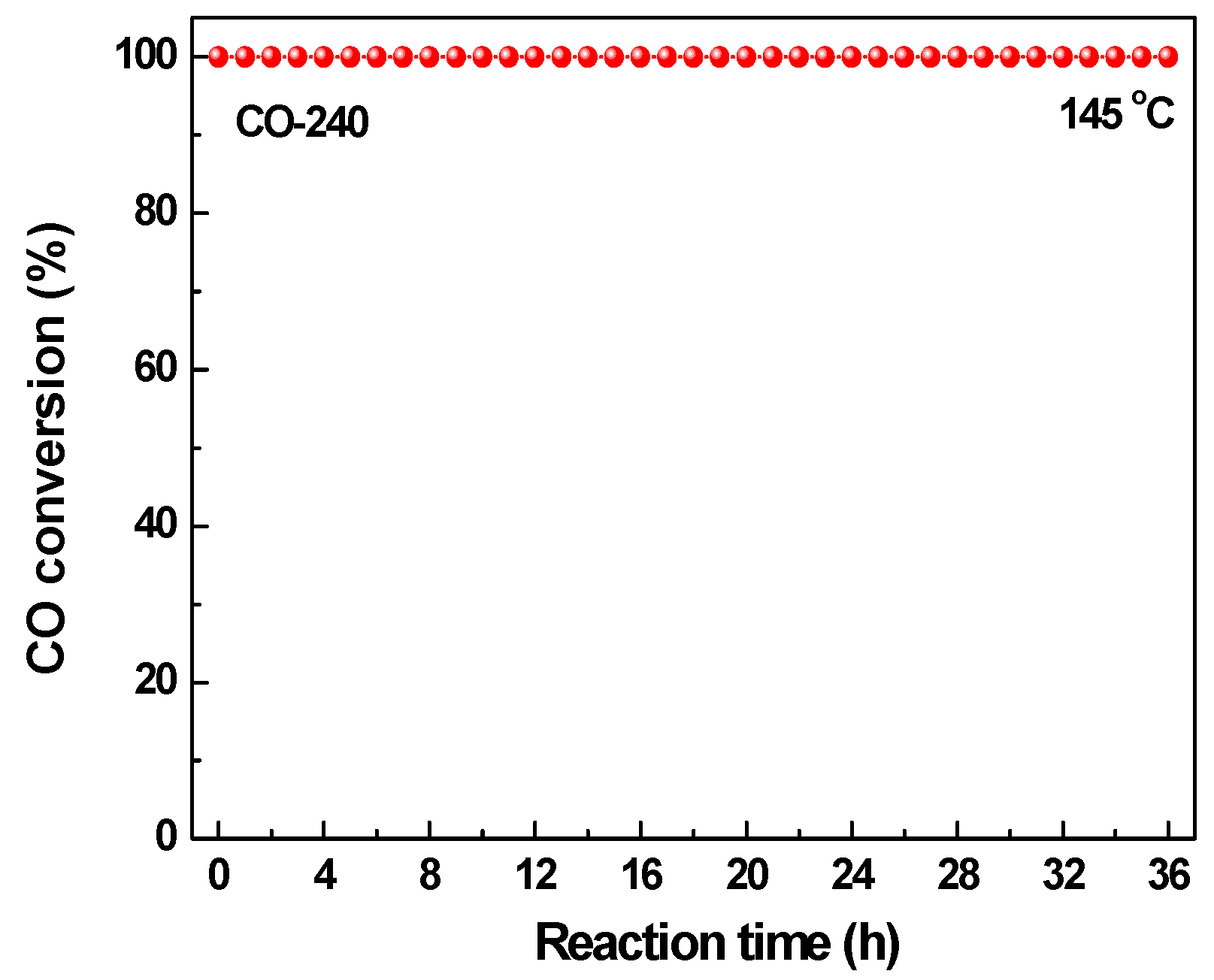
2.2. Effect of Activating Atmosphere on CuBTC Catalysts
3. Materials and Methods
3.1. Materials
3.2. Preparation and Thermal Activation of the CuBTC Catalysts
3.3. Catalysts Characterization
3.4. CO Oxidation Activity Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haruta, M.; Tsubota, S.; Kobayashi, T.; Kageyama, H.; Genet, M.J.; Delmon, B. Low-temperature oxidation of CO over gold supported on TiO2, α-Fe2O3, and Co3O4. J. Catal. 1993, 144, 175–192. [Google Scholar] [CrossRef]
- Schubert, M.M.; Hackenberg, S.; Van Veen, A.C.; Muhler, M.; Plzak, V.; Behm, R.J. CO oxidation over supported gold catalysts—“Inert” and “Active” support materials and their role for the oxygen supply during reaction. J. Catal. 2001, 197, 113–122. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Fukumoto, K.; Ishima, T.; Yamamoto, H.; Sano, M.; Miyake, T. Preparation of copper-containing mesoporous manganese oxides and their catalytic performance for CO oxidation. Appl. Catal. B Environ. 2009, 89, 420–424. [Google Scholar] [CrossRef]
- Barbato, P.S.; Di Benedetto, A.; Landi, G.; Lisi, L. CuO/CeO2 based monoliths for CO preferential oxidation in H2-rich streams. Chem. Eng. J. 2015, 279, 983–993. [Google Scholar] [CrossRef]
- Miguel-Garcia, I.; Navlani-Garcia, M.; Garcia-Aguilar, J.; Berenguer-Murcia, A.; Lozano-Castello, D.; Cazorla-Amoros, D. Capillary microreactors based on hierarchial SiO2 monoliths incorporating noble metal nanoparticles for the preferential oxidation of CO. Chem. Eng. J. 2015, 275, 71–78. [Google Scholar] [CrossRef]
- Di, L.; Duan, D.; Zhang, X.; Qi, B.; Zhan, Z. Effect of TiO2 crystal phase and preparation method on the catalytic performance of Au/TiO2 for CO oxidation. IEEE Trans. Plasma Sci. 2016, 44, 2692–2698. [Google Scholar] [CrossRef]
- Xu, W.; Zhan, Z.; Di, L.; Zhang, X. Enhanced activity for CO oxidation over Pd/Al2O3 catalysts prepared by atmospheric-pressure cold plasma. Catal. Today 2015, 256, 148–152. [Google Scholar] [CrossRef]
- Manasilp, A.; Gulari, E. Selective CO oxidation over Pt/alumina catalysts for fuel cell applications. Appl. Catal. B Environ. 2002, 37, 17–25. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, A.Q.; Wang, X.D.; Mou, C.Y.; Zhang, T. Au-Cu Alloy nanoparticles confined in SBA-15 as a highly efficient catalyst for CO oxidation. Chem. Commun. 2008, 27, 3187–3189. [Google Scholar] [CrossRef] [PubMed]
- Di, L.B.; Xu, W.J.; Zhan, Z.B.; Zhang, X.L. Synthesis of alumina supported Pd-Cu alloy nanoparticles for CO oxidation via a fast and facile method. RSC Adv. 2015, 5, 71854–71858. [Google Scholar] [CrossRef]
- Xie, X.W.; Li, Y.; Liu, Z.Q.; Haruta, M.; Shen, W.J. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 458, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.M.; Rao, K.N.; Bharali, P. Copper promoted cobalt and nickel catalysts supported on ceria-alumina mixed oxide: Structural characterization and CO oxidation activity. Ind. Eng. Chem. Res. 2009, 48, 8478–8486. [Google Scholar] [CrossRef]
- Yu, Y.; Takei, T.; Ohashi, H.; He, H.; Zhang, X.L.; Haruta, M. Pretreatments of Co3O4 at moderate temperature for CO oxidation at −80 °C. J. Catal. 2009, 267, 121–128. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T. Selective CO oxidation over CuO-CeO2 catalysts prepared via the urea–nitrate combustion method. Appl. Catal. A Gen. 2003, 244, 155–167. [Google Scholar] [CrossRef]
- Zou, R.Q.; Sakurai, H.; Han, S.; Zhong, R.Q.; Xu, Q. Probing the lewis acid sites and CO catalytic oxidation activity of the porous metal-organic polymer [Cu (5-methylisophthalate)]. J. Am. Chem. Soc. 2007, 129, 8402–8403. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.Y.; Liu, C.J. Cu3(BTC)2: CO oxidation over MOF based catalysts. Chem. Commun. 2011, 47, 2167–2169. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.G.; Wang, Y.; Li, C.Q.; Zhan, Z.C.; Zi, X.H.; Zhang, G.Z.; Wang, R.; He, H. Effect of activation temperature on catalytic performance of CuBTC for CO oxidation. Chin. J. Catal. 2012, 33, 986–992. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Metal-organic frameworks: A new class of porous materials. Micropor. Mesopor. Mat. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Yong, L.J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–organic framework materials as chemical sensors. Chem. Rev. 2011, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chua, Y.S.; Krungleviciute, V.; Tyagi, M.; Chen, P.; Yildirim, T.; Zhou, W. Unusual and highly tunable missing-linker defects in zirconium metal–organic framework UiO-66 and their important effects on gas adsorption. J. Am. Chem. Soc. 2013, 135, 10525–10532. [Google Scholar] [CrossRef] [PubMed]
- Kaye, S.S.; Dailly, A.; Yaghi, O.M.; Long, J.R. Impact of preparation and handling on the hydrogen storage properties of Zn4O (1,4-benzenedicarboxylate)3 (MOF-5). J. Am. Chem. Soc. 2007, 129, 14176–14177. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Langmi, H.W.; North, B.C.; Mathe, M.; Bessarabov, D. Modulated synthesis of zirconium-metal organic framework (Zr-MOF) for hydrogen storage applications. Int. J. Hydrogen Energy 2014, 39, 890–895. [Google Scholar] [CrossRef]
- Alaerts, L.; Séguin, E.; Poelman, H.; Thibault-Starzyk, F.; Jacobs, P.A.; De Vos, D.E. Probing the Lewis Acidity and Catalytic Activity of the Metal-Organic Framework [Cu3(BTC)2] (BTC = Benzene-1,3,5-tricarboxylate). Chem. Eur. J. 2006, 12, 7353–7363. [Google Scholar] [CrossRef] [PubMed]
- Xamena, F.X.L.I.; Casanova, O.; Tailleur, R.G.; Garcia, H.; Corma, A. Metal organic frameworks (MOFs) as catalysts: A combination of Cu2+ and Co2+ MOFs as an efficient catalyst for tetralin oxidation. J. Catal. 2008, 255, 220–227. [Google Scholar]
- Valvekens, P.; Vermoortele, F.; De Vos, D. Metal-organic frameworks as catalysts: The role of metal active sites. Catal. Sci. Technol. 2013, 3, 1435–1445. [Google Scholar] [CrossRef]
- Zamaro, J.M.; Pérez, N.C.; Miró, E.E.; Casado, C.; Seoane, B.; Téllez, C.; Coronas, J. HKUST-1 MOF: A matrix to synthesize CuO and CuO-CeO2 nanoparticle catalysts for CO oxidation. Chem. Eng. J. 2012, 195, 180–187. [Google Scholar] [CrossRef]
- Rösler, C.; Fischer, R.A. Metal–organic frameworks as hosts for nanoparticles. Crystengcomm 2015, 17, 199–217. [Google Scholar] [CrossRef]
- Jiang, H.L.; Liu, B.; Akita, T.; Haruta, M.; Sakurai, H.; Xu, Q. Au@ZIF-8: CO oxidation over gold nanoparticles deposited to metal-organic framework. J. Am. Chem. Soc. 2009, 131, 11302–11303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhong, C.L.; Liu, C.J. Enhanced CO oxidation over thermal treated Ag/Cu-BTC. Catal. Commun. 2013, 38, 74–76. [Google Scholar] [CrossRef]
- Liang, Q.; Zhao, Z.; Liu, J.; Wei, Y.C.; Jiang, G.Y.; Duan, A.J. Pd Nanoparticles deposited on metal-organic framework of MIL-53(Al) an active catalyst for CO oxidation. Acta Phys. Chim. Sin. 2014, 30, 129–134. [Google Scholar]
- Wang, W.X.; Li, Y.W.; Zhang, R.J.; He, D.H.; Liu, H.J.; Liao, S.J. Metal-organic framework as a host for synthesis of nanoscale Co3O4 as an active catalyst for CO oxidation. Catal. Commun. 2012, 12, 875–879. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, C.; Xiao, W.M.; Xu, L.; Zhang, N. CuO/CeO2 catalysts with well-dispersed active sites prepared from Cu3(BTC)2 metal–organic framework precursor for preferential CO oxidation. Catal. Commun. 2012, 26, 25–29. [Google Scholar] [CrossRef]
- Das, R.; Pachfule, P.; Banerjee, R.; Poddar, P. Metal and metal oxide nanoparticle synthesis from metal organic frameworks (MOFs): Finding the border of metal and metal oxides. Nanoscale 2012, 4, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Mondloch, J.E.; Karagiaridi, O.; Farha, O.K.; Hupp, J.T. Activation of metal–organic framework materials. Crystengcomm 2013, 15, 9258–9264. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; Groy, T.L.; Yaghi, O.M. Establishing microporosity in open metal-organic frameworks: Gas sorption isotherms for Zn(BDC) (BDC = 1, 4-benzenedicarboxylate). J. Am. Chem. Soc. 1998, 120, 8571–8572. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar]
- Liu, J.C.; Culp, J.T.; Natesakhawat, S.; Bockrath, B.C.; Zande, B.; Sankar, S.G.; Garberoglio, G.; Johnson, J.K. Experimental and theoretical studies of gas adsorption in Cu3(BTC)2: An effective activation procedure. J. Phys. Chem. C 2007, 111, 9305–9313. [Google Scholar] [CrossRef]
- Yang, Y.; Shukla, P.; Wang, S.; Rudolph, V.; Chen, X.M.; Zhu, Z. Significant improvement of surface area and CO2 adsorption of Cu–BTC via solvent exchange activation. RSC Adv. 2013, 3, 17065–17072. [Google Scholar] [CrossRef]
- Ma, L.; Jin, A.; Xie, Z.; Lin, W. Freeze drying significantly increases permanent porosity and hydrogen uptake in 4, 4-connected metal-organic frameworks. Angew. Chem. 2009, 121, 10089–10092. [Google Scholar] [CrossRef]
- Lohe, M.R.; Rose, M.; Kaskel, S. Metal–organic framework (MOF) aerogels with high micro-and macroporosity. Chem. Commun. 2009, 40, 6056–6058. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.P.; Farha, O.K.; Mulfort, K.L.; Hupp, J.T. Supercritical processing as a route to high internal surface areas and permanent microporosity in metal-organic framework materials. J. Am. Chem. Soc. 2008, 131, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.; Volosskiy, B.; Demir, S.; Gándara, F.; McGrier, P.L.; Furukawa, H.; Cascio, D.; Stoddart, J.F.; Yaghi, O.M. Synthesis, structure, and metalation of two new highly porous zirconium metal-organic frameworks. Inorg. Chem. 2012, 51, 6443–6445. [Google Scholar] [CrossRef]
- Kim, H.K.; Yun, W.S.; Kim, M.B.; Kim, J.Y.; Bae, Y.S.; Lee, J.; Jeong, N.C. A chemical route to activation of open metal sites in the copper-based metal–organic framework materials HKUST-1 and Cu-MOF-2. J. Am. Chem. Soc. 2015, 137, 10009–10015. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Duan, D.; Zhan, Z.; Zhang, X. Gas-liquid cold plasma for synthesizing copper hydroxide nitrate nanosheets with high adsorption capacity. Adv. Mater. Interfaces 2016, 3, 1600760. [Google Scholar] [CrossRef]
- Di, L.; Li, Z.; Lee, B.; Park, D.-W. An alternative atmospheric-pressure cold plasma method for synthesizing Pd/P25 catalysts with the assistance of ethanol. Int. J. Hydrogen Energy 2017. [Google Scholar] [CrossRef]


| Sample | Dp (nm) | Vp (cm3·g−1) | SBET (m2·g−1) | T50% (°C) | T100% (°C) |
|---|---|---|---|---|---|
| As-prepared CuBTC | 3.84 | 0.412 | 1102.9 | 255 | 260 |
| CuBTC | 3.80 | 0.266 | 616.7 | 255 | 260 |
| CO-240 | 17.75 | 0.415 | 253.4 | 110 | 145 |
| Ar-240 | 3.79 | 0.291 | 759.4 | 237 | 255 |
| O2-240 | 3.80 | 0.430 | 385.6 | 144 | 170 |
| H2-240 | 3.36 | 0.007 | 2.0 | 245 | 255 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhan, Z.; Li, Z.; Di, L. Thermal Activation of CuBTC MOF for CO Oxidation: The Effect of Activation Atmosphere. Catalysts 2017, 7, 106. https://doi.org/10.3390/catal7040106
Zhang X, Zhan Z, Li Z, Di L. Thermal Activation of CuBTC MOF for CO Oxidation: The Effect of Activation Atmosphere. Catalysts. 2017; 7(4):106. https://doi.org/10.3390/catal7040106
Chicago/Turabian StyleZhang, Xiuling, Zhibin Zhan, Zhuang Li, and Lanbo Di. 2017. "Thermal Activation of CuBTC MOF for CO Oxidation: The Effect of Activation Atmosphere" Catalysts 7, no. 4: 106. https://doi.org/10.3390/catal7040106