Environmental Benign Synthesis of Lithium Silicates and Mg-Al Layered Double Hydroxide from Vermiculite Mineral for CO2 Capture
Abstract
:1. Introduction
2. Results and Discussion
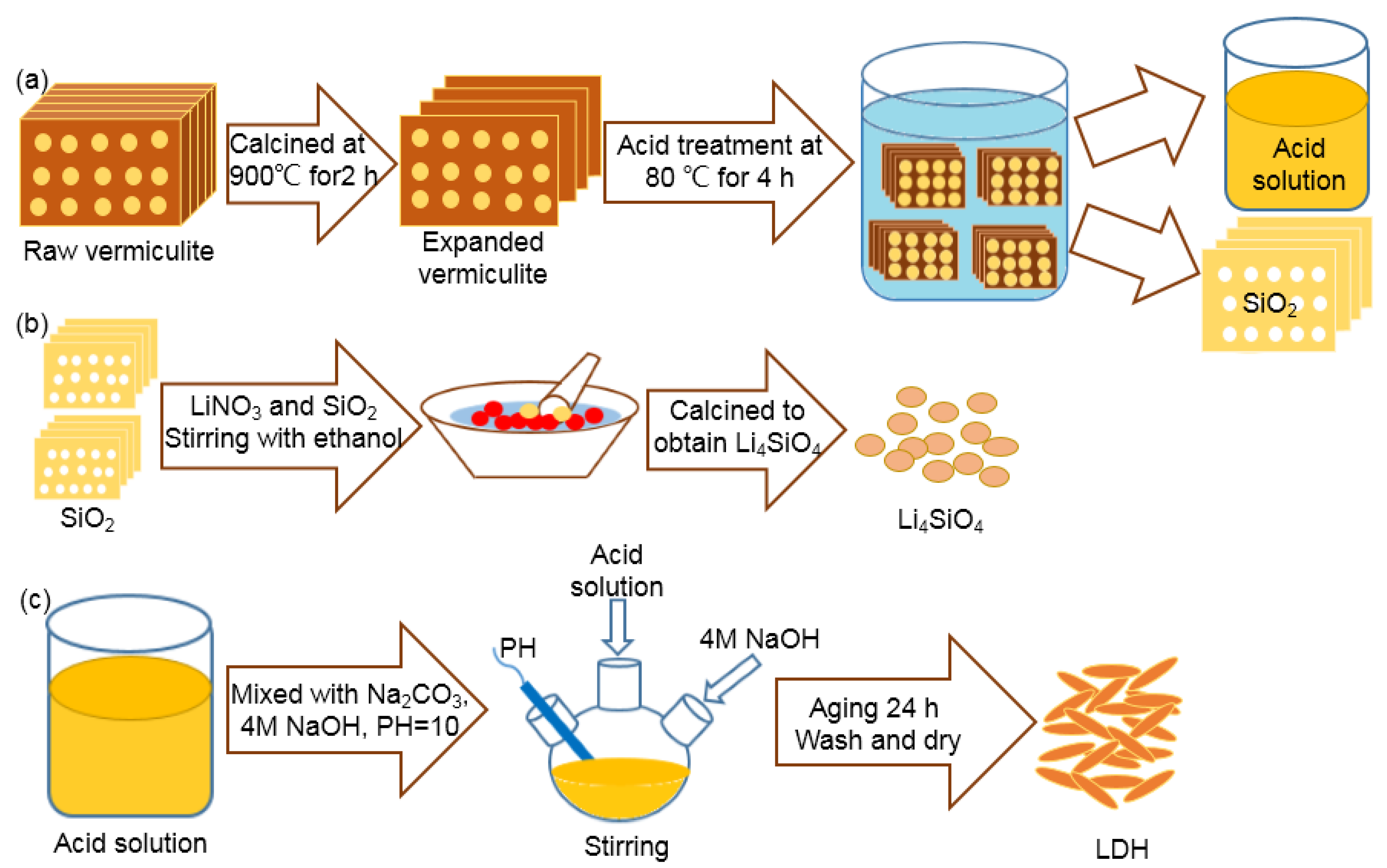
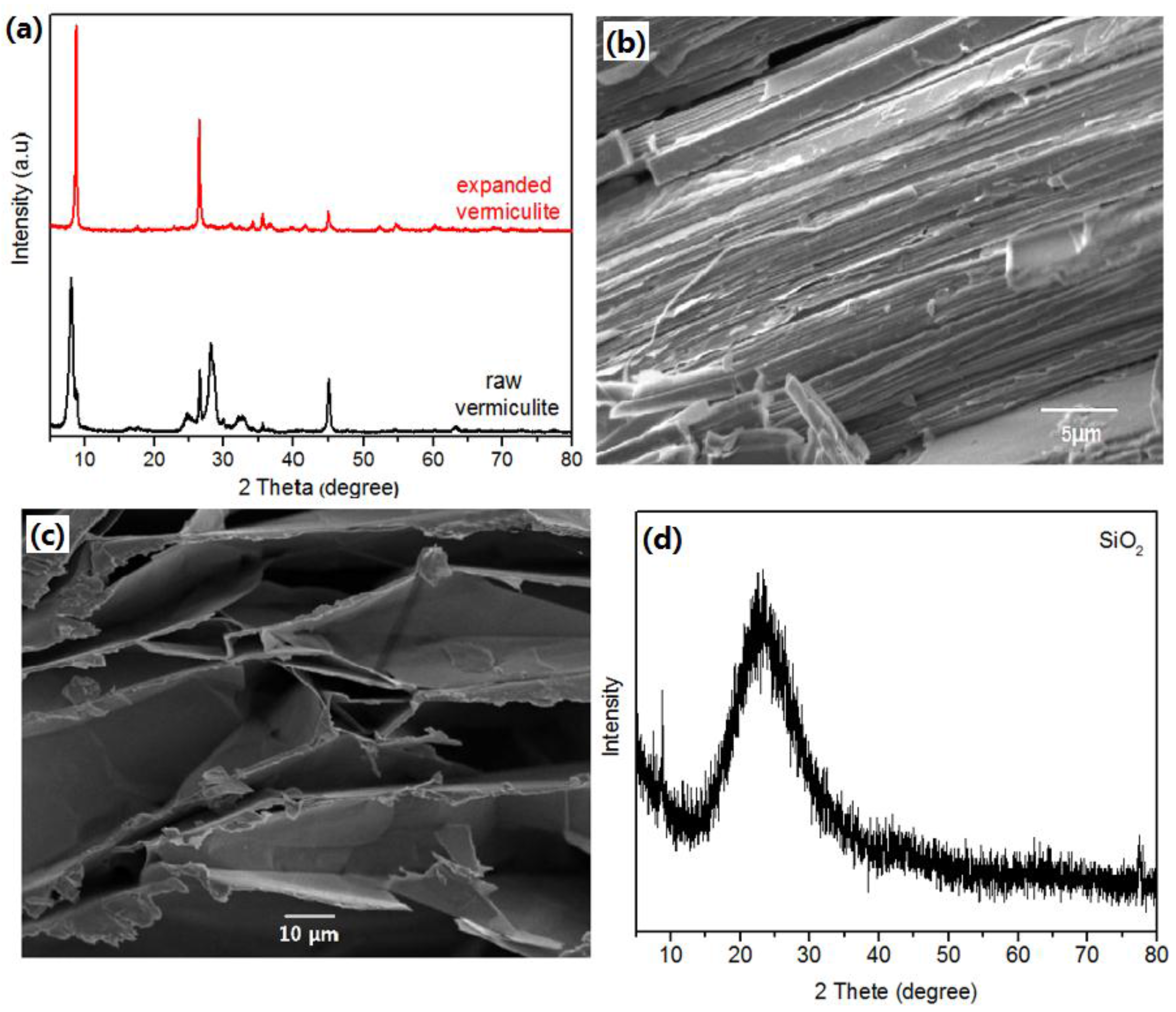
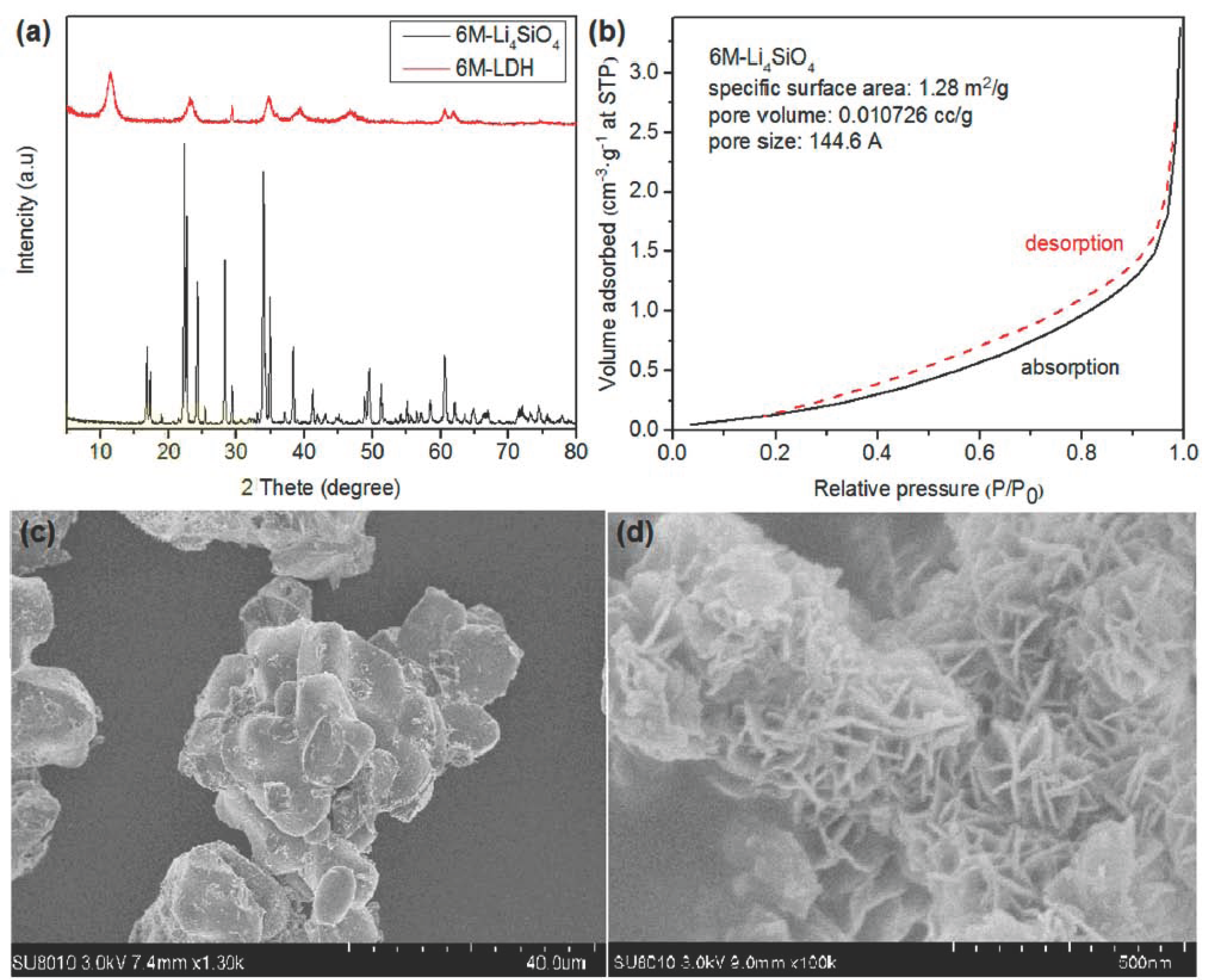
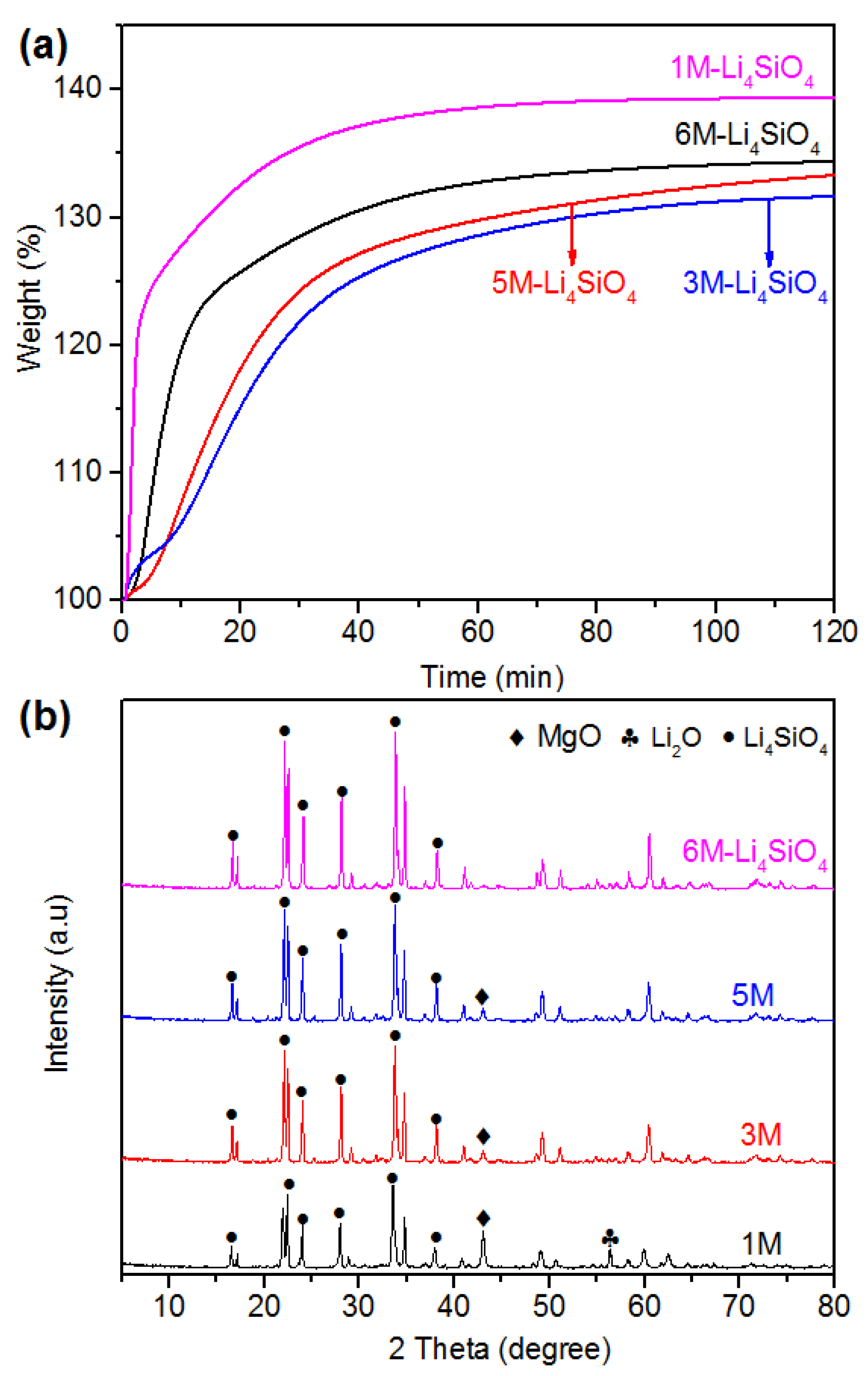
2.1. Environmentally Benign Synthesis and Characterization of Li4SiO4 and LDHs
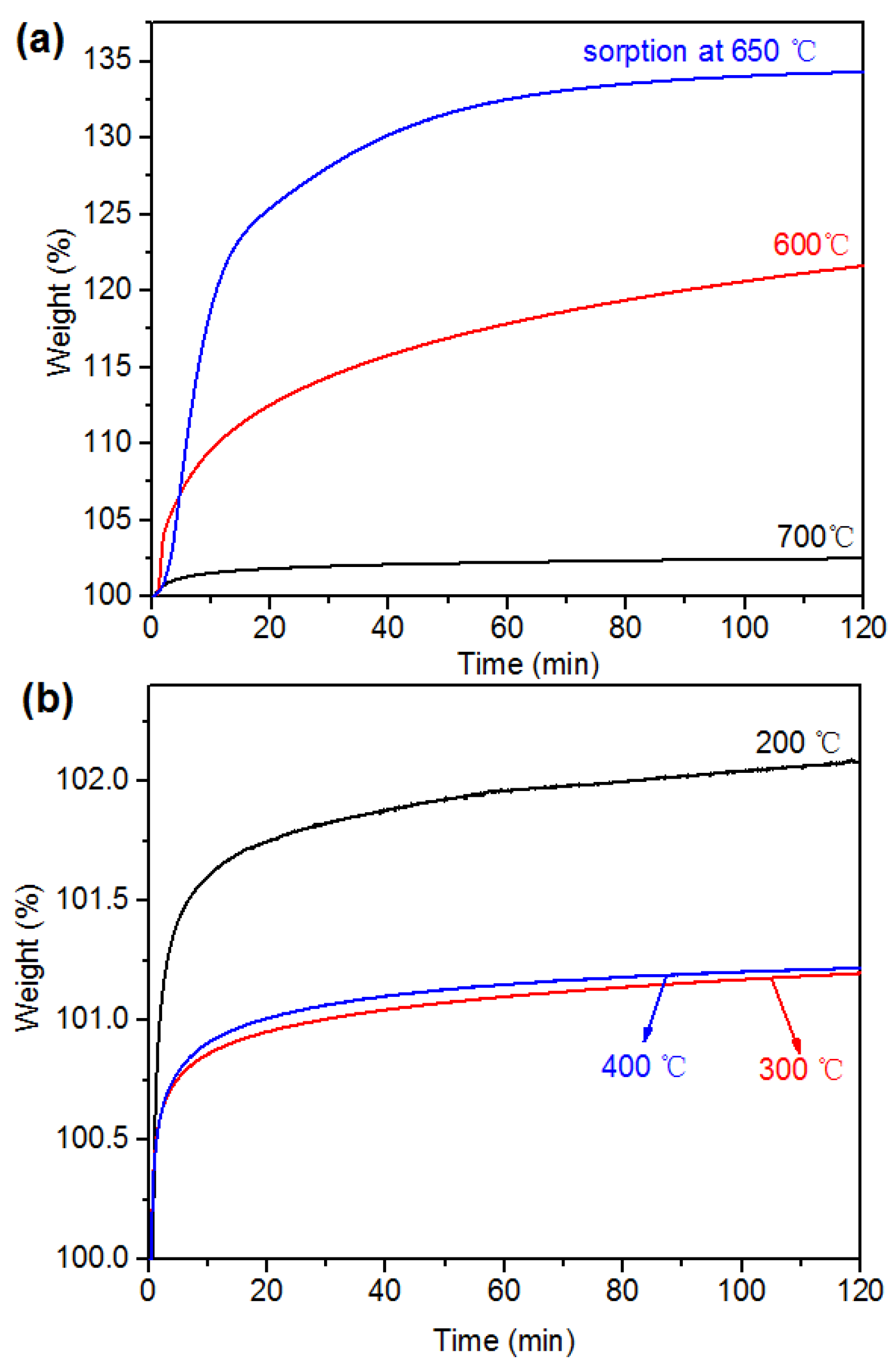
2.2. CO2 Capture Performance of Synthesized Li4SiO4 and LDH-Based Sorbents
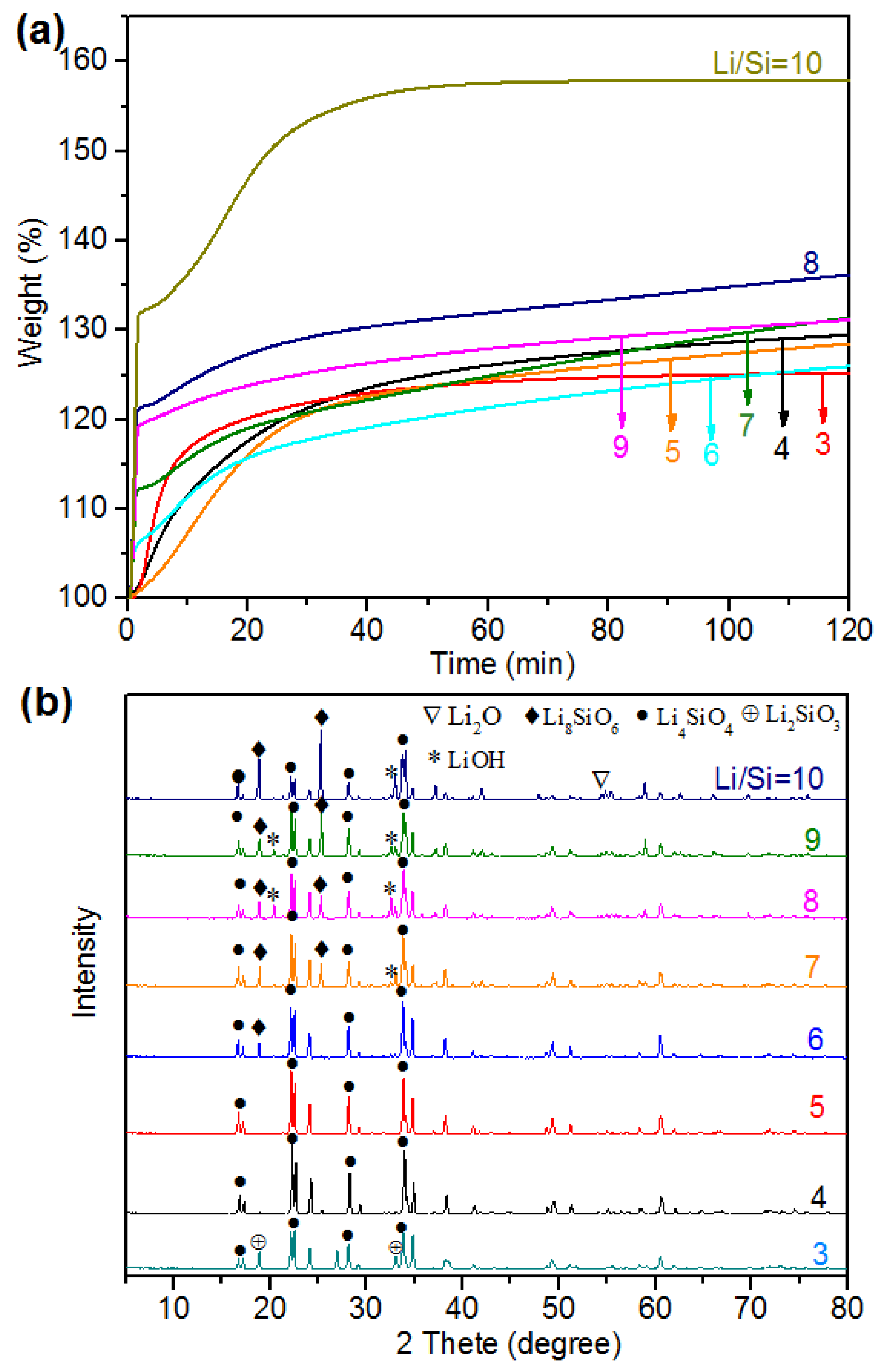
2.3. Optimization of the Synthesis Strategy
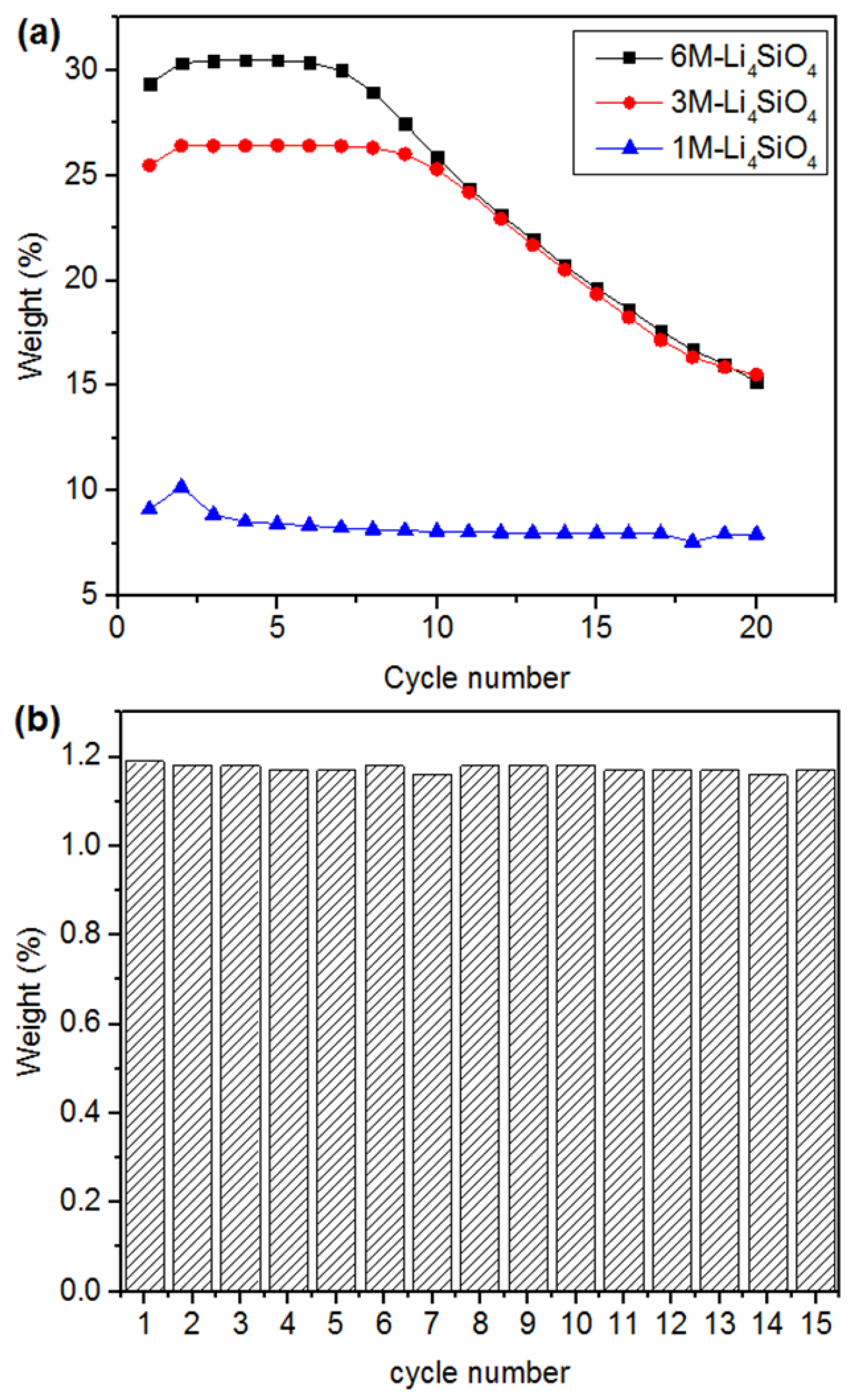
2.4. Cycling Stability of Obtained CO2 Sorbents
3. Materials and Methods
3.1. Synthesis of Li4SiO4 and LDH from Vermiculite
3.2. Characterization of Samples
3.3. Evaluation of CO2 Capture Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kato, M.; Yoshikawa, S.; Nnkagawa, K. Carbon dioxide absorption by lithium orthosilicate in a wide range of temperature and carbon dioxide concentrations. J. Mater. Sci. Lett. 2002, 21, 485–487. [Google Scholar] [CrossRef]
- Basinas, P.; Wu, Y.H.; Grammelis, P.; Anthony, E.J.; Grace, J.R.; Lim, C.J. Effect of pressure and gas concentration on CO2 and SO2 capture performance of limestones. Fuel 2014, 122, 236–246. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Shen, C.; Ni, Y.H.; Wu, Y.Q.; Wu, Q.F.; Zhu, Z.B. Self-Activation Mechanism Investigations on Large K2CO3-Doped Li4SiO4 Sorbent Particles. Ind. Eng. Chem. Res. 2015, 54, 7292–7300. [Google Scholar] [CrossRef]
- Rusten, H.K.; Ochoa-Fernandez, E.; Lindborg, H.; Chen, D.; Jakobsen, H.A. Hydrogen production by sorption-enhanced steam methane reforming using lithium oxides as CO2-acceptor. Ind. Eng. Chem. Res. 2007, 46, 8729–8737. [Google Scholar] [CrossRef]
- Kato, M.; Maezawa, Y.; Takeda, S.; Hagiwara, Y.; Kogo, R. Precombustion CO2 capture using ceramic absorbent and methane steam reforming. J. Ceram. Soc. Jpn. 2005, 113, 252–254. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Gallorini, F.; Servili, S. Hydrogen production through sorption-enhanced steam methane reforming and membrane technology: A review. Energy 2008, 33, 554–570. [Google Scholar] [CrossRef]
- Bretado, M.E.; Vigil, M.D.D.; Martinez, V.H.C.; Ortiz, A.L. Thermodynamic analysis for the production of hydrogen through Sorption Enhanced Water Gas Shift (SEWGS). Int. J. Chem. React. Eng. 2008, 6, 77–90. [Google Scholar]
- Chen, Y.; Zhao, Y.; Zhang, J.; Zheng, C. Hydrogen production through CO2 sorption-enhanced methane steam reforming: Comparison between different adsorbents. Sci. China Technol. Sci. 2011, 54, 2999–3008. [Google Scholar] [CrossRef]
- Kim, K.; Kim, D.; Park, Y.K.; Lee, K.S. A solid sorbent-based multi-stage fluidized bed process with inter-stage heat integration as an energy efficient carbon capture process. Int. J. Greenh. Gas Control 2014, 26, 135–146. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, C.; Zhang, S.; Wu, Y.Q. Steam methane reforming reaction enhanced by a novel K2CO3-Doped Li4SiO4 sorbent: Investigations on the sorbent and catalyst coupling behaviors and sorbent regeneration strategy. Int. J. Hydrog. Energy 2016, 41, 4831–4842. [Google Scholar] [CrossRef]
- Wang, J.Y.; Huang, L.; Yang, R.Y.; Zhang, Z.; Wu, J.W.; Gao, Y.S.; Wang, Q.; O’Hare, D.; Zhong, Z.Y. Recent advances in solid sorbents for CO2 capture and new development trends. Energy. Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Wang, Q.; Tay, H.H.; Zhong, Z.Y.; Luo, J.Z.; Borgna, A. Synthesis of high-temperature CO2 adsorbents from organo-layered double hydroxides with markedly improved CO2 capture capacity. Energy Environ. Sci. 2012, 5, 7526–7530. [Google Scholar] [CrossRef]
- Wang, J.Y.; Mei, X.Y.; Huang, L.; Zheng, Q.W.; Qiao, Y.Q.; Zang, K.T.; Mao, S.C.; Yang, R.Y.; Zhang, Z.; Gao, Y.S.; Guo, Z.H.; Huang, Z.G.; et al. Synthesis of layered double hydroxides/graphene oxide nanocomposite as a novel high-temperature CO2 adsorbent. J. Energy Chem. 2015, 24, 127–137. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Z.; Wu, J.; Yi, X.; Zheng, A.; Umar, A.; O’Hare, D.; Wang, Q. Comprehensive investigation of CO2 adsorption on Mg–Al–CO3 LDH-derived mixed metal oxides. J. Mater. Chem. A 2013, 1, 12782. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, J.; Zhang, Y.; Gao, W.; Harada, T.; Huang, L.; Hatton, T.A.; Wang, Q. Alkali Nitrates Molten Salt Modified Commercial MgO for Intermediate-Temperature CO2 Capture: Optimization of the Li/Na/K Ratio. Ind. Eng. Chem. Res. 2017, 56, 1509–1517. [Google Scholar] [CrossRef]
- Al-Jeboori, M.J.; Fennell, P.S.; Nguyen, M.; Peng, K. Effects of Different Dopants and Doping Procedures on the Reactivity of CaO-based Sorbents for CO2 Capture. Energy Fuels 2012, 26, 6584–6594. [Google Scholar]
- Chen, S.Y.; Xiang, W.G.; Wang, D.; Xue, Z.P. Incorporating IGCC and CaO sorption-enhanced process for power generation with CO2 capture. Appl. Energy 2012, 95, 285–294. [Google Scholar] [CrossRef]
- Pfeiffer, H.; Lima, E.; Bosch, P. Lithium-Sodium Metazirconate Solid Solutions, Li2-xNaxZrO3 (0 <= x <= 2): A Hierarchical Architecture. Chem. Mater. 2006, 18, 2642–2647. [Google Scholar] [CrossRef]
- Zheng, Q.W.; Huang, L.; Zhang, Y.; Wang, J.Y.; Zhao, C.Z.; Zhang, Q.; Zheng, W.J.; Cao, D.P.; O’Hared, D.; Wang, Q. Unexpected highly reversible topotactic CO2 sorption/desorption capacity for potassium dititanate. J. Mater. Chem. A 2016, 4, 12889–12896. [Google Scholar] [CrossRef]
- Rodríguez, M.T.; Pfeiffer, H. Sodium metasilicate (Na2SiO3): A thermo-kinetic analysis of its CO2 chemical sorption. Thermochim. Acta 2008, 473, 92–95. [Google Scholar] [CrossRef]
- Romero-Ibarra, I.C.; Ortiz-Landeros, J.; Pfeiffer, H. Microstructural and CO2 chemisorption analyses of Li4SiO4: Effect of surface modification by the ball milling process. Thermochim. Acta 2013, 567, 118–124. [Google Scholar] [CrossRef]
- Reddy, M.K.R.; Xu, Z.P.; Lu, G.Q.; da Costa, J.C.D. Influence of water on high-temperature CO2 capture using layered double hydroxide derivatives. Ind. Eng. Chem. Res. 2008, 47, 2630–2635. [Google Scholar] [CrossRef]
- Qiao, Y.Q.; Wang, J.Y.; Huang, L.; Zheng, Q.W.; O’Hare, D.; Wang, Q. LDH/MgCO3 hybrid multilayer on an aluminium substrate as a novel high-temperature CO2 adsorbent. RSC Adv. 2015, 5, 82777–82780. [Google Scholar] [CrossRef]
- Qin, Q.; Wang, J.; Zhou, T.; Zheng, Q.; Huang, L.; Zhang, Y.; Lu, P.; Umar, A.; Louis, B.; Wang, Q. Impact of organic interlayer anions on the CO2 adsorption performance of Mg-Al layered double hydroxides derived mixed oxides. J. Energy Chem. 2017. [Google Scholar] [CrossRef]
- Sanna, A.; Ramli, I.; Maroto-Valer, M.M. Development of sodium/lithium/fly ash sorbents for high temperature post-combustion CO2 capture. Appl. Energy 2015, 156, 197–206. [Google Scholar] [CrossRef]
- Wang, K.; Guo, X.; Zhao, P.; Wang, F.; Zheng, C. High temperature capture of CO2 on lithium-based sorbents from rice husk ash. J. Hazard. Mater. 2011, 189, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.Y.; Li, X.Y.; Ouyang, J.; Yang, H.M. Lithium orthosilicate with halloysite as silicon source for high temperature CO2 capture. RSC Adv. 2016, 6, 44106–44112. [Google Scholar] [CrossRef]
- Nguyen, A.N.; Reinert, L.; Leveque, J.M.; Beziat, A.; Dehaudt, P.; Juliaa, J.F.; Duclaux, L. Preparation and characterization of micron and submicron-sized vermiculite powders by ultrasonic irradiation. Appl. Clay Sci. 2013, 72, 9–17. [Google Scholar] [CrossRef]
- Duman, O.; Tunc, S.; Polat, T.G. Determination of adsorptive properties of expanded vermiculite for the removal of C.I. Basic Red 9 from aqueous solution: Kinetic, isotherm and thermodynamic studies. Appl. Clay Sci. 2015, 109, 22–32. [Google Scholar] [CrossRef]
- Stawinski, W.; Freitas, O.; Chmielarz, L.; Wegrzyn, A.; Komedera, K.; Blachowski, A.; Figueiredo, S. The influence of acid treatments over vermiculite based material as adsorbent for cationic textile dyestuffs. Chemosphere 2016, 153, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Adpakpang, K.; Patil, S.B.; Oh, S.M.; Kang, J.H.; Lacroix, M.; Hwang, S.J. Effective Chemical Route to 2D Nanostructured Silicon Electrode Material: Phase Transition from Exfoliated Clay Nanosheet to Porous Si Nanoplate. Electrochim. Acta 2016, 204, 60–68. [Google Scholar] [CrossRef]
- Ryu, J.; Hong, D.; Choi, S.; Park, S. Synthesis of Ultrathin Si Nanosheets from Natural Clays for Lithium-Ion Battery Anodes. ACS Nano 2016, 10, 2843–2851. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Jang, Y.J.; Choi, S.; Kang, H.J.; Park, H.; Lee, J.S.; Park, S. All-in-one synthesis of mesoporous silicon nanosheets from natural clay and their applicability to hydrogen evolution. NPG Asia Mater. 2016, 8, e248. [Google Scholar] [CrossRef]
- Olivares-Marín, M.; Drage, T.C.; Maroto-Valer, M.M. Novel lithium-based sorbents from fly ashes for CO2 capture at high temperatures. Int. J. Greenh. Gas Control 2010, 4, 623–629. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, P.; Guo, X.; Li, Y.; Han, D.; Chao, Y. Enhancement of reactivity in Li4SiO4-based sorbents from the nano-sized rice husk ash for high-temperature CO2 capture. Energy Convers. Manag. 2014, 81, 447–454. [Google Scholar] [CrossRef]
- Li, R.G.; Zhu, J.Q.; Zhou, W.B.; Cheng, X.M.; Li, Y.Y. Thermal properties of sodium nitrate-expanded vermiculite form-stable composite phase change materials. Mater. Des. 2016, 104, 190–196. [Google Scholar] [CrossRef]
- Tian, W.L.; Kong, X.G.; Jiang, M.H.; Lei, X.D.; Duan, X. Hierarchical layered double hydroxide epitaxially grown on vermiculite for Cr(VI) removal. Mater. Lett. 2016, 175, 110–113. [Google Scholar] [CrossRef]
- Perez-Maqueda, L.A.; Maqueda, C.; Perez-Rodriguez, J.L.; Subrt, J.; Cerny, Z.; Balek, V. Thermal behaviour of ground and unground acid leached vermiculite. J. Therm. Anal. Calorim. 2011, 107, 431–438. [Google Scholar] [CrossRef]
- El Mouzdahir, Y.; Elmchaouri, A.; Mahboub, R.; Gil, A.; Korili, S.A. Synthesis of nano-layered vermiculite of low density by thermal treatment. Powder Technol. 2009, 189, 2–5. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Z.; Tay, H.H.; Chen, L.; Liu, Y.; Chang, J.; Zhong, Z.; Luo, J.; Borgna, A. High temperature adsorption of CO2 on Mg–Al hydrotalcite: Effect of the charge compensating anions and the synthesis pH. Catal. Today 2011, 164, 198–203. [Google Scholar] [CrossRef]
- Wang, Q.; Tay, H.H.; Ng, D.J.; Chen, L.; Liu, Y.; Chang, J.; Zhong, Z.; Luo, J.; Borgna, A. The effect of trivalent cations on the performance of Mg-M-CO3 layered double hydroxides for high-temperature CO2 capture. ChemSusChem 2010, 3, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tay, H.H.; Chen, L.; Liu, Y.; Chang, J.; Zhong, Z.; Luo, J.; Borgna, A. Preparation and CO2 Capture Capacity of Alkali Metal Carbonates Promoted Hydrotalcite. J. Nanoeng. Nanomanuf. 2011, 1, 298–303. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Y.; Luo, J.; Zhong, Z.; Borgna, A.; Guo, Z.; O’Hare, D. Synthesis of nano-sized spherical Mg3Al–CO3 layered double hydroxide as a high-temperature CO2 adsorbent. RSC Adv. 2013, 3, 3414. [Google Scholar] [CrossRef]
- Wang, Q.; Tay, H.H.; Guo, Z.; Chen, L.; Liu, Y.; Chang, J.; Zhong, Z.; Luo, J.; Borgna, A. Morphology and composition controllable synthesis of Mg–Al–CO3 hydrotalcites by tuning the synthesis pH and the CO2 capture capacity. Appl. Clay Sci. 2012, 55, 18–26. [Google Scholar] [CrossRef]
- Duan, Y.; Pfeiffer, H.; Li, B.; Romero-Ibarra, I.C.; Sorescu, D.C.; Luebke, D.R.; Halley, J.W. CO2 capture properties of lithium silicates with different ratios of Li2O/SiO2: An ab initio thermodynamic and experimental approach. Phys. Chem. Chem. Phys. 2013, 15, 13538–13558. [Google Scholar] [CrossRef] [PubMed]


| Acid | 1 M (HNO3) | 3 M (HNO3) | 5 M (HNO3) | 6 M (Aqua Regia) |
|---|---|---|---|---|
| SiO2 (g g−1) | 0.583 | 0.511 | 0.419 | 0.373 |
| LDH (g g−1) | 0.365 | 0.422 | 0.475 | 0.667 |
| Li/Si Molar Ration | 3:1 | 4:1 | 5:1 | 6:1 | 7:1 | 8:1 | 9:1 | 10:1 |
|---|---|---|---|---|---|---|---|---|
| CO2 sorption capacity (wt %) | 25.21 | 30.47 | 28.56 | 25.97 | 31.43 | 36.21 | 31.56 | 57.93 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhou, T.; Louis, B.; Yu, F.; Dan, J.; Wang, Q. Environmental Benign Synthesis of Lithium Silicates and Mg-Al Layered Double Hydroxide from Vermiculite Mineral for CO2 Capture. Catalysts 2017, 7, 105. https://doi.org/10.3390/catal7040105
Zhang Y, Zhou T, Louis B, Yu F, Dan J, Wang Q. Environmental Benign Synthesis of Lithium Silicates and Mg-Al Layered Double Hydroxide from Vermiculite Mineral for CO2 Capture. Catalysts. 2017; 7(4):105. https://doi.org/10.3390/catal7040105
Chicago/Turabian StyleZhang, Yu, Tuantuan Zhou, Benoit Louis, Feng Yu, Jianming Dan, and Qiang Wang. 2017. "Environmental Benign Synthesis of Lithium Silicates and Mg-Al Layered Double Hydroxide from Vermiculite Mineral for CO2 Capture" Catalysts 7, no. 4: 105. https://doi.org/10.3390/catal7040105
APA StyleZhang, Y., Zhou, T., Louis, B., Yu, F., Dan, J., & Wang, Q. (2017). Environmental Benign Synthesis of Lithium Silicates and Mg-Al Layered Double Hydroxide from Vermiculite Mineral for CO2 Capture. Catalysts, 7(4), 105. https://doi.org/10.3390/catal7040105








