Stabilization of a Lipolytic Enzyme for Commercial Application
Abstract
:1. Introduction
2. Results
2.1. T. lanouginosa Lipase (Lipex) CLEA Hyperactivation
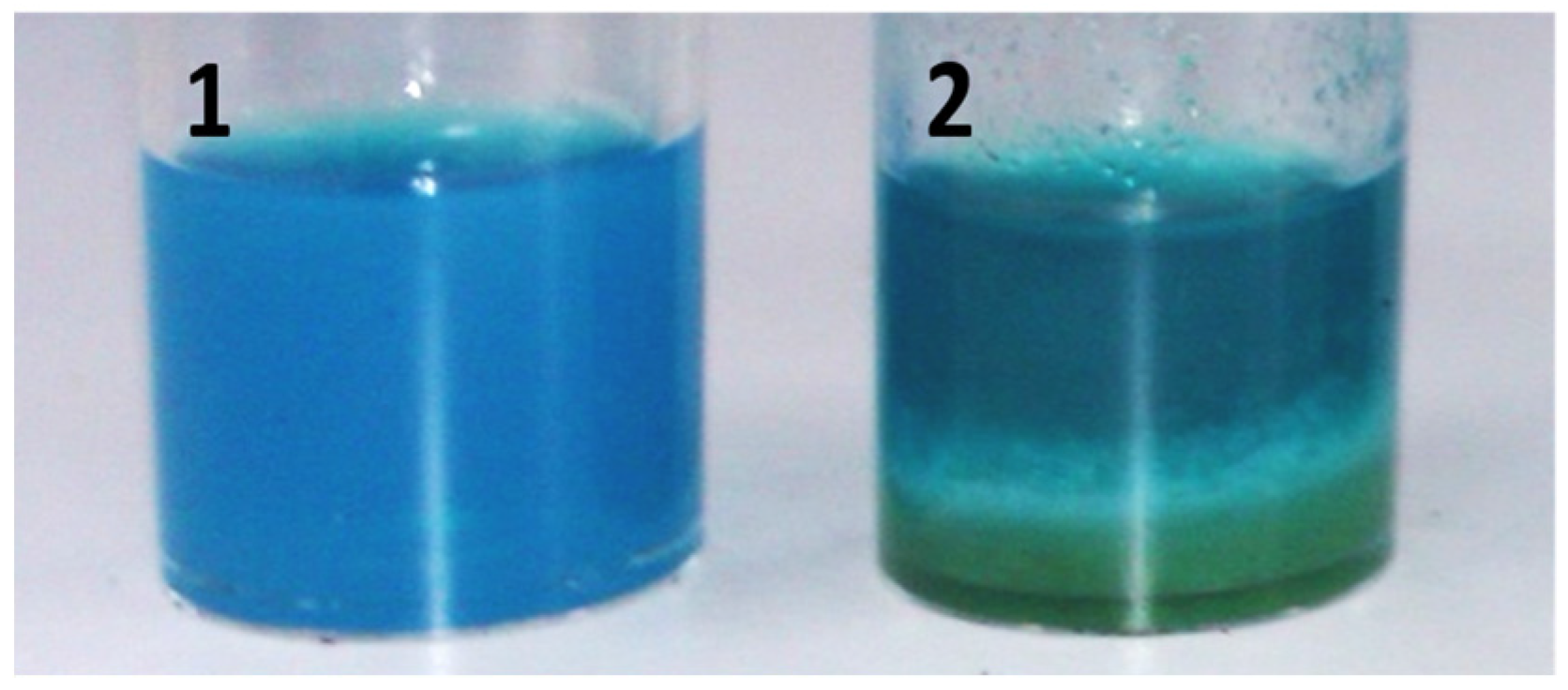
2.2. CLEAs Suspension in Formulation
2.3. CLEA Particle Size Determination
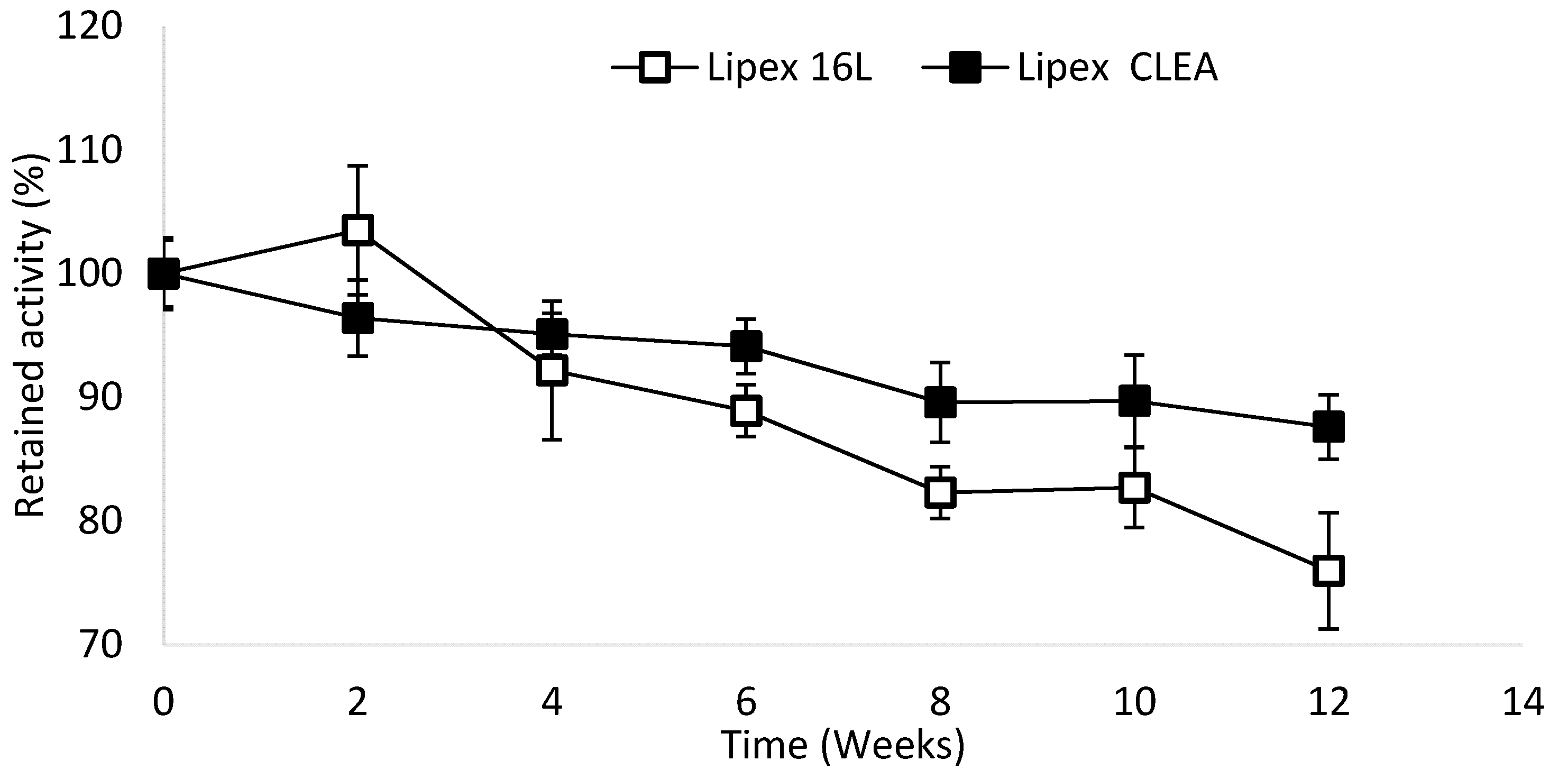
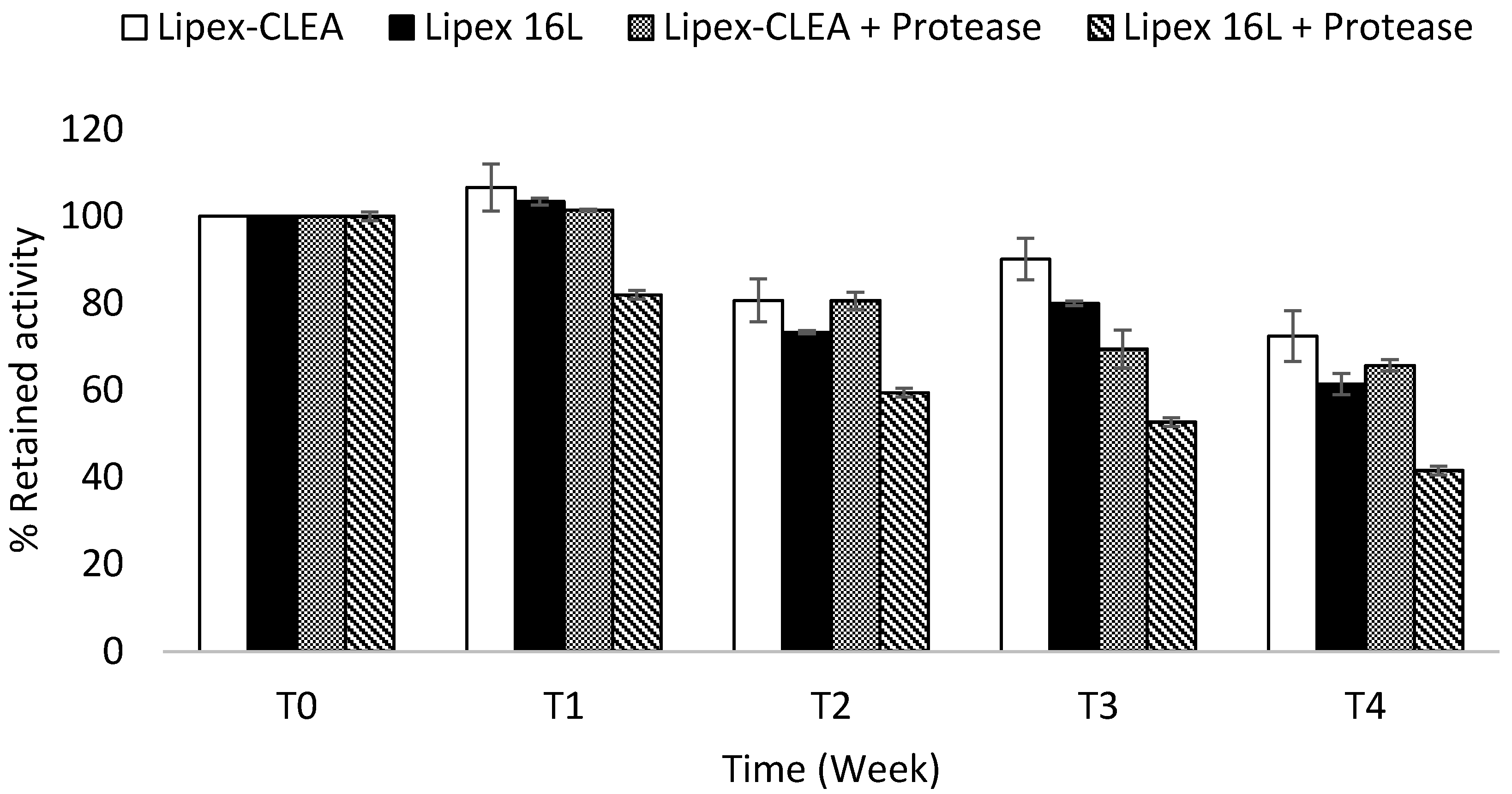
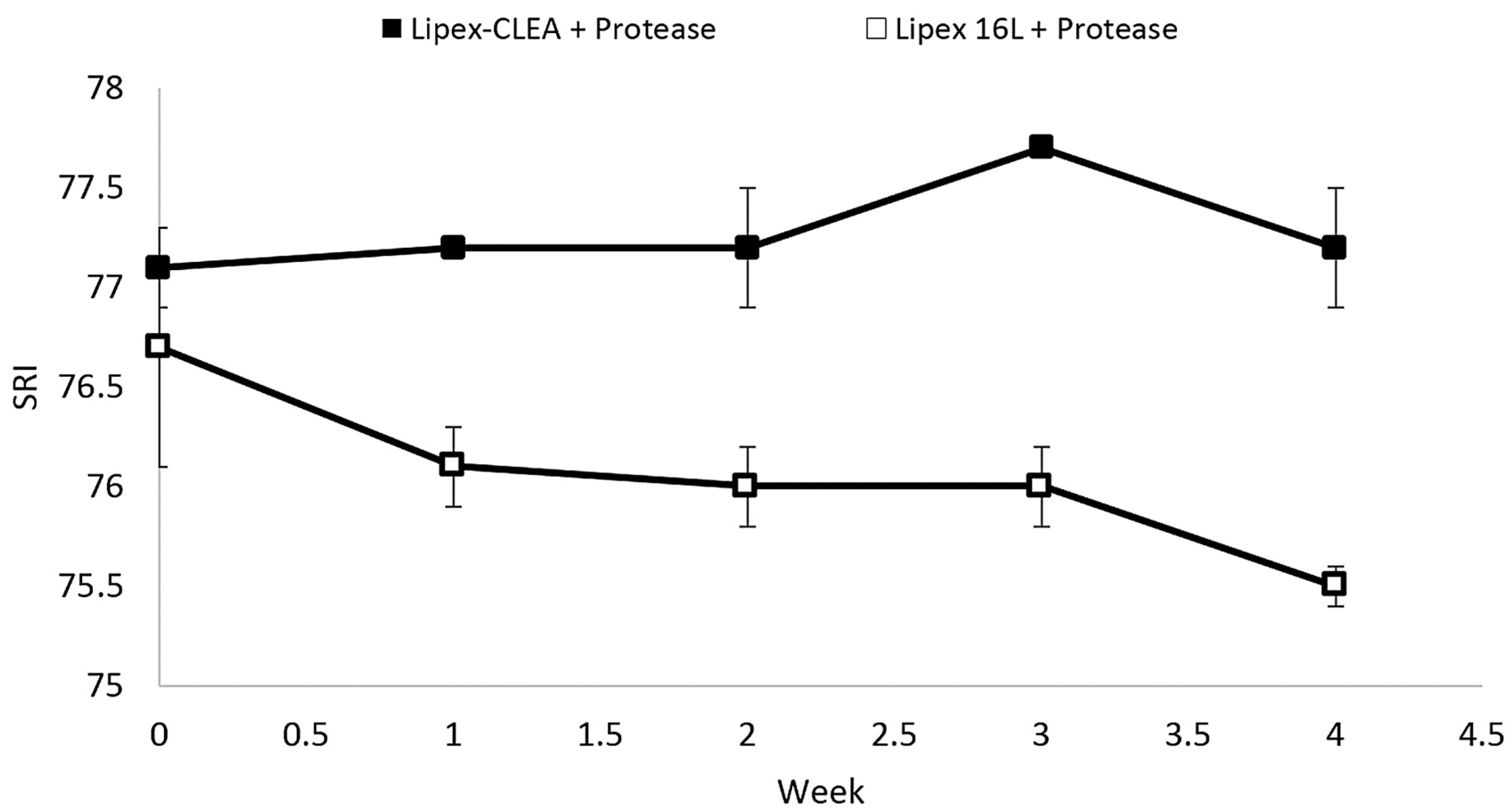
2.4. Lipex CLEA Stability
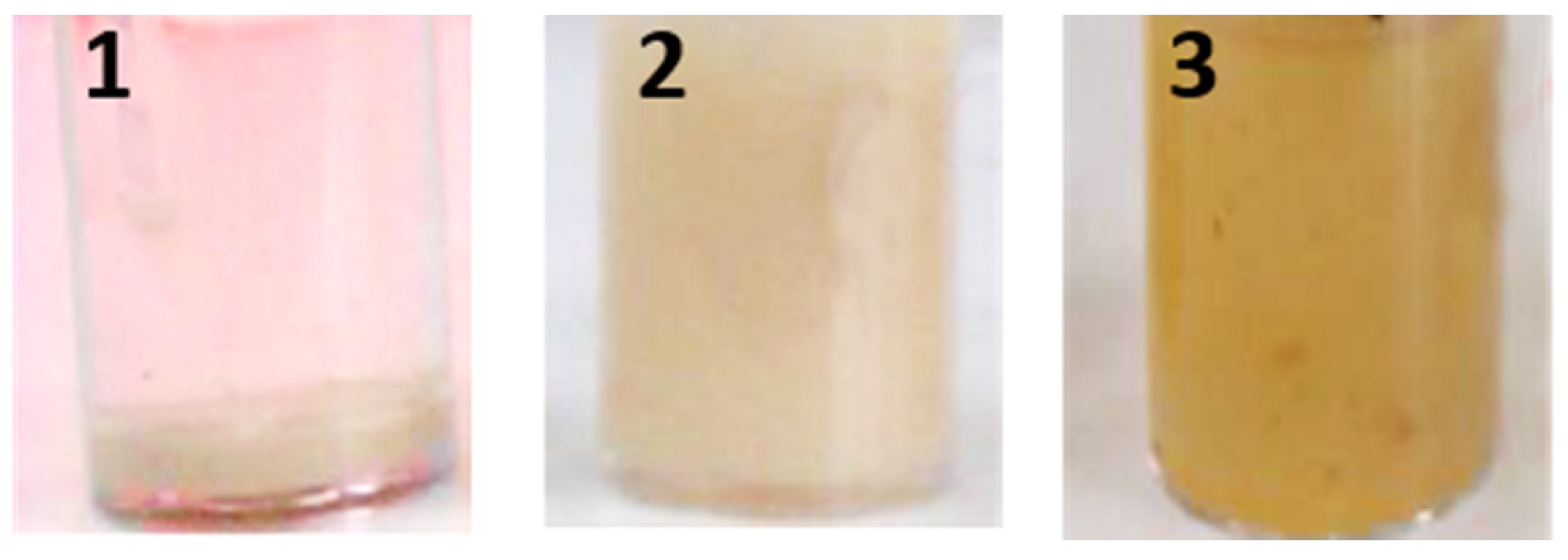
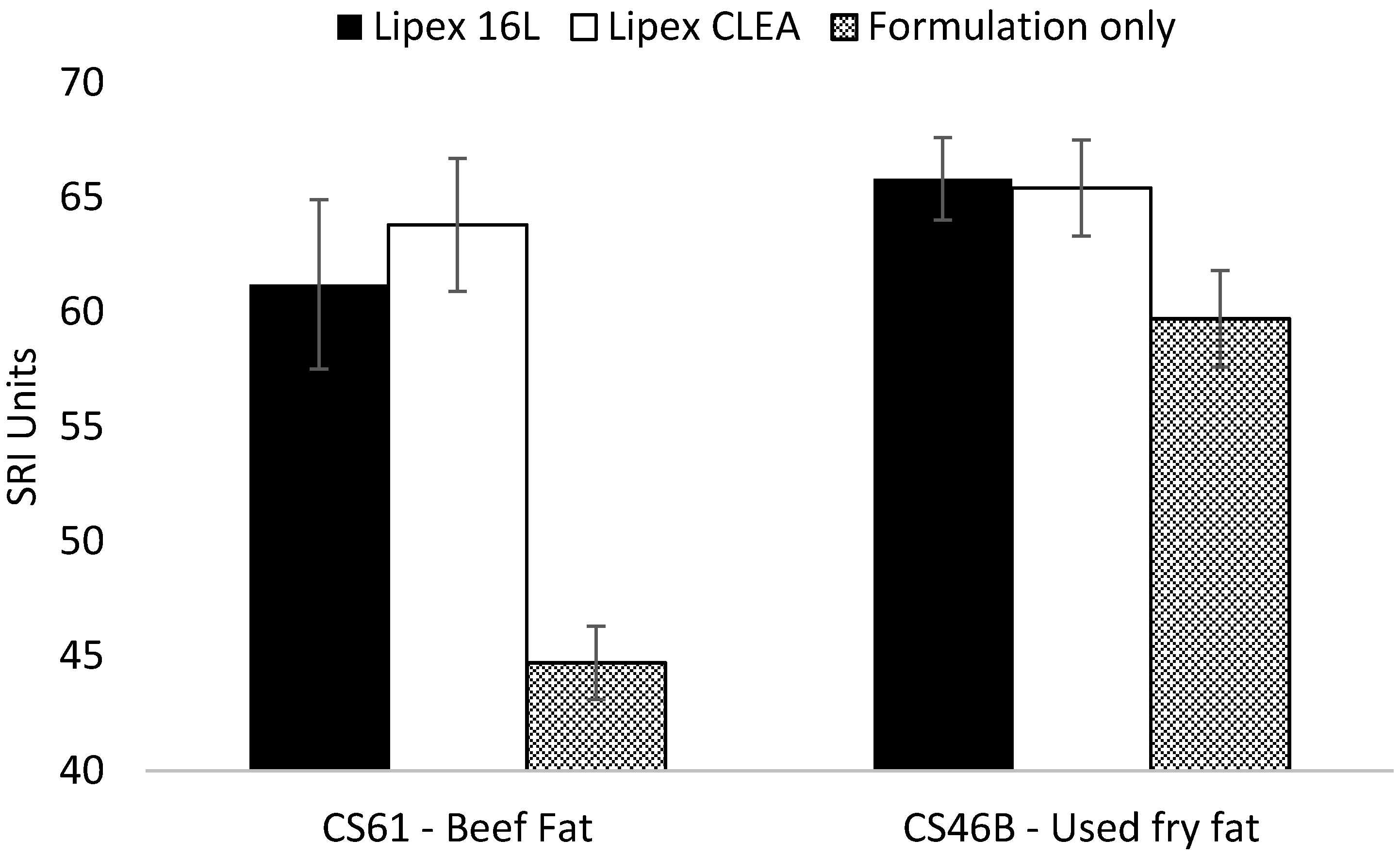
2.5. Wash Performance
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Lipex CLEA
4.3. Size Determination of the CLEA
4.4. Activity Assay
4.5. CLEAs Dispersion in Aqueous Environment
4.6. Storage Stability
4.7. Wash Performance Methods
4.7.1. Micro-Titre End-Point Stain Removal Assays
4.7.2. Terg-O-Tometer Wash Performance
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eco-friendly Detergents Boosting Unilever’s Sales · Environmental Leader · Environmental Management News. Available online: https://www.environmentalleader.com/2008/12/eco-friendly-detergents-boosting-unilevers-sales/ (accessed on 1 September 2016).
- Posorske, L.H. Industrial-Scale Application of Enzymes to the Fats and Oil Industry. J. Am. Oil Chem. Soc. 1984, 61, 1758–1760. [Google Scholar] [CrossRef]
- Denmark Ecolabelling European Ecolabel Revision of Ecolabel Criteria for Laundry Detergents. Packag. (Boston Mass.) 2009, 1–39. [CrossRef]
- Galante, Y.A.; Formantici, C. Enzyme applications in detergency and in manufacturing industries. Curr. Org. Chem. 2003, 7, 1399–1422. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ayub, M.A.Z. Effects of the combined use of Thermomyces lanuginosus and Rhizomucor miehei lipases for the transesterification and hydrolysis of soybean oil. Process Biochem. 2011, 46, 682–688. [Google Scholar] [CrossRef]
- Turan, S.; Karabulut, I.; Vural, H. Effects of reaction parameters on the incorporation of caprylic acid into soybean oil for production of structured lipids. J. Food Lipids 2006, 13, 306–317. [Google Scholar] [CrossRef]
- Vikbjerg, A.F.; Peng, L.; Mu, H.; Xu, X. Continuous production of structured phospholipids in a packed bed reactor with lipase from Thermomyces lanuginosa. J. Am. Oil Chem. Soc. 2005, 82, 237–242. [Google Scholar] [CrossRef]
- Derewenda, U.; Swenson, L.; Green, R.; Wei, Y.; Yamaguchi, S.; Joerger, R.; Haas, M.J.; Derewenda, Z.S. Current progress in crystallographic studies of new lipases from filamentous fungi. Protein Eng. 1994, 7, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lafuente, R. Lipase from Thermomyces lanuginosus: Uses and prospects as an industrial biocatalyst. J. Mol. Catal. B Enzym. 2010, 62, 197–212. [Google Scholar] [CrossRef]
- Derewenda, U.; Brzozowski, A.M.; David, M.; Lawson, Z.S.D. Catalysis at the interface: the anatomy of a conformational change in a triglyceride lipase. Biochemistry 1992, 31, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Verger, R. “Interfacial activation” of lipases: Facts and artifacts. Trends Biotechnol. 1997, 15, 32–38. [Google Scholar] [CrossRef]
- Fernandes, M.L. M.; Krieger, N.; Baron, A.M.; Zamora, P.P.; Ramos, L.P.; Mitchell, D.A. Hydrolysis and synthesis reactions catalysed by Thermomyces lanuginosa lipase in the AOT/Isooctane reversed micellar system. J. Mol. Catal. B Enzym. 2004, 30, 43–49. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzyme Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Polizzi, K.M.; Bommarius, A.S.; Broering, J.M.; Chaparro-Riggers, J.F. Stability of biocatalysts. Curr. Opin. Chem. Biol. 2007, 11, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization-Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Petersen, S.B.; Harald Jonson, P.; Fojan, P.; Petersen, E.I.; Neves Petersen, M.T.; Hansen, S.; Ishak, R.J.; Hough, E. Protein engineering the surface of enzymes. J. Biotechnol. 1998, 66, 11–26. [Google Scholar] [CrossRef]
- Scharnagl, C.; Reif, M. Stability of proteins: Temperature, pressure and the role of the solvent. Biochim. Biophys. Acta - Proteins Proteom. 2005, 1749, 187–213. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Van Pelt, S. Enzyme immobilisation in biocatalysis: why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Schoevaart, R.; Wolbers, M.W.; Golubovic, M.; Ottens, M.; Kieboom, A.P. G.; van Rantwijk, F.; Van Der Wielen, L.A.M.; Sheldon, R.A. Preparation, optimization, and structures, of cross-linked enzyme aggregates (CLEAs). Biotechnol. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Doscher, M.S.; Richards, F.M. The Activity of an Enzyme in the Crystalline State: Ribonuclease {S}. J. Biol. Chem. 1963, 238, 2399–2406. [Google Scholar]
- Schmidt, M.; Bornscheuer, U.T. High-throughput assays for lipases and esterases. Biomol. Eng. 2005, 22, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Quiocho, F.A.; Richards, F.M. Intermolecular Cross Linking of a Protein in the Crystalline State: Carboxypeptidase-a. Proc. Natl. Acad. Sci. USA 1964, 52, 833–839. [Google Scholar] [CrossRef] [PubMed]
- St Clair, N.L.; Navia, M.A. Cross-linked Enzyme Crystals as Robust Biocatalysts. Science 1992, 80, 7314–7316. [Google Scholar] [CrossRef]
- Cao, L.; van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates: a simple and effective method for the immobilization of penicillin acylase. Org. Lett. 2000, 2, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R. A Cross-linked enzyme aggregates (CLEAs): stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Kartal, F.; Kilinc, A. Crosslinked aggregates of Rhizopus oryzae lipase as industrial biocatalysts: Preparation, optimization, characterization, and application for enantioselective resolution reactions. Biotechnol. Prog. 2012, 28, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Czichocki, G.; Dautzenberg, H.; Capan, E.; Vorlop, K.-D. New and effective entrapment of polyelectrolyte-enzyme-complexes in LentiKats. Biotechnol. Lett. 2001, 23, 1303–1307. [Google Scholar] [CrossRef]
- Shah, S.; Sharma, A.; Gupta, M.N. Preparation of cross-linked enzyme aggregates by using bovine serum albumin as a proteic feeder. Anal. Biochem. 2006, 351, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Coupling chemical modification and immobilization to improve the catalytic performance of enzymes. Adv. Synth. Catal. 2011, 353, 2216–2238. [Google Scholar] [CrossRef]
- Guauque Torres, M.D. P.; Foresti, M.L.; Ferreira, M.L. Cross-linked enzyme aggregates (CLEAs) of selected lipases: a procedure for the proper calculation of their recovered activity. AMB Express 2013, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.; Dutt, K.; Misra, S.; Raghuwanshi, S.; Saxena, R.K. Characterization of cross-linked immobilized lipase from thermophilic mould Thermomyces lanuginosa using glutaraldehyde. Bioresour. Technol. 2009, 100, 4074–4076. [Google Scholar] [CrossRef] [PubMed]
- Kartal, F.; Janssen, M.H.A.; Hollmann, F.; Sheldon, R.A.; Kılınc, A. Improved esterification activity of Candida rugosa lipase in organic solvent by immobilization as Cross-linked enzyme aggregates (CLEAs). J. Mol. Catal. B Enzym. 2011, 71, 85–89. [Google Scholar] [CrossRef]
- López-Serrano, P.; Cao, L.; Van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates with enhanced activity: Application to lipases. Biotechnol. Lett. 2002, 24, 1379–1383. [Google Scholar] [CrossRef]

| Compound | Final Concentration Test (Concentration that Shows the Highest Activity Retained) | Highest Activity Retained (%) |
|---|---|---|
| Lipex 16L | - | 100.0 |
| Lipex CLEAs without compound | - | 86.3 |
| Tween 85 | 2.4% v/v | 47.5 |
| Tween 80 | 6.5–50.4 mM (19 mM) | 129.7 |
| Tween 40 | 19.5 mM | 55.6 |
| Tween 20 | 6.7–27.1 mM | 57.8 |
| Span 20 | 72.1 mM | 35.0 |
| Span 60 | 58.1 mM | 55.4 |
| Span 80 | 58.3 mM | 61.2 |
| Tributyrin | 110–661 mM (220 mM) | 36.2 |
| Sodium dodecyl sulfate | 5.8–115.6 mM (5.8 mM) | 21.6 |
| Orlistat | 67–336 µM (67 µM) | 45.7 |
| Arginine | 1.9–9.6 mM (1.9 mM) | 3.5 |
| Coconut oil | 1.65%–3.3% v/v (1.65 v/v) | 31.6 |
| Rhamnolipid | 1.6%–9.9% v/v (3.3 v/v) | 51.8 |
| Sample | Velocity (cm/s) | Diameter (µm) | Mean Diameter (µm) | Retained Activity Compared to Free Enzyme (%) |
|---|---|---|---|---|
| Lipex CLEA without terminator step | 4.86 × 10-5 | 2.99 | 4.01 | 67.9 ± 4.5 |
| 8.96 × 10-5 | 4.05 | |||
| 9.05 × 10-5 | 4.07 | |||
| 8.33 × 10-5 | 3.91 | |||
| Lipex CLEA with terminator step | 1.43 × 10-5 | 1.62 | 1.85 | 123.8 ± 8.0 |
| 1.32 × 10-5 | 1.56 | |||
| 2.84 × 10-5 | 2.28 | |||
| 2.08 × 10-5 | 1.96 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Rose, S.A.; Novak, H.; Dowd, A.; Singh, S.; Lang, D.A.; Littlechild, J. Stabilization of a Lipolytic Enzyme for Commercial Application. Catalysts 2017, 7, 91. https://doi.org/10.3390/catal7030091
De Rose SA, Novak H, Dowd A, Singh S, Lang DA, Littlechild J. Stabilization of a Lipolytic Enzyme for Commercial Application. Catalysts. 2017; 7(3):91. https://doi.org/10.3390/catal7030091
Chicago/Turabian StyleDe Rose, Simone Antonio, Halina Novak, Andrew Dowd, Sukriti Singh, Dietmar Andreas Lang, and Jennifer Littlechild. 2017. "Stabilization of a Lipolytic Enzyme for Commercial Application" Catalysts 7, no. 3: 91. https://doi.org/10.3390/catal7030091






