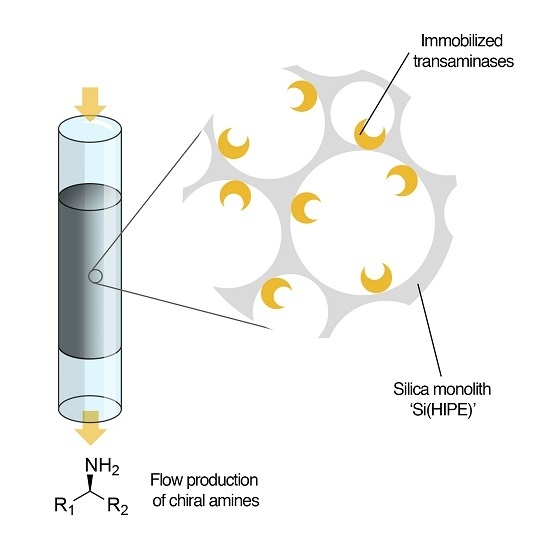
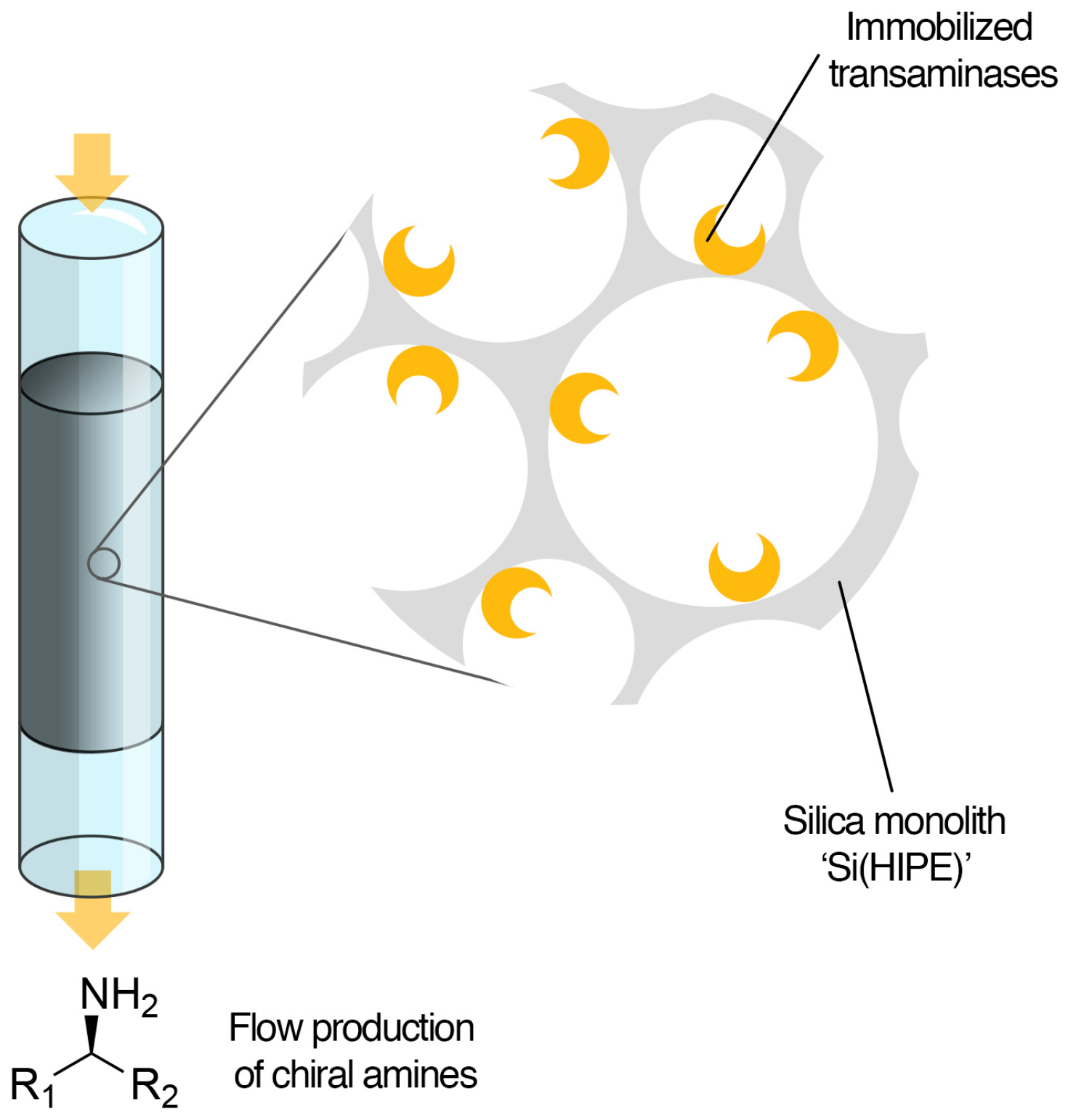
Enantioselective Transamination in Continuous Flow Mode with Transaminase Immobilized in a Macrocellular Silica Monolith
Abstract
:1. Introduction
2. Results and Discussion
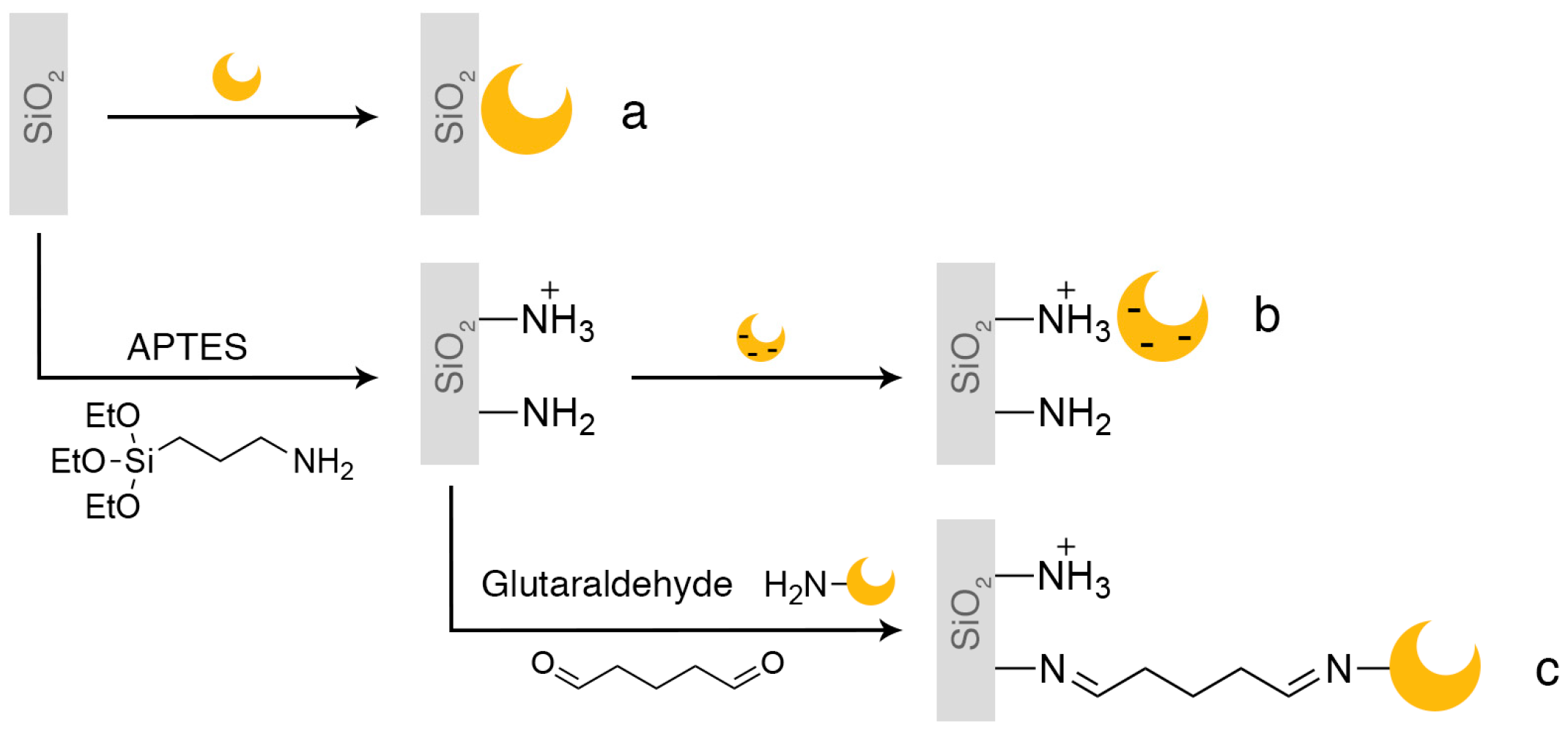
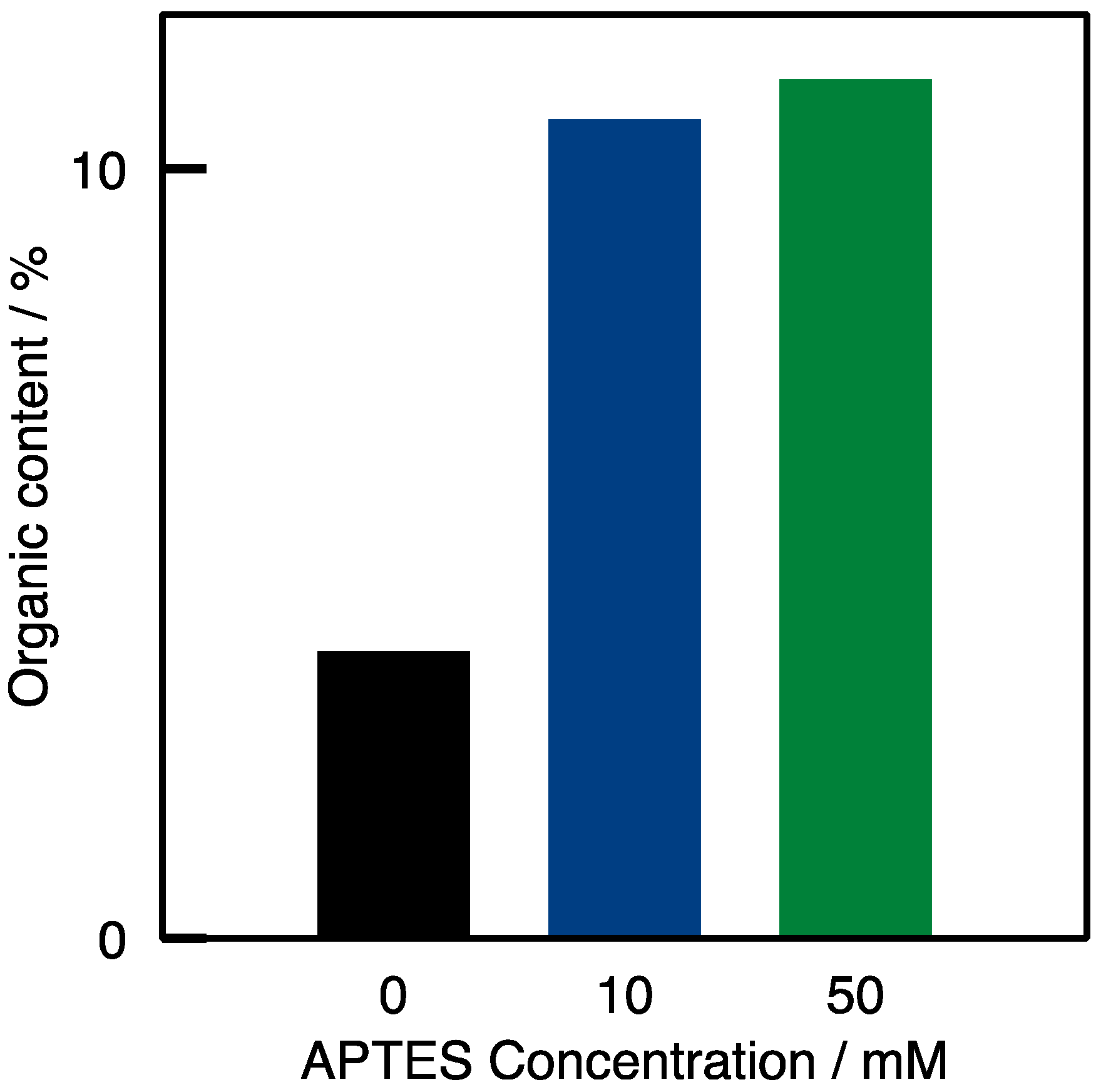
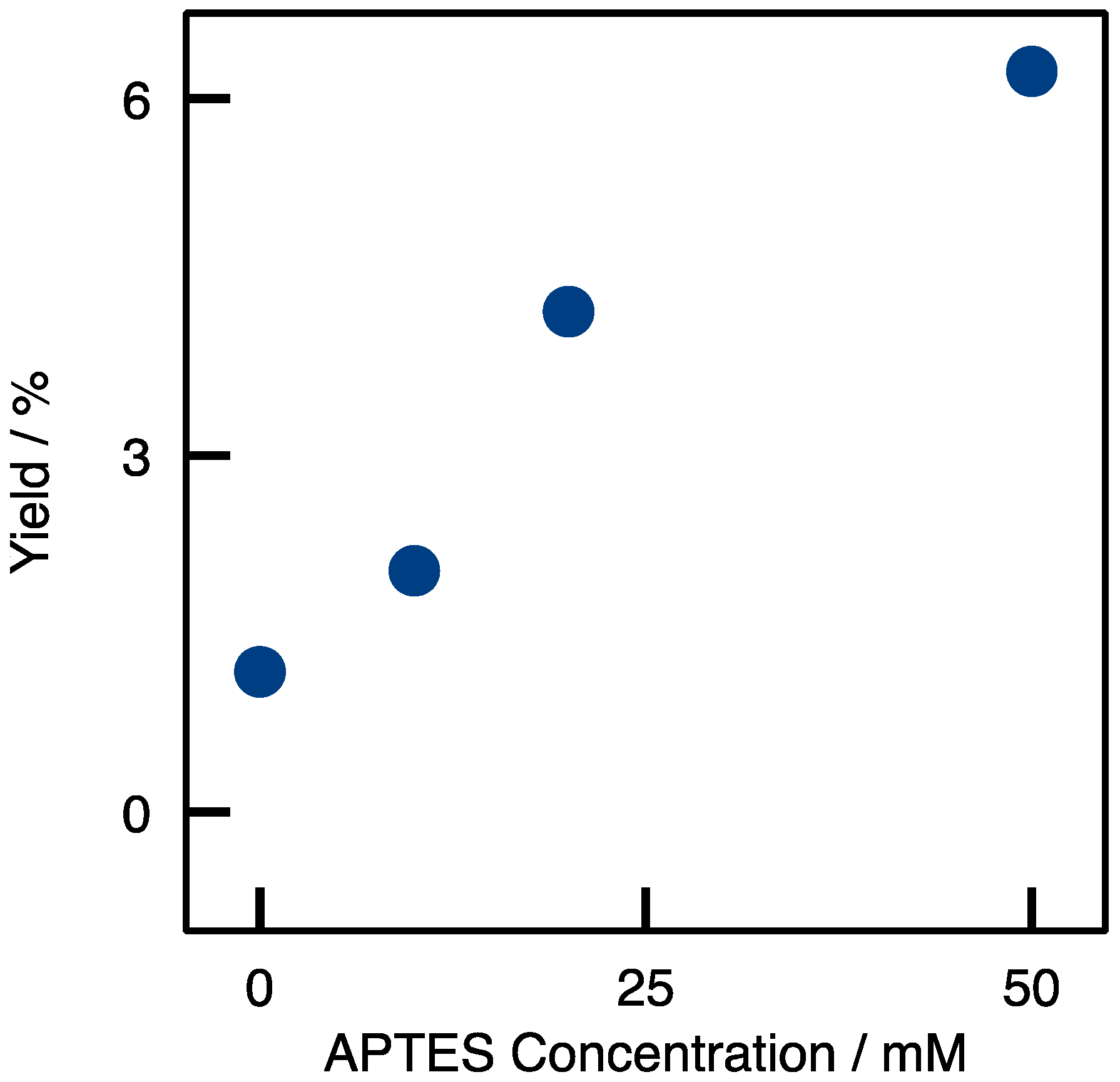
2.1. Enzyme Immobilization on Si(HIPE) Monoliths
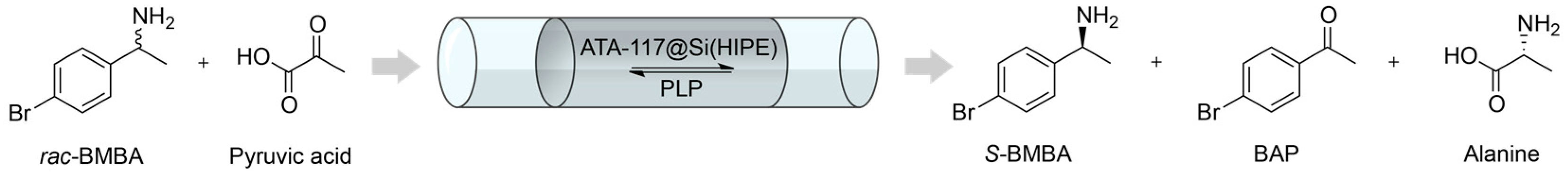
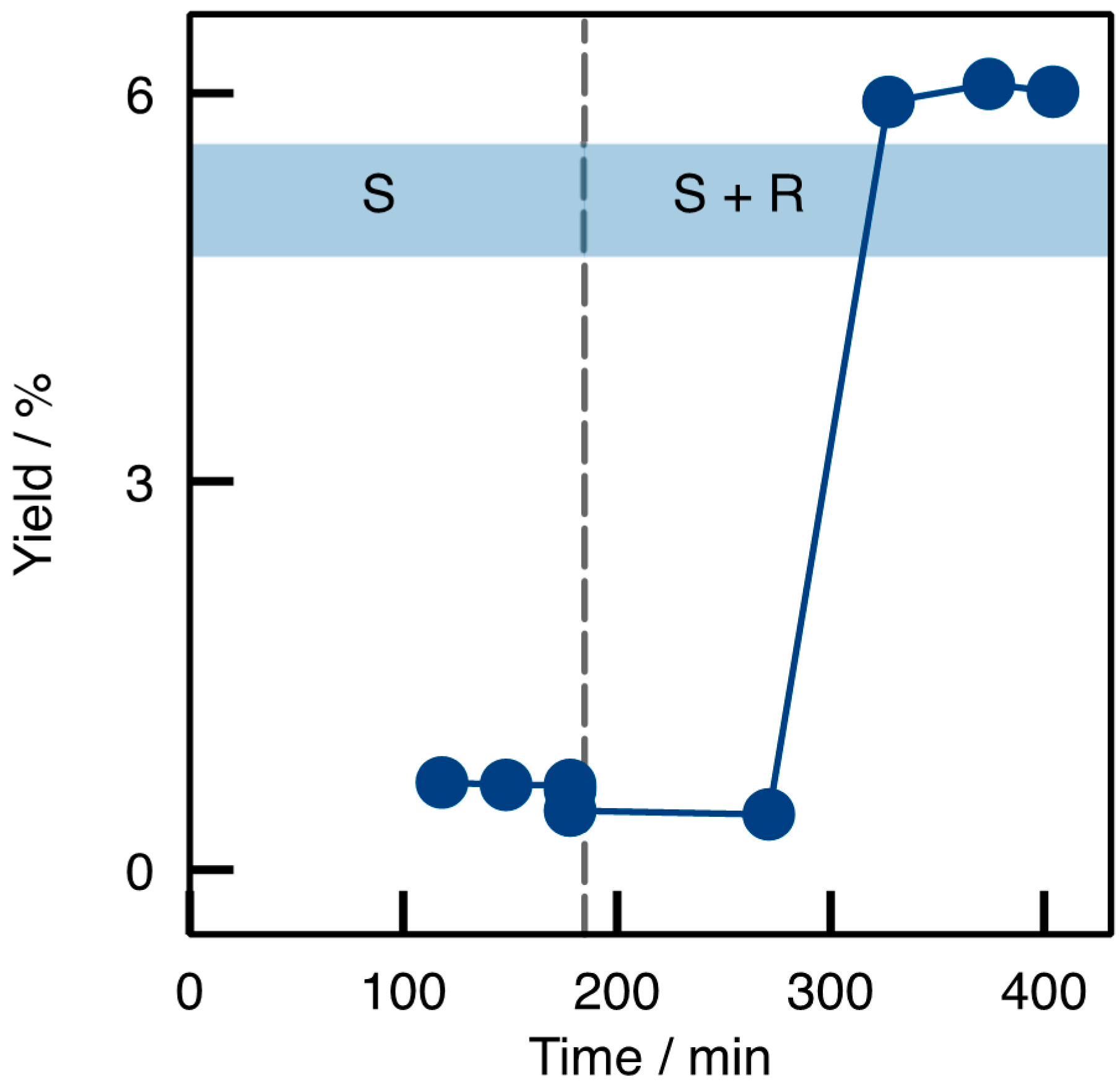
2.2. Transaminations in Continuous Flow Mode
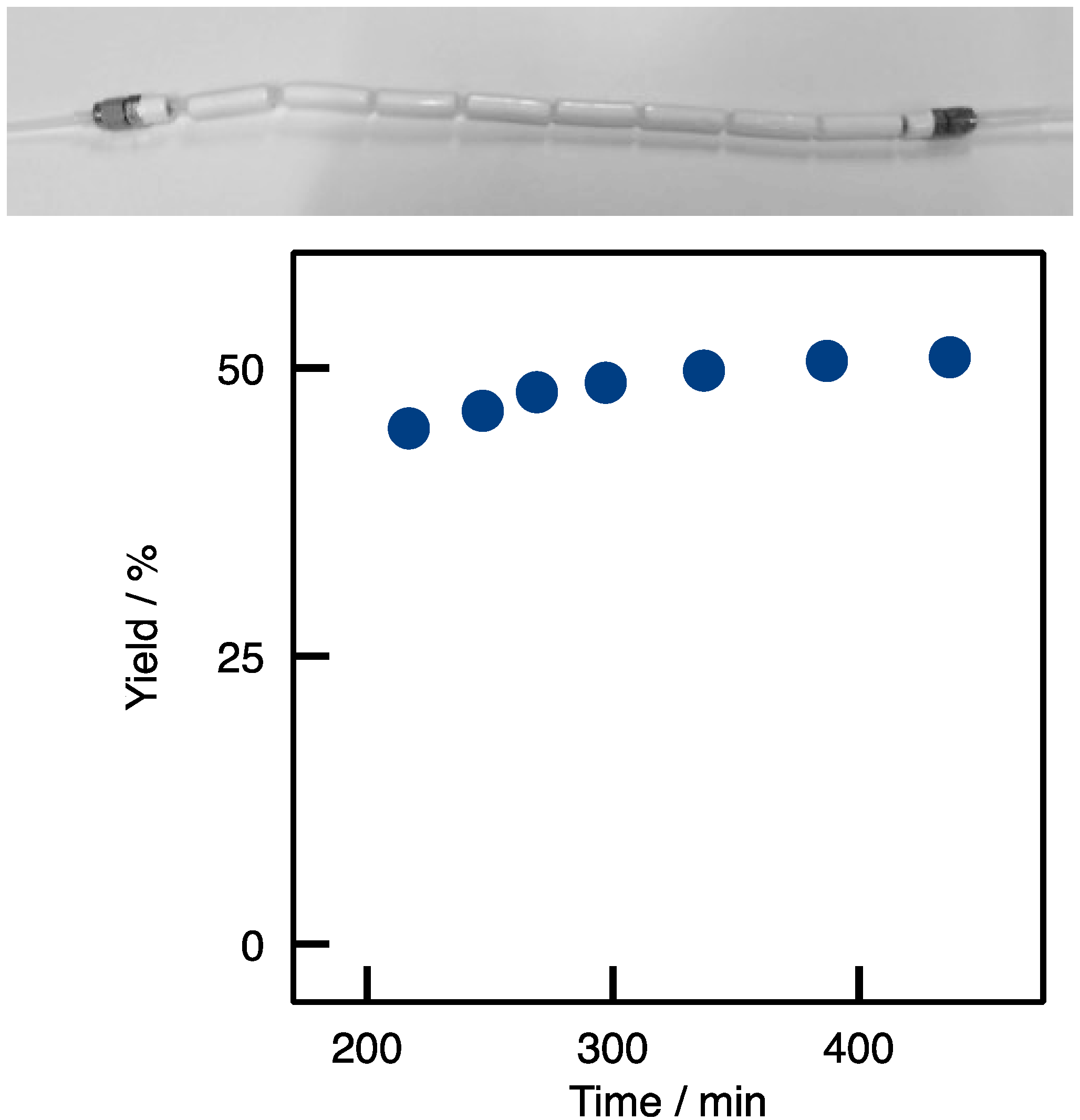
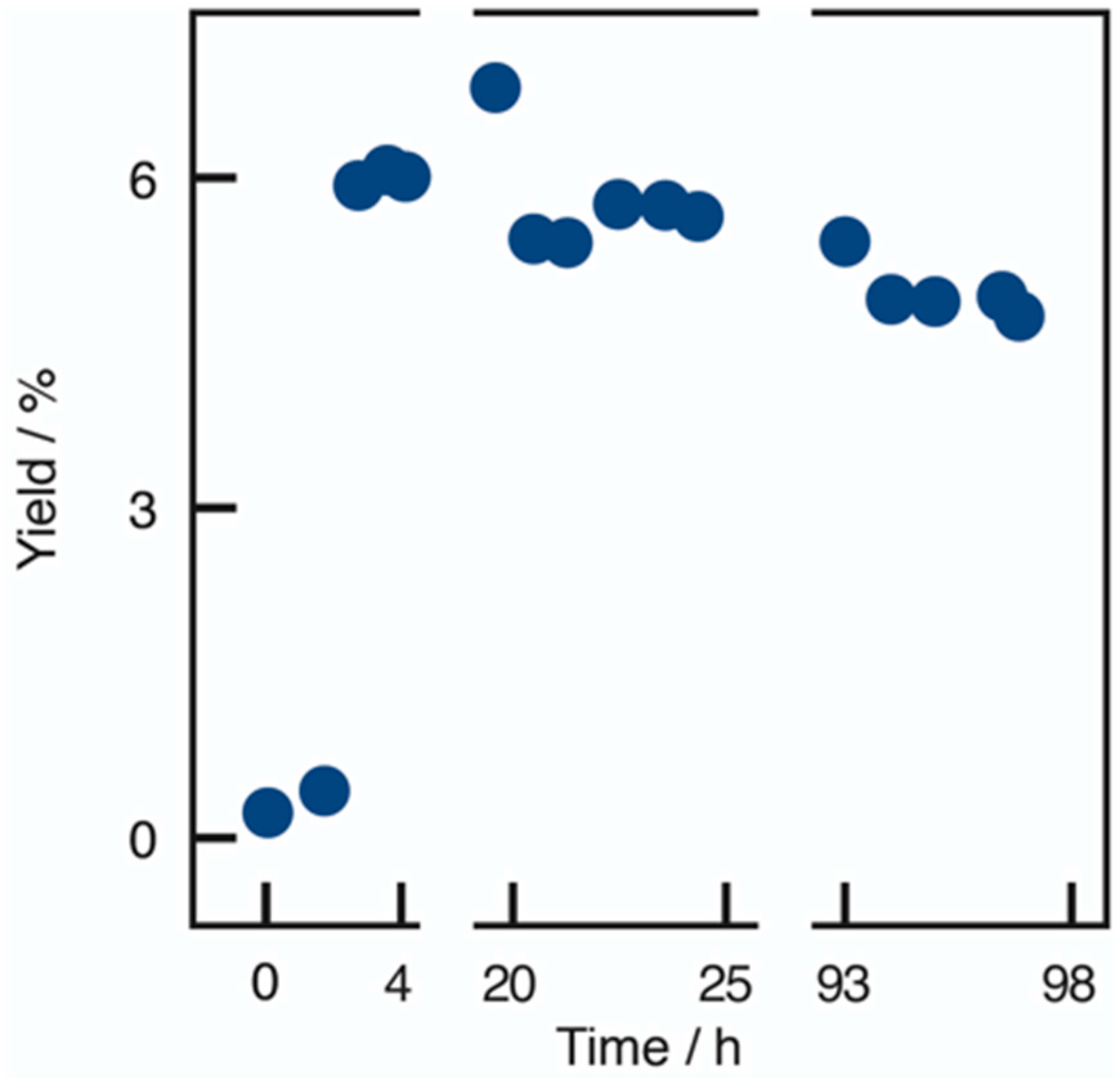
2.3. Applicability and Robustness of the Biocatalytic Flow Reactor
3. Materials and Methods
3.1. Materials
3.2. Support Synthesis—Si(HIPE) Monolith
3.3. Functionalization of the Monolith Surface
3.4. Characterizations
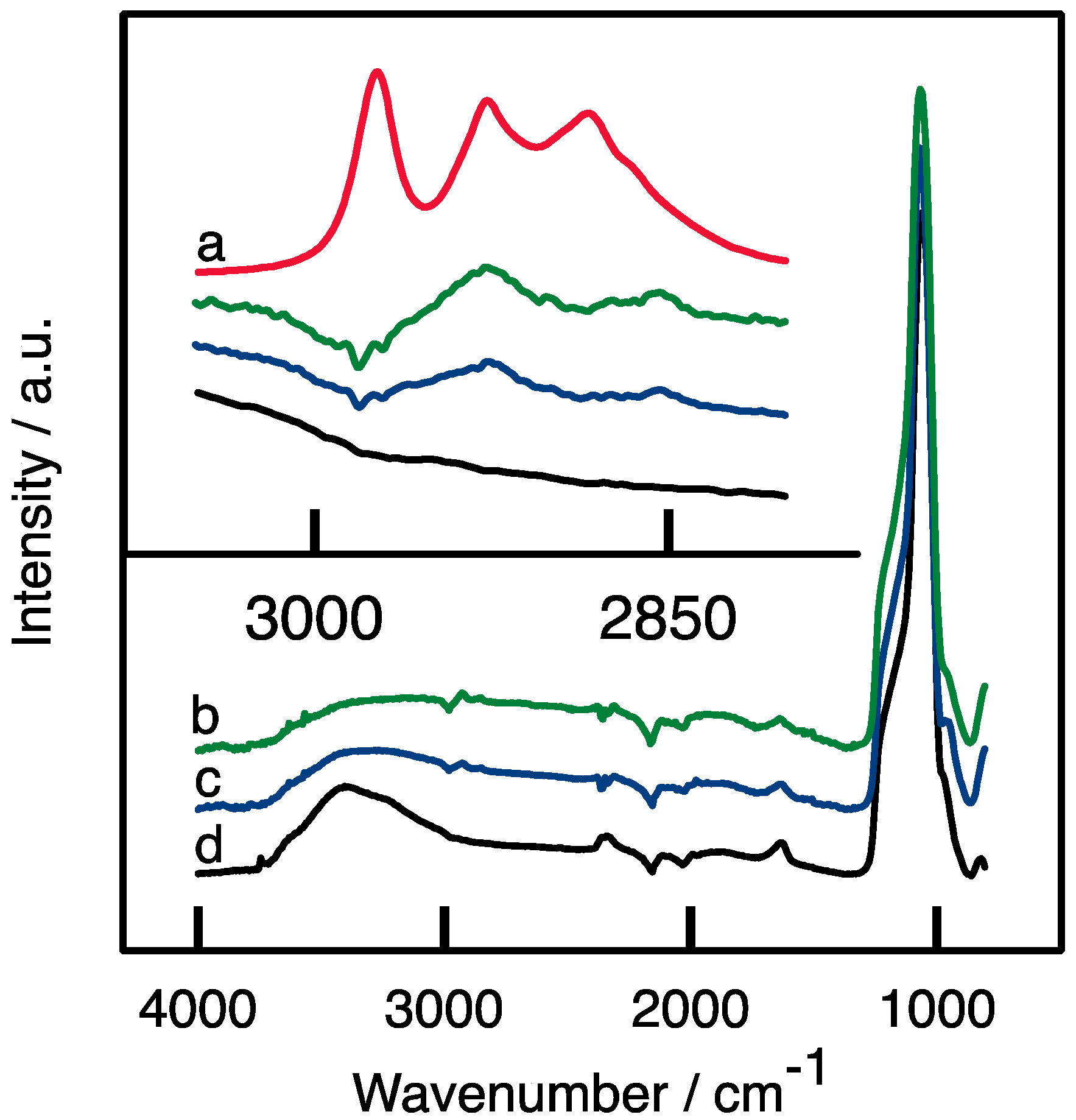
3.4.1. Attenuated Total Reflectance—InfraRed spectroscopy (ATR-FTIR)
3.4.2. Thermo Gravimetric Analysis (TGA)
3.4.3. Scanning Electron Microscopy (SEM)
3.4.4. Nitrogen Physisorption
3.5. Transamination Activity Determination
3.6. Enantiomeric Excess Determination
3.7. Enzyme Quantitation
3.8. Dynamic Light Scattering (DLS)
3.9. Flow Reactions
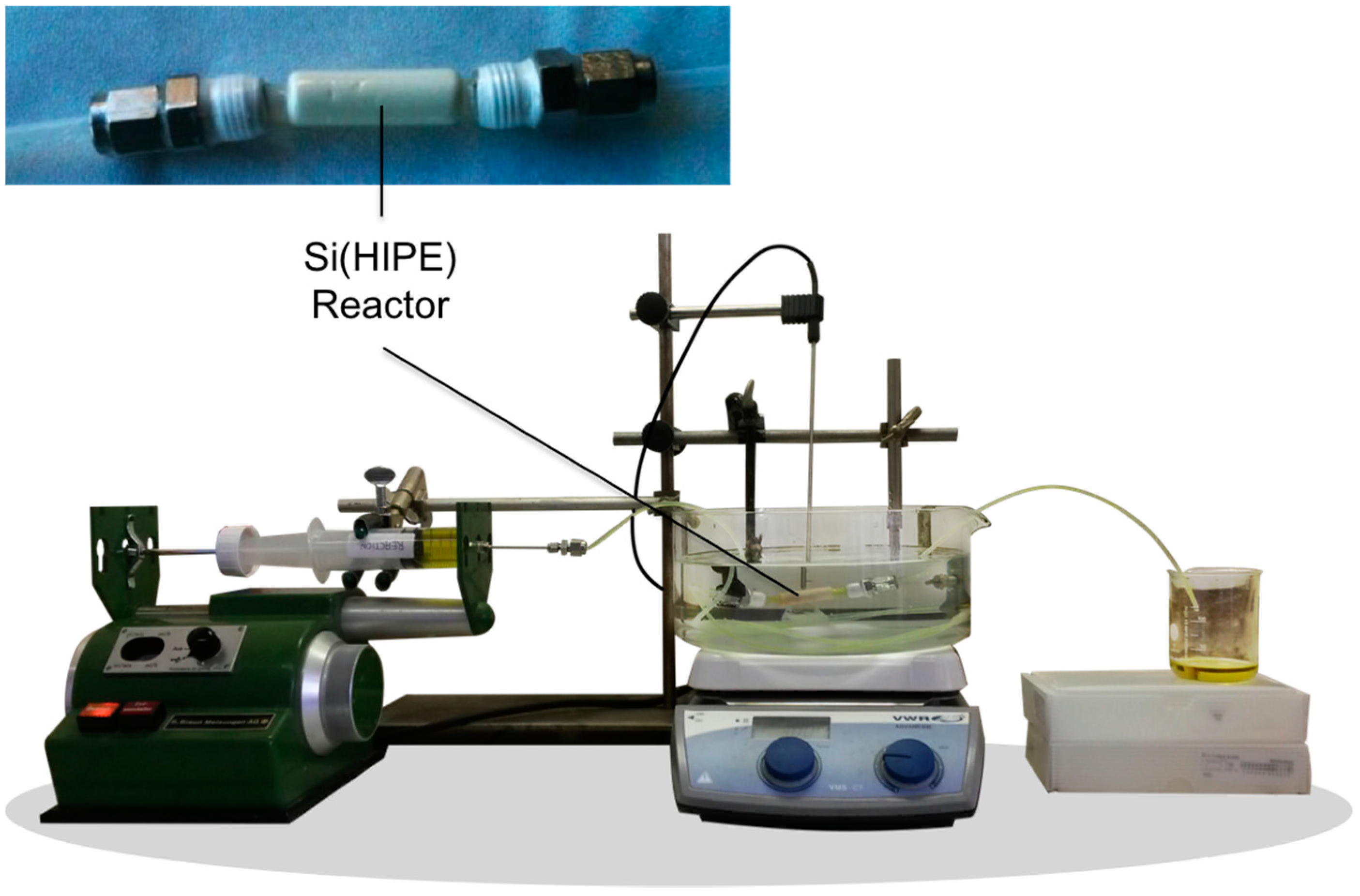
3.9.1. Flow Reactor
3.9.2. Transaminases Immobilization
3.9.3. Flow Transamination Reaction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bornscheuer, U.T. Immobilizing enzymes: How to create more suitable biocatalysts. Angew. Chem. Int. Ed. 2003, 42, 3336–3337. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Tiwari, M.K.; Singh, R.; Lee, J.K. From protein engineering to immobilization: Promising strategies for the upgrade of industrial enzymes. Int. J. Mol. Sci. 2013, 14, 1232–1277. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Cao, L. Carrier-Bound Immobilized Enzymes; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Savile, C.K.; Janey, J.M.; Mundorff, E.C.; Moore, J.C.; Tam, S.; Jarvis, W.R.; Colbeck, J.C.; Krebber, A.; Fleitz, F.J.; Brands, J.; et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science 2010, 329, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Sehl, T.; Hailes, H.C.; Ward, J.M.; Wardenga, R.; von Lieres, E.; Offermann, H.; Westphal, R.; Pohl, M.; Rother, D. Two steps in one pot: Enzyme cascade for the synthesis of nor(pseudo)ephedrine from inexpensive starting materials. Angew. Chem. Int. Ed. 2013, 52, 6772–6775. [Google Scholar] [CrossRef] [PubMed]
- Girardin, M.; Ouellet, S.G.; Gauvreau, D.; Moore, J.C.; Hughes, G.; Devine, P.N.; O’Shea, P.D.; Campeau, L.C. Convergent kilogram-scale synthesis of dual orexin receptor antagonist. Org. Process Res. Dev. 2012, 17, 61–68. [Google Scholar] [CrossRef]
- Cárdenas-Fernández, M.; Khalikova, E.; Korpela, T.; López, C.; Álvaro, G. Co-immobilised aspartase and transaminase for high-yield synthesis of l-phenylalanine. Biochem. Eng. J. 2015, 93, 173–178. [Google Scholar] [CrossRef]
- Yi, S.S.; Lee, C.W.; Kim, J.; Kyung, D.; Kim, B.G.; Lee, Y.S. Covalent immobilization of ω-transaminase from Vibrio fluvialis JS17 on chitosan beads. Process Biochem. 2007, 42, 895–898. [Google Scholar] [CrossRef]
- Truppo, M.D.; Strotman, H.; Hughes, G. Development of an Immobilized Transaminase Capable of Operating in Organic Solvent. ChemCatChem 2012, 4, 1071–1074. [Google Scholar] [CrossRef]
- Mallin, H.; Menyes, U.; Vorhaben, T.; Höhne, M.; Bornscheuer, U.T. Immobilization of two (R)-Amine Transaminases on an Optimized Chitosan Support for the Enzymatic Synthesis of Optically Pure Amines. ChemCatChem 2013, 5, 588–593. [Google Scholar] [CrossRef]
- Mallin, H.; Höhne, M.; Bornscheuer, U.T. Immobilization of (R)- and (S)-amine transaminases on chitosan support and their application for amine synthesis using isopropylamine as donor. J. Biotechnol. 2014, 191, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Hyeon, T. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Koszelewski, D.; Müller, N.; Schrittwieser, J.H.; Faber, K.; Kroutil, W. Immobilization of ω-transaminases by encapsulation in a sol–gel/celite matrix. J. Mol. Catal. B Enzym. 2010, 63, 39–44. [Google Scholar] [CrossRef]
- Yang, L.; Shi, J.; Chen, C.; Wang, S.; Zhu, L.; Xie, W.; Guo, L. Dual-enzyme, co-immobilized capillary microreactor combined with substrate recycling for high-sensitive glutamate determination based on CE. Electrophoresis 2009, 30, 3527–3533. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. The E Factor: Fifteen years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Jas, G.; Kirschning, A. Continuous Flow Techniques in Organic Synthesis. Chem. Eur. J. 2003, 9, 5708–5723. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.H.; Kroutil, W.; Jamison, T.F. Continuous Flow Synthesis of Chiral Amines in Organic Solvents: Immobilization of E. coli Cells Containing Both ω-Transaminase and PLP. Org. Lett. 2014, 16, 6092–6095. [Google Scholar]
- Debecker, D.P.; Boissière, C.; Laurent, G.; Huet, S.; Eliaers, P.; Sanchez, C.; Backov, R. First acidic macro-mesocellular aluminosilicate monolithic foams “SiAl(HIPE)” and their catalytic properties. Chem. Commun. 2015, 51, 14018–14021. [Google Scholar] [CrossRef] [PubMed]
- Brun, N.; Babeau-Garcia, A.; Achard, M.-F.; Sanchez, C.; Durand, F.; Laurent, G.; Birot, M.; Deleuze, H.; Backov, R. Enzyme-based biohybrid foams designed for continuous flow heterogeneous catalysis and biodiesel production. Energy Environ. Sci. 2011, 4, 2840–2844. [Google Scholar] [CrossRef]
- Ungureanu, S.; Laurent, G.; Deleuze, H.; Babot, O.; Achard, M.-F.; Popa, M.I.; Sanchez, C.; Backov, R. Syntheses and characterization of new organically grafted silica foams. Colloids Surfacea A 2010, 360, 85–93. [Google Scholar] [CrossRef]
- Kirschning, A.; Solodenko, W.; Mennecke, K. Combining enabling techniques in organic synthesis: Continuous flow processes with heterogenized catalysts. Chem. Eur. J. 2006, 12, 5972–5990. [Google Scholar] [CrossRef] [PubMed]
- Nandiyanto, A.B.D.; Kim, S.G.; Iskandar, F.; Okuyama, K. Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameters. Microporous Mesoporous Mater. 2009, 120, 447–453. [Google Scholar] [CrossRef]
- Lazghab, M.; Saleh, K.; Guigon, P. Functionalisation of porous silica powders in a fluidised-bed reactor with glycidoxypropyltrimethoxysilane (GPTMS) and aminopropyltriethoxysilane (APTES). Chem. Eng. Res. Des. 2010, 88, 686–692. [Google Scholar] [CrossRef]
- Gaffney, D.; Cooney, J.; Magner, E. Modification of mesoporous silicates for immobilization of enzymes. Top. Catal. 2012, 55, 1101–1106. [Google Scholar] [CrossRef]
- Bai, Y.-X.; Li, Y.-F.; Yang, Y.; Yi, L.-X. Covalent immobilization of triacylglycerol lipase onto functionalized novel mesoporous silica supports. Process Biochem. 2006, 41, 770–777. [Google Scholar] [CrossRef]
- Brun, N.; Babeau Garcia, A.; Deleuze, H.; Achard, M.F.; Sanchez, C.; Durand, F.; Oestreicher, V.; Backov, R. Enzyme-based hybrid macroporous foams as highly efficient biocatalysts obtained through integrative chemistry. Chem. Mater. 2010, 22, 4555–4562. [Google Scholar] [CrossRef]
- Zaidan, U.H.; Rahman, M.B.A.; Basri, M.; Othman, S.S.; Rahman, R.N.Z.R.A.; Salleh, A.B. Silylation of mica for lipase immobilization as biocatalysts in esterification. Appl. Clay Sci. 2010, 47, 276–282. [Google Scholar] [CrossRef]
- Tran, D.N.; Balkus, K.J. Perspective of recent progress in immobilization of enzymes. ACS Catal. 2011, 1, 956–968. [Google Scholar] [CrossRef]
- Pasternack, R.M.; Amy, S.R.; Chabal, Y.J. Attachment of 3-(Aminopropyl) triethoxysilane on Silicon Oxide Surfaces: Dependence on Solution Temperature. Langmuir 2008, 24, 12963–12971. [Google Scholar] [CrossRef] [PubMed]
- Putz, A.M.; Putz, M.V. Spectral inverse quantum (Spectral-IQ) method for modeling mesoporous systems: Application on Silica films by FTIR. Int. J. Mol. Sci. 2012, 13, 15925–15941. [Google Scholar] [CrossRef] [PubMed]
- Etienne, M.; Walcarius, A. Analytical investigation of the chemical reactivity and stability of aminopropyl-grafted silica in aqueous medium. Talanta 2003, 59, 1173–1188. [Google Scholar] [CrossRef]
- Kursunlu, A.N.; Guler, E.; Dumrul, H.; Kocyigit, O.; Gubbuk, I.H. Chemical modification of silica gel with synthesized new Schiff base derivatives and sorption studies of cobalt (II) and nickel (II). Appl. Surf. Sci. 2009, 255, 8798–8803. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, L.; Wen, X. Mesoporous silicas synthesis and application for lignin peroxidase immobilization by covalent binding method. J. Environ. Sci. 2012, 25, 181–187. [Google Scholar] [CrossRef]
- Tufvesson, P.; Jensen, J.S.; Kroutil, W.; Woodley, J.M. Experimental determination of thermodynamic equilibrium in biocatalytic transamination. Biotechnol. Bioeng. 2012, 109, 2159–2162. [Google Scholar] [CrossRef] [PubMed]
- Tufvesson, P.; Bach, C.; Woodley, J.M. A model to assess the feasibility of shifting reaction equilibrium by acetone removal in the transamination of ketones using 2-propylamine. Biotechnol. Bioeng. 2014, 111, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zor, T.; Selinger, Z. Linearization of the Bradford Protein Assay Increases Its Sensitivity: Theoretical and Experimental Studies. Anal. Biochem. 1996, 236, 302–308. [Google Scholar] [CrossRef] [PubMed]
 ) Si(HIPE); (
) Si(HIPE); (  ) TA-A10; (▲) TA-A10-GA; and (◆), reagent solution (containing rac-BMBA).
) TA-A10; (▲) TA-A10-GA; and (◆), reagent solution (containing rac-BMBA).
 ) Si(HIPE); (
) Si(HIPE); (  ) TA-A10; (▲) TA-A10-GA; and (◆), reagent solution (containing rac-BMBA).
) TA-A10; (▲) TA-A10-GA; and (◆), reagent solution (containing rac-BMBA).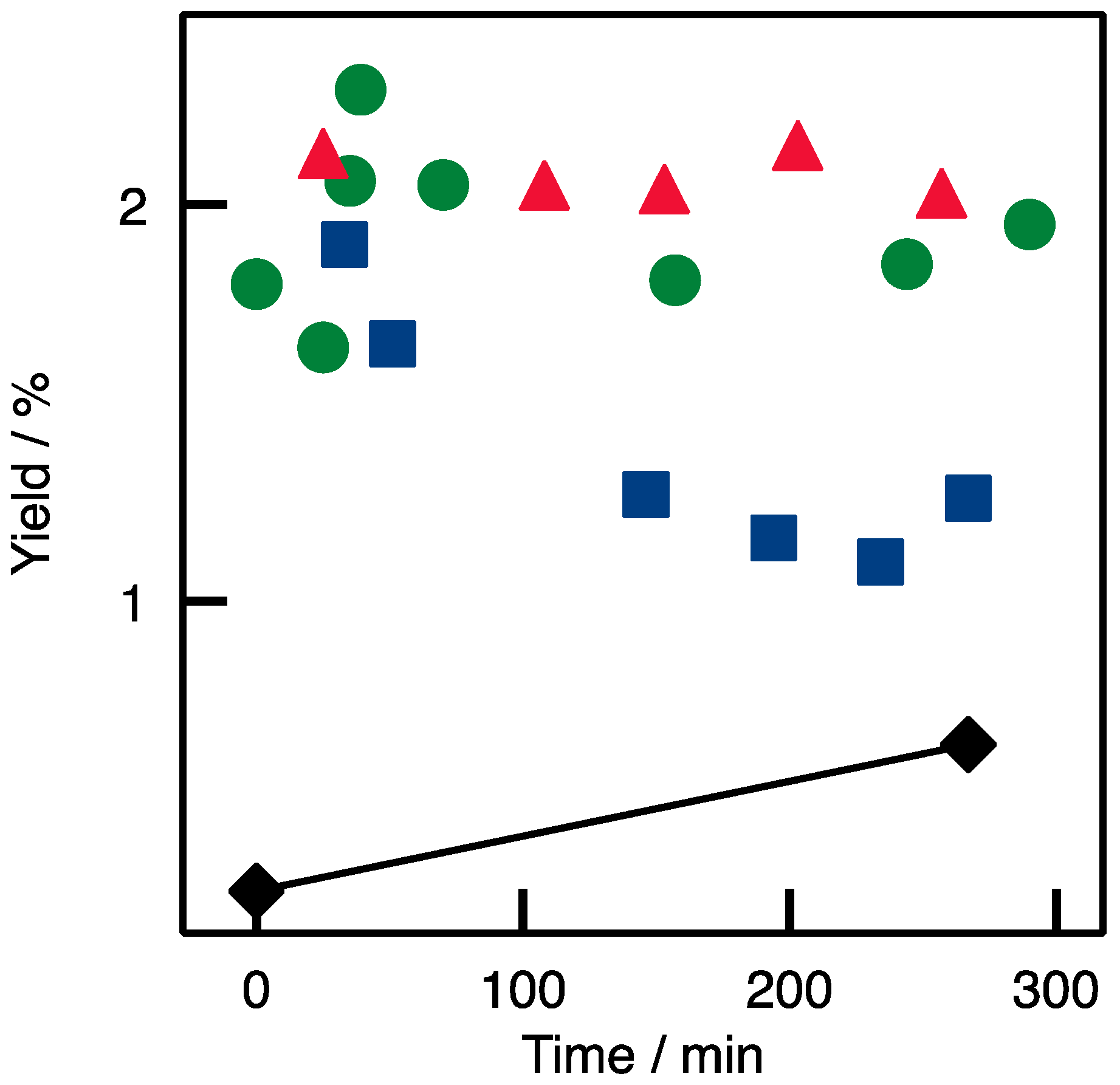
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van den Biggelaar, L.; Soumillion, P.; Debecker, D.P. Enantioselective Transamination in Continuous Flow Mode with Transaminase Immobilized in a Macrocellular Silica Monolith. Catalysts 2017, 7, 54. https://doi.org/10.3390/catal7020054
Van den Biggelaar L, Soumillion P, Debecker DP. Enantioselective Transamination in Continuous Flow Mode with Transaminase Immobilized in a Macrocellular Silica Monolith. Catalysts. 2017; 7(2):54. https://doi.org/10.3390/catal7020054
Chicago/Turabian StyleVan den Biggelaar, Ludivine, Patrice Soumillion, and Damien P. Debecker. 2017. "Enantioselective Transamination in Continuous Flow Mode with Transaminase Immobilized in a Macrocellular Silica Monolith" Catalysts 7, no. 2: 54. https://doi.org/10.3390/catal7020054