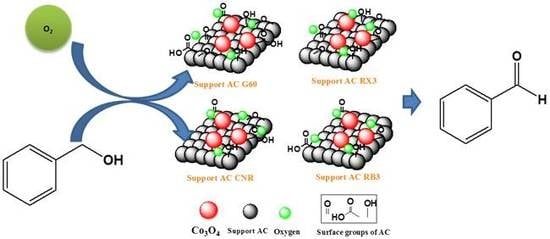
Catalytic Performance of Co3O4 on Different Activated Carbon Supports in the Benzyl Alcohol Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Supports and Catalysts
2.2. Catalytic Tests
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Larock, R.C. Comprehensive Organic Transformations; CVH: New York, NY, USA, 1999; pp. 1234–1250. [Google Scholar]
- Zhan, B.-Z.; Thompson, A. Recent developments in the aerobic oxidation of alcohols. Tetrahedron 2004, 60. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Jayaram, R.V. Liquid phase catalytic oxidation of alcohols over mixed metal oxides. Catal. Commun. 2008, 9, 186–193. [Google Scholar] [CrossRef]
- Steves, J.E.; Stahl, S.S. Copper-Catalyzed Aerobic Alcohol Oxidation. In Liquid Phase Aerobic Oxidation Catalysis: Industrial Applications and Academic Perspectives; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2016; pp. 85–96. [Google Scholar]
- Arends, I.W.C.E.; Sheldon, R.A. Modern Oxidation of Alcohols Using Environmentally Benign Oxidants. In Modern Oxidation Methods; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2010; pp. 147–185. [Google Scholar]
- Villa, A.; Wang, D.; Dimitratos, N.; Su, D.; Trevisan, V.; Prati, L. Pd on carbon nanotubes for liquid phase alcohol oxidation. Catal. Today 2010, 150, 8–15. [Google Scholar] [CrossRef]
- Korovchenko, P.; Donze, C.; Gallezot, P.; Besson, M. Oxidation of primary alcohols with air on carbon-supported platinum catalysts for the synthesis of aldehydes or acids. Catal. Today 2007, 121, 13–21. [Google Scholar] [CrossRef]
- Wang, B.; Lin, M.; Ang, T.P.; Chang, J.; Yang, Y.; Borgna, A. Liquid phase aerobic oxidation of benzyl alcohol over Pd and Rh catalysts on N-doped mesoporous carbon: Effect of the surface acido-basicity. Catal. Commun. 2012, 25, 96–101. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Qiu, J. Aerobic oxidation of alcohols over carbon nanotube-supported Ru catalysts assembled at the interfaces of emulsion droplets. Appl. Catal. A Gen. 2010, 382, 131–137. [Google Scholar] [CrossRef]
- Cruz, P.; Pérez, Y.; del Hierro, I.; Fajardo, M. Copper, copper oxide nanoparticles and copper complexes supported on mesoporous SBA-15 as catalysts in the selective oxidation of benzyl alcohol in aqueous phase. Microporous Mesoporous Mater. 2016, 220, 136–147. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, X.; Chen, Y.; Liu, T.; Yang, Y. Characterization and catalytic application of highly dispersed manganese oxides supported on activated carbon. J. Mol. Catal. A Chem. 2009, 301, 24–30. [Google Scholar] [CrossRef]
- Punniyamurthy, T.; Velusamy, S.; Iqbal, J. Recent Advances in Transition Metal Catalyzed Oxidation of Organic Substrates with Molecular Oxygen. Chem. Rev. 2005, 105, 2329–2364. [Google Scholar] [CrossRef] [PubMed]
- Taghavimoghaddam, J.; Knowles, G.P.; Chaffee, A.L. Preparation and characterization of mesoporous silica supported cobalt oxide as a catalyst for the oxidation of cyclohexanol. J. Mol. Catal. A Chem. 2012, 258, 79–88. [Google Scholar] [CrossRef]
- Zhu, J.; Faria, J.L.; Figueiredo, J.L.; Thomas, A. Reaction Mechanism of Aerobic Oxidation of Alcohols Conducted on Activated-Carbon-Supported Cobalt Oxide Catalysts. Chem. A Eur. J. 2011, 17, 7112–7117. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, S.; Hu, J.; Fu, X.; Peng, L.; Kan, Q.; Huo, Q.; Guan, J. Highly efficient N-doped magnetic cobalt-graphene composite for selective oxidation of benzyl alcohol. Catal. Commun. 2016, 87, 90–93. [Google Scholar] [CrossRef]
- Nie, R.; Shi, J.; Du, W.; Ning, W.; Hou, Z.; Xiao, F.-S. A sandwich N-doped graphene/Co3O4 hybrid: An efficient catalyst for selective oxidation of olefins and alcohols. J. Mater. Chem. A 2013, 1, 9037–9045. [Google Scholar] [CrossRef]
- Pan, C.-J.; Tsai, M.-C.; Su, W.-N.; Rick, J.; Akalework, N.G.; Agegnehu, A.K.; Cheng, S.-Y.; Hwang, B.-J. Tuning/exploiting Strong Metal-Support Interaction (SMSI) in Heterogeneous Catalysis. J. Taiwan Inst. Chem. Eng. 2017, 74, 154–186. [Google Scholar] [CrossRef]
- Qiao, D.; Xu, C.; Xu, J. Aerobic oxidation of benzyl alcohol over Co3O4/rehydrated hydrotalcite catalysts: The promotional effect of hydrotalcite support. Catal. Commun. 2014, 45, 44–48. [Google Scholar] [CrossRef]
- Zhu, J.; Kailasam, K.; Fischer, A.; Thomas, A. Supported Cobalt Oxide Nanoparticles As Catalyst for Aerobic Oxidation of Alcohols in Liquid Phase. ACS Catal. 2011, 1, 342–347. [Google Scholar] [CrossRef]
- Shi, P.; Su, R.; Wan, F.; Zhu, M.; Li, D.; Xu, S. Co3O4 nanocrystals on graphene oxide as a synergistic catalyst for degradation of Orange II in water by advanced oxidation technology based on sulfate radicals. Appl. Catal. B Environ. 2012, 123–124, 265–272. [Google Scholar] [CrossRef]
- Onoe, T.; Iwamoto, S.; Inoue, M. Synthesis and activity of the Pt catalyst supported on CNT. Catal. Commun. 2007, 8, 701–706. [Google Scholar] [CrossRef]
- Francisco, R.-R. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Auer, E.; Freund, A.; Pietsch, J.; Tacke, T. Carbons as supports for industrial precious metal catalysts. Appl. Catal. A Gen. 1998, 173, 259–271. [Google Scholar] [CrossRef]
- Krishna Murthy, J.; Chandra Shekar, S.; Siva Kumar, V.; Rama Rao, K.S. Highly selective zirconium oxychloride modified Pd/C catalyst in the hydrodechlorination of dichlorodifluoromethane to difluoromethane. Catal. Commun. 2002, 3, 145–149. [Google Scholar] [CrossRef]
- Pinna, F.; Signoretto, M.; Strukul, G.; Benedetti, A.; Malentacchi, M.; Pernicone, N. Ruthenium as a Dispersing Agent in Carbon-Supported Palladium. J. Catal. 1995, 155, 166–169. [Google Scholar] [CrossRef]
- Boonamnuayvitaya, V.; Sae-ung, S.; Tanthapanichakoon, W. Preparation of activated carbons from coffee residue for the adsorption of formaldehyde. Sep. Purif. Technol. 2005, 42, 159–168. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Zhuang, Q.L.; Kyotani, T.; Tomita, A. DRIFT and TK/TPD analyses of surface oxygen complexes formed during carbon gasification. Energy Fuels 1994, 8, 714–718. [Google Scholar] [CrossRef]
- Christoskova, S.G.; Stoyanova, M.; Georgieva, M.; Mehandjiev, D. Preparation and characterization of a higher cobalt oxide. Mater. Chem. Phys. 1999, 60, 39–43. [Google Scholar] [CrossRef]
- Tang, C.-W.; Wang, C.-B.; Chien, S.-H. Characterization of cobalt oxides studied by FT-IR, Raman, TPR and TG-MS. Thermochim. Acta 2008, 473, 68–73. [Google Scholar] [CrossRef]
- Aksoylu, A.E.; Madalena, M.; Freitas, A.; Pereira, M.F.R.; Figueiredo, J.L. The effects of different activated carbon supports and support modifications on the properties of Pt/AC catalysts. Carbon 2001, 39, 175–185. [Google Scholar] [CrossRef]
- Román-Martínez, M.C.; Cazorla-Amorós, D.; Linares-Solano, A.; de Lecea, C.S.-M. Tpd and TPR characterization of carbonaceous supports and Pt/C catalysts. Carbon 1993, 31, 895–902. [Google Scholar] [CrossRef]
- Elodie, G.; Rodrigues, M.F.R.P.; Chen, X.; Delgado, J.J.; Órfão, J.J.M. Influence of activated carbon surface chemistry on the activity of Au/AC catalysts in glycerol oxidation. J. Catal. 2011, 281, 119–127. [Google Scholar]
- Luo, J.; Yu, H.; Wang, H.; Wang, H.; Peng, F. Aerobic oxidation of benzyl alcohol to benzaldehyde catalyzed by carbon nanotubes without any promoter. Chem. Eng. J. 2014, 240, 434–442. [Google Scholar] [CrossRef]
- Slot, T.K.; Eisenberg, D.; van Noordenne, D.; Jungbacker, P.; Rothenberg, G. Cooperative Catalysis for Selective Alcohol Oxidation with Molecular Oxygen. Chem. A Eur. J. 2016, 22, 12307–12311. [Google Scholar] [CrossRef] [PubMed]
- Zielke, U.; Hüttinger, K.J.; Hoffman, W.P. Surface-oxidized carbon fibers: I. Surface structure and chemistry. Carbon 1996, 34, 983–998. [Google Scholar] [CrossRef]
- Fu, T.; Jiang, Y.; Lv, J.; Li, Z. Effect of carbon support on Fischer–Tropsch synthesis activity and product distribution over Co-based catalysts. Fuel Process. Technol. 2013, 110, 141–149. [Google Scholar] [CrossRef]
- Zhang, H.; Alhamed, Y.A.; Al-Zahrani, A.; Daous, M.; Inokawa, H.; Kojima, Y.; Petrov, L.A. Tuning catalytic performances of cobalt catalysts for clean hydrogen generation via variation of the type of carbon support and catalyst post-treatment temperature. Int. J. Hydrog. Energy 2014, 39, 17573–17582. [Google Scholar] [CrossRef]
- Zhang, H.; Lancelot, C.; Chu, W.; Hong, J.; Khodakov, A.Y.; Chernavskii, P.A.; Zheng, J.; Tong, D. The nature of cobalt species in carbon nanotubes and their catalytic performance in Fischer-Tropsch reaction. J. Mater. Chem. 2009, 19, 9241–9249. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. NIST Standard Reference Database; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2007.
- Christoskova, S.G.; Stojanova, M.; Georgieva, M.; Mehandzhiev, D. Study on the thermal stability of a high Co-oxide used as low-temperature catalyst and oxidant for complete oxidation. Thermochim. Acta 1997, 292, 77–83. [Google Scholar] [CrossRef]
- Mate, V.R.; Shirai, M.; Rode, C.V. Heterogeneous Co3O4 catalyst for selective oxidation of aqueous veratryl alcohol using molecular oxygen. Catal. Commun. 2013, 33 (Suppl. C), 66–69. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Xamena, L.F.X.; Casanova, O.; Galiasso, T.R.; Garcia, H.; Corma, A. Metal organic frameworks (MOFs) as catalysts: A combination of Cu2+ and Co2+ MOFs as an efficient catalyst for tetralin oxidation. J. Catal. 2008, 255, 220–227. [Google Scholar] [CrossRef]
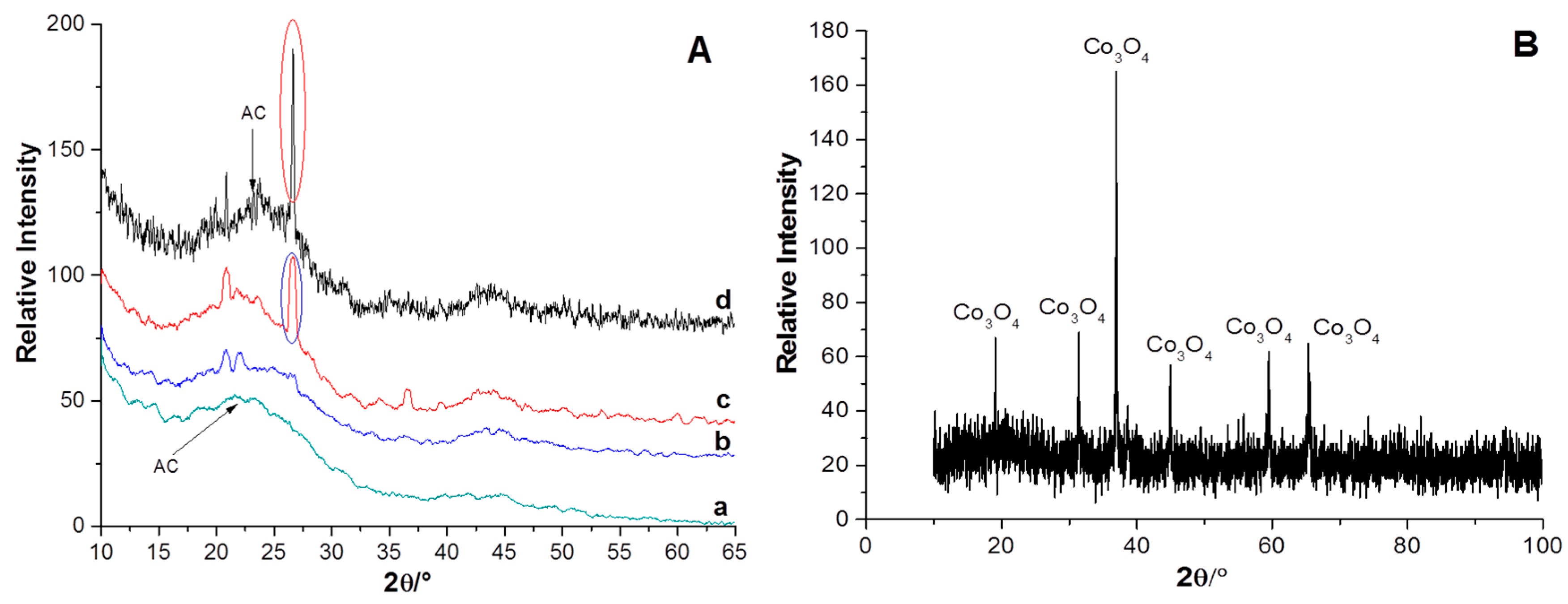

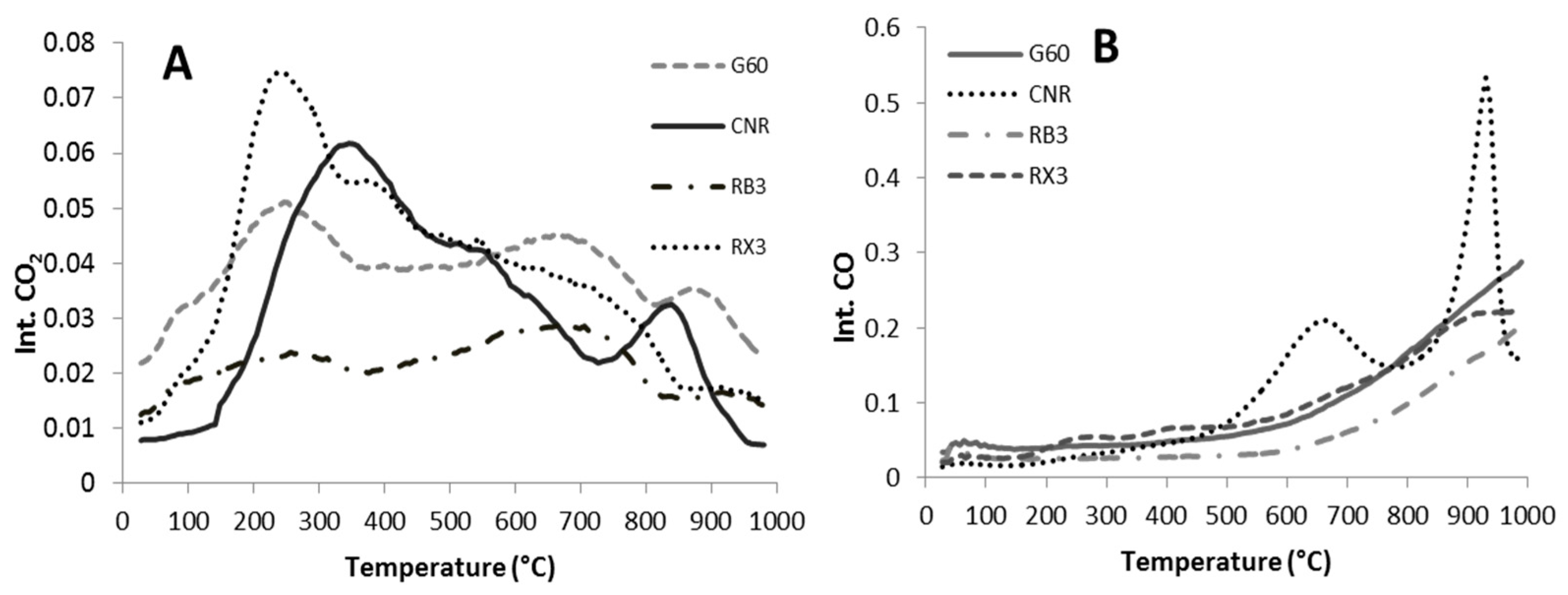
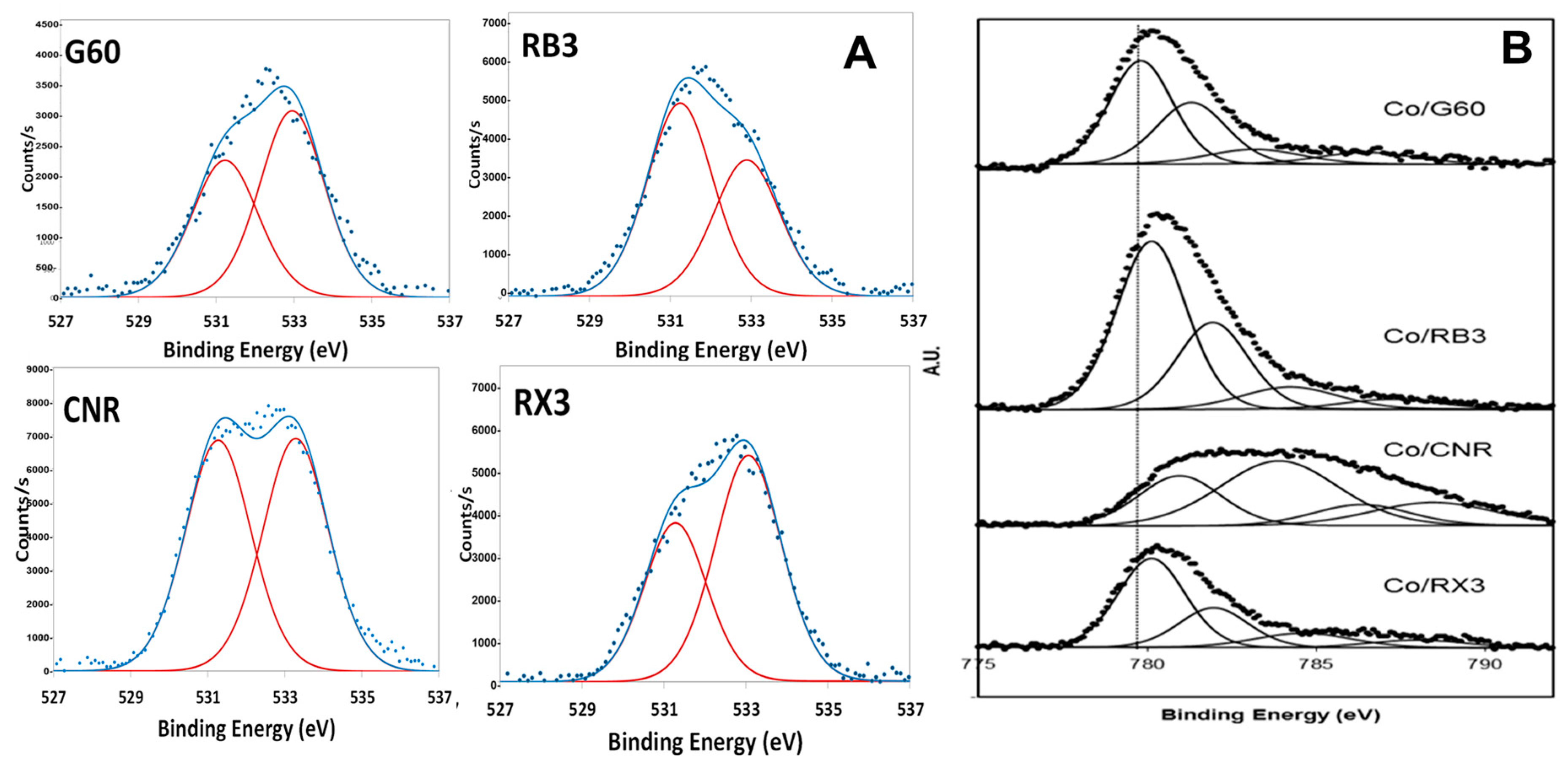

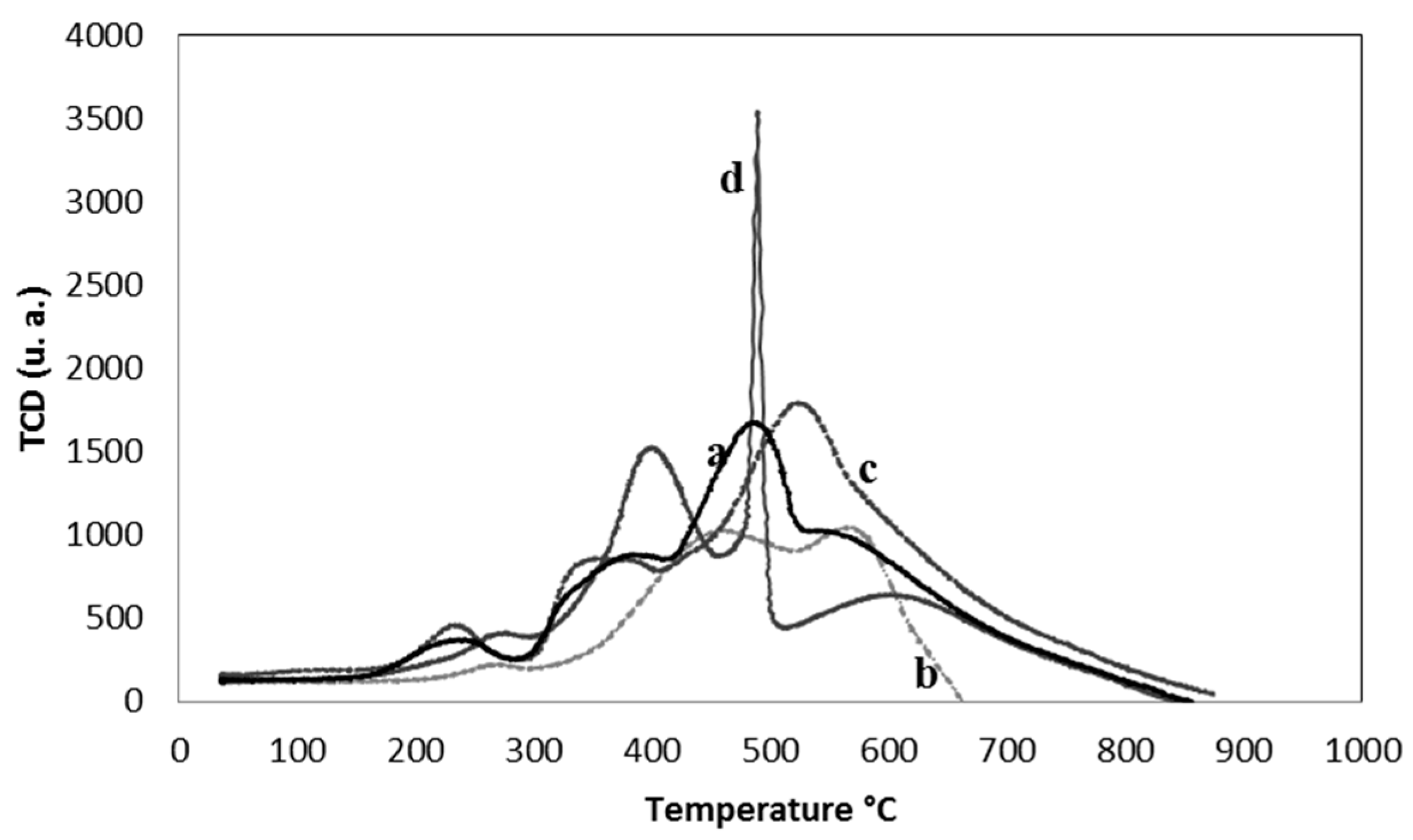

| Sample | SBET (m2 g−1) | Vµ (cm3 g−1) | Pore Size (nm) | TPR | XPS | |
|---|---|---|---|---|---|---|
| Area (a) | Tmax (°C) (b) | BE O 1s (eV) | ||||
| G60 | 978 | 0.336 | 41.1 | 41,159 | 680 | 531.2–532.9 |
| RB3 | 1133 | 0.520 | 24.3 | 38,296 | 696 | 531.2–532.9 |
| RX3 | 1524 | 0.622 | 26.7 | 42,368 | 688 | 531.3–533.1 |
| CNR | 1503 | 0.680 | 24.4 | 39,707 | 739 | 531.3–533.3 |
| Sample | CO (µmol/g) | CO2 (µmol/g) | O2 (µmol/g) | Total Area |
|---|---|---|---|---|
| G60 | 91.0 | 37.2 | 16.6 | 144.8 |
| RB3 | 55.9 | 20.6 | 11.7 | 88.2 |
| RX3 | 94.1 | 36.9 | 16.9 | 147.9 |
| CNR | 115.6 | 30.3 | 9.9 | 155.8 |
| Catalyst | SBET (m2 g−1) | Metal Loading (wt %) | TPR Area (a) | XPS | |||
|---|---|---|---|---|---|---|---|
| BE Co 2p3/2 (eV) | Co3+/Co2+ (at./at.) | Co/C (at./at.) | BE O 1s (eV) | ||||
| Co3O4/G60 | 777 | 15.2 | 202,855 | 779.9 781.4 | 1.07 | 0.041 | 530.2 532.1 |
| Co3O4/RB3 | 452 | 8.4 | 114,036 | 779.8 781.3 | 0.99 | 0.092 | 529.9 531.5 533.3 |
| Co3O4/RX3 | 1007 | 10.8 | 129,193 | 779.9 781.2 | 0.62 | 0.033 | 530.3 532.1 |
| Co3O4/CNR | 969 | 9.8 | 66,562 | 780.6 782.4 | 0.56 | 0.044 | 531.2 533.4 535.3 |
| Co3O4/G60 Used Catalyst | 729 | 9.5 | 200,025 | 779.9 781.7 | 1.28 | 0.046 | 530.3 532.1 534.0 |
| System | Conversion (%) * | Selectivity (%) * | Cobalt (wt %) | Catalytic Activity (mmol BA ) |
|---|---|---|---|---|
| Co3O4 | 48.7 | ≥99 | - | - |
| Co3O4/G60 | 100 | ≥99 | 15.2 | 4.2 |
| Co3O4/RX3 | 90.1 | ≥99 | 10.8 | 3.4 |
| Co3O4/CNR | 76.3 | ≥99 | 9.8 | 0.25 |
| Co3O4/RB3 | 66.5 | ≥99 | 8.4 | 0.30 |
| Co3O4/G60 (Used Catalyst) | 89 | ≥99 | 9.5 | 3.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordoba, M.; Miranda, C.; Lederhos, C.; Coloma-Pascual, F.; Ardila, A.; Fuentes, G.A.; Pouilloux, Y.; Ramírez, A. Catalytic Performance of Co3O4 on Different Activated Carbon Supports in the Benzyl Alcohol Oxidation. Catalysts 2017, 7, 384. https://doi.org/10.3390/catal7120384
Cordoba M, Miranda C, Lederhos C, Coloma-Pascual F, Ardila A, Fuentes GA, Pouilloux Y, Ramírez A. Catalytic Performance of Co3O4 on Different Activated Carbon Supports in the Benzyl Alcohol Oxidation. Catalysts. 2017; 7(12):384. https://doi.org/10.3390/catal7120384
Chicago/Turabian StyleCordoba, Misael, Cristian Miranda, Cecilia Lederhos, Fernando Coloma-Pascual, Alba Ardila, Gustavo A. Fuentes, Yannick Pouilloux, and Alfonso Ramírez. 2017. "Catalytic Performance of Co3O4 on Different Activated Carbon Supports in the Benzyl Alcohol Oxidation" Catalysts 7, no. 12: 384. https://doi.org/10.3390/catal7120384