Microwave-Assisted Silver-Catalyzed Protodecarboxylation and Decarboxylative Iodination of Aromatic Carboxylic Acids
Abstract
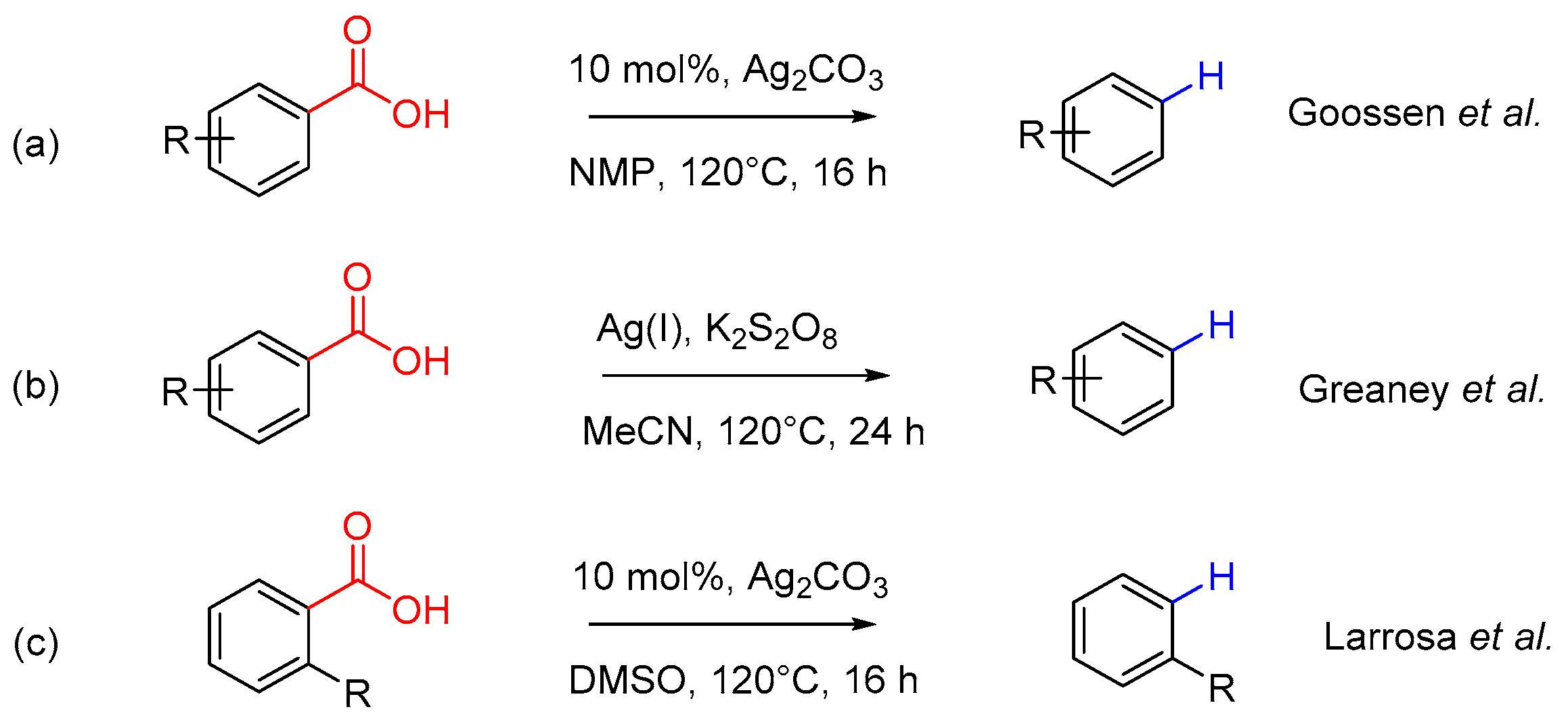
:1. Introduction
2. Results and Discussion
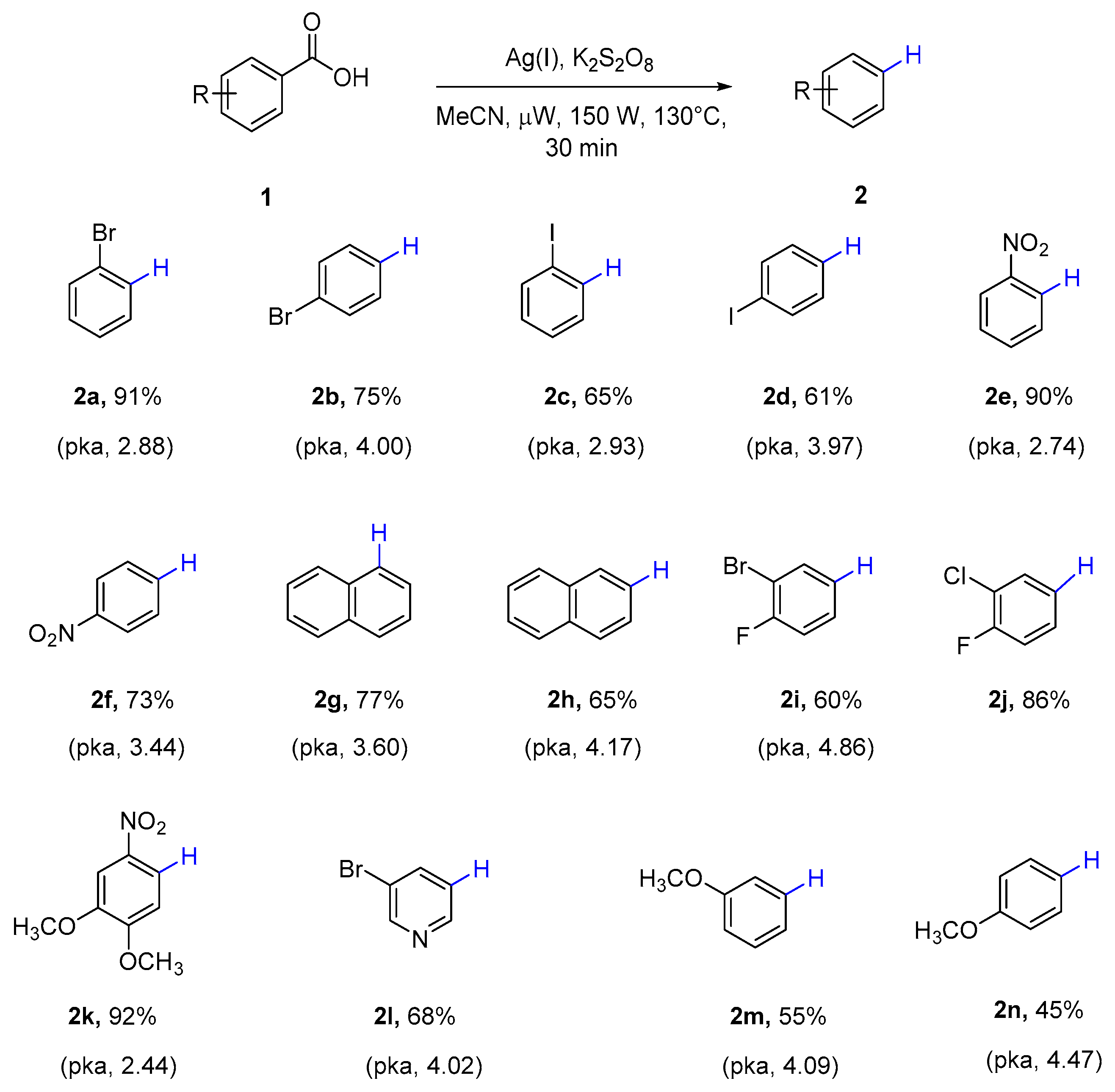
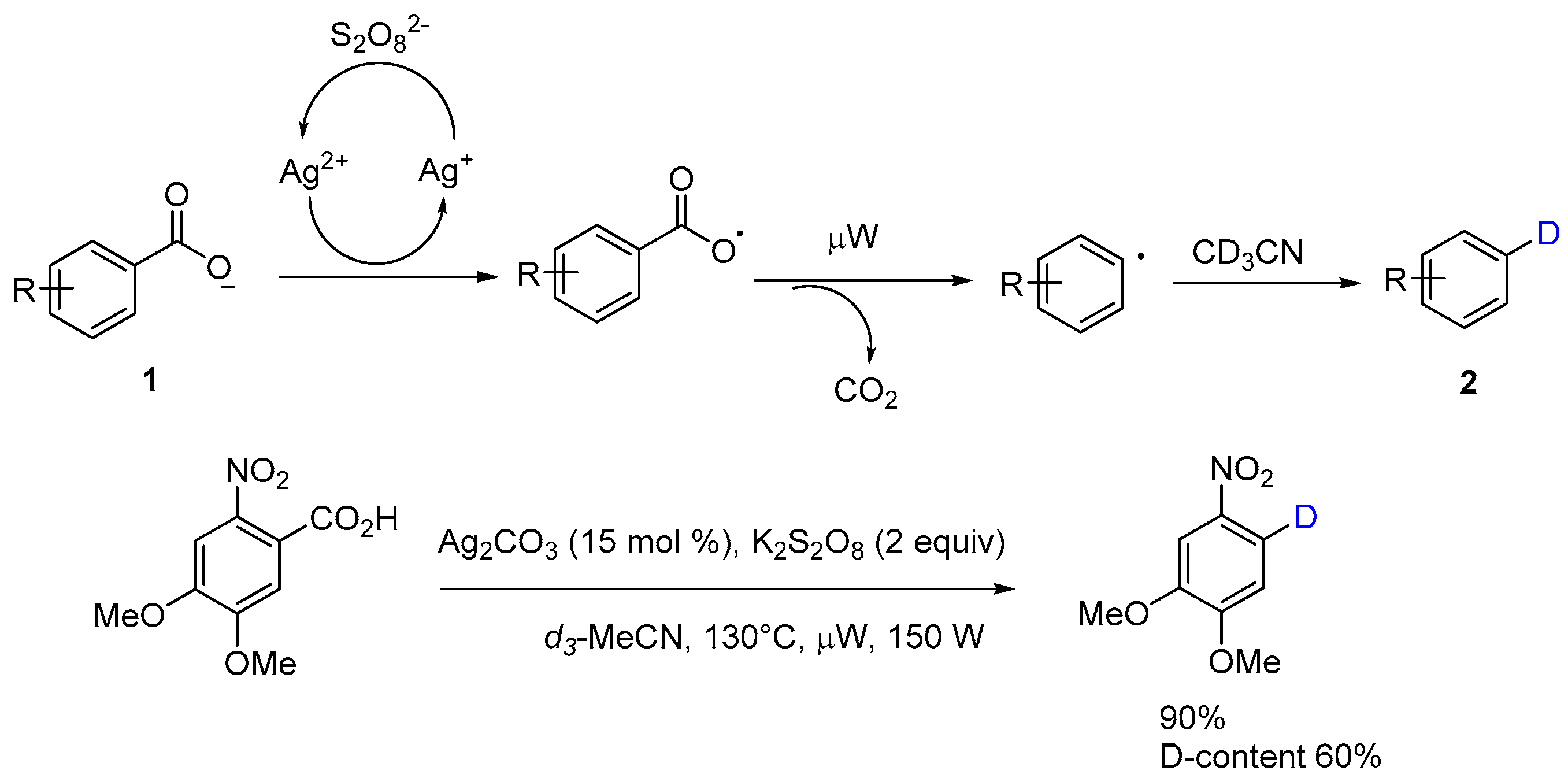
2.1. Microwave-Assisted Protodecarboxylation Reaction of Aromatic Carboxylic Acids
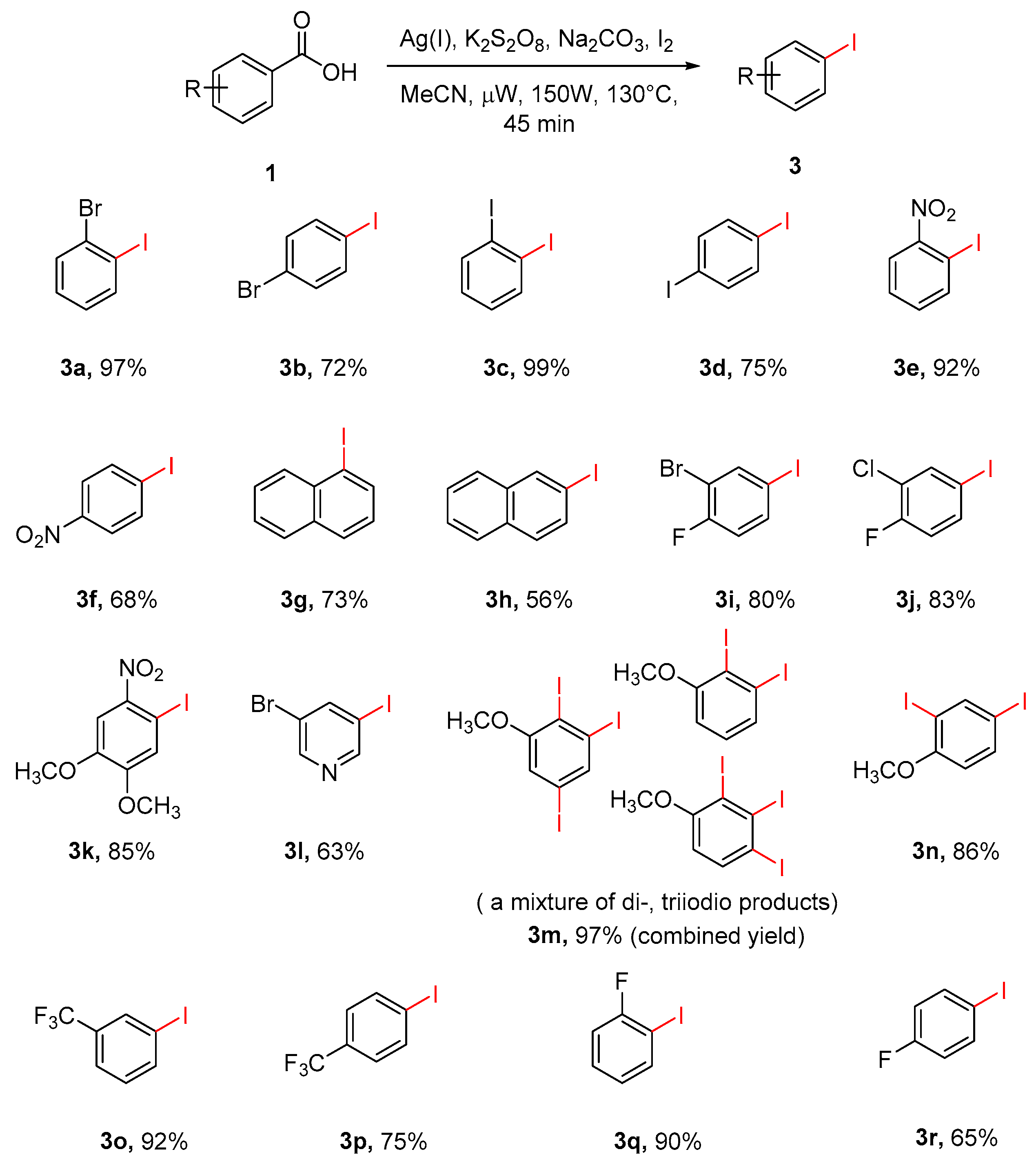
2.2. Microwave-Assisted Decarboxylative Iodination of Aromatic Carboxylic Acid
3. Experimental Details
3.1. General Procedure for Microwave-Assisted Protodecarboxylation Reaction of Aromatic Carboxylic Acid
3.2. General Procedure for Microwave-Assisted Decarboxylative Iodination Reaction of Aromatic Carboxylic Acid
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goossen, L.J.; Rodriguez, N.; Goossen, K. Carboxylic acids as substrates in homogeneous catalysis. Angew. Chem. Int. Ed. Engl. 2008, 47, 3100–3120. [Google Scholar] [CrossRef] [PubMed]
- Goossen, L.J.; Goossen, K.; Rodriguez, N.; Blanchot, M.; Linder, C.; Zimmermann, B. New catalytic transformations of carboxylic acids. Pure Appl. Chem. 2008, 80, 1725–1733. [Google Scholar] [CrossRef]
- Font, M.; Quibell, J.M.; Perry, G.J.P.; Larrosa, I. The use of carboxylic acids as traceless directing groups for regioselective C-H bond functionalisation. Chem. Commun. 2017, 53, 5584–5597. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.; Goossen, L.J. Decarboxylative coupling reactions: A modern strategy for C-C-bond formation. Chem. Soc. Rev. 2011, 40, 5030–5048. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Zhang, G.; Wang, H.; Huang, Z.; Wang, J.; Singh, A.K.; Lei, A. Recent advances in radical C-H activation/radical cross-coupling. Chem. Rev. 2017, 117, 9016–9085. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Arcos, U.A.; Rojas-Ocampo, J.; Porcel, S. Oxidative cyclization of alkenoic acids promoted by AgOAc. Dalton Trans. 2016, 45, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Jafarpour, F.; Zarei, S.; Olia, M.B.; Jalalimanesh, N.; Rahiminejadan, S. Palladium-catalyzed decarboxylative cross-coupling reactions: A route for regioselective functionalization of coumarins. J. Org. Chem. 2013, 78, 2957–2964. [Google Scholar] [CrossRef] [PubMed]
- Arroniz, C.; Ironmonger, A.; Rassias, G.; Larrosa, I. Direct ortho-arylation of ortho-substituted benzoic acids: Overriding Pd-catalyzed protodecarboxylation. Org. Lett. 2013, 15, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, S.; Dzik, W.I.; Goossen, L.J. Synthesis of aryl ethers from benzoates through carboxylate-directed C-H-activating alkoxylation with concomitant protodecarboxylation. Angew. Chem. Int. Ed. Engl. 2013, 52, 2959–2962. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, S.; Nolan, S.P. Gold(I)-catalyzed protodecarboxylation of (hetero)aromatic carboxylic acids. Chemistry 2013, 19, 14034–14038. [Google Scholar] [CrossRef] [PubMed]
- Goossen, L.J.; Thiel, W.R.; Rodriguez, N.; Linder, C.; Melzer, B. Copper-catalyzed protodecarboxylation of aromatic carboxylic acids. Adv. Synth. Catal. 2007, 349, 2241–2246. [Google Scholar] [CrossRef]
- Cornella, J.; Sanchez, C.; Banawa, D.; Larrosa, I. Silver-catalysed protodecarboxylation of ortho-substituted benzoic acids. Chem. Commun. 2009, 7176–7178. [Google Scholar] [CrossRef] [PubMed]
- Grainger, R.; Cornella, J.; Blakemore, D.C.; Larrosa, I.; Campanera, J.M. The ortho-substituent effect on the Ag-catalysed decarboxylation of benzoic acids. Chemistry 2014, 20, 16680–16687. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Taylor, J.B.; Greaney, M.F. Protodecarboxylation of benzoic acids under radical conditions. Chem. Commun. 2012, 48, 8270–8272. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Cai, Q. Copper/amino acid catalyzed cross-couplings of aryl and vinyl halides with nucleophiles. Acc. Chem. Res. 2008, 41, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Evano, G.; Blanchard, N.; Toumi, M. Copper-mediated coupling reactions and their applications in natural products and designed biomolecules synthesis. Chem. Rev. 2008, 108, 3054–3131. [Google Scholar] [CrossRef] [PubMed]
- Barluenga, J.; Gonzalez, J.M.; Garciamartin, M.A.; Campos, P.J.; Asensio, G. Acid-mediated reaction of bis(pyridine)iodonium(I) tetrafluoroborate with aromatic-compounds—A selective and general iodination method. J. Org. Chem. 1993, 58, 2058–2060. [Google Scholar] [CrossRef]
- Bunevicius, R.; Kazanavicius, G.; Zalinkevicius, R.; Prange, A.J., Jr. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N. Engl. J. Med. 1999, 340, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Hallouard, F.; Anton, N.; Choquet, P.; Constantinesco, A.; Vandamme, T. Iodinated blood pool contrast media for preclinical X-ray imaging applications—A review. Biomaterials 2010, 31, 6249–6268. [Google Scholar] [CrossRef] [PubMed]
- Pimlott, S.L.; Sutherland, A. Molecular tracers for the PET and spect imaging of disease. Chem. Soc. Rev. 2011, 40, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Seevers, R.H.; Counsell, R.E. Radioiodination techniques for small organic-molecules. Chem. Rev. 1982, 82, 575–590. [Google Scholar] [CrossRef]
- Baird, W.C.; Surridge, J.H. Halogenation with copper(II) halides—Synthesis of aryl iodides. J. Org. Chem. 1970, 35, 3436–3442. [Google Scholar] [CrossRef]
- Naumann, D.; Ruther, G. Synthesis of aryl iodine(III) difluorides by direct fluorination of aryl iodides. J. Fluorine Chem. 1980, 15, 213–222. [Google Scholar] [CrossRef]
- Cant, A.A.; Bhalla, R.; Pimlott, S.L.; Sutherland, A. Nickel-catalysed aromatic Finkelstein reaction of aryl and heteroaryl bromides. Chem. Commun. 2012, 48, 3993–3995. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ichikawa, S.; Buchwald, S.L. Rapid and efficient copper-catalyzed Finkelstein reaction of (hetero)aromatics under continuous-flow conditions. Angew. Chem. Int. Ed. Engl. 2015, 54, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Klapars, A.; Buchwald, S.L. Copper-catalyzed halogen exchange in aryl halides: An aromatic Finkelstein reaction. J. Am. Chem. Soc. 2002, 124, 14844–14845. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, W.; Zeng, H.; Mu, X.; Cosa, G.; Mi, Z.; Li, C.J. Photo-induced metal-catalyst-free aromatic Finkelstein reaction. J. Am. Chem. Soc. 2015, 137, 8328–8331. [Google Scholar] [CrossRef] [PubMed]
- Perry, G.J.P.; Quibell, J.M.; Panigrahi, A.; Larrosa, I. Transition-metal-free decarboxylative iodination: New routes for decarboxylative oxidative cross-couplings. J. Am. Chem. Soc. 2017, 139, 11527–11536. [Google Scholar] [CrossRef] [PubMed]
- Mavandadi, F.; Pilotti, A. The impact of microwave-assisted organic synthesis in drug discovery. Drug Discov. Today 2006, 11, 165–174. [Google Scholar] [CrossRef]
- Lidstrom, P.; Westman, J.; Lewis, A. Enhancement of combinatorial chemistry by microwave-assisted organic synthesis. Comb. Chem. High Throughput Screen. 2002, 5, 441–458. [Google Scholar] [CrossRef] [PubMed]
- Goossen, L.J.; Manjolinho, F.; Khan, B.A.; Rodriguez, N. Microwave-assisted Cu-catalyzed protodecarboxylation of aromatic carboxylic acids. J. Org. Chem. 2009, 74, 2620–2623. [Google Scholar] [CrossRef] [PubMed]
- Ferro, S.; Grazia, S.D.; De Luca, L.; Gitto, R.; Faliti, C.E.; Debyzer, Z.; Chimirri, A. Microwave assisted organic synthesis (MAOS) of small molecules as potential HIV-1 integrase inhibitors. Molecules 2011, 16, 6858–6870. [Google Scholar] [CrossRef] [PubMed]



| Entry | Catalyst Loading (mol %) | Ag(I) Catalyst (mol %) | K2S2O8 (equiv.) | Temperature (°C) | Time (h) | Yield (%) 1 |
|---|---|---|---|---|---|---|
| 1 | 10 | Ag2CO3 | 2 | 130 | 1 | 64 |
| 2 | 15 | Ag2CO3 | 2 | 130 | 1 | 87 |
| 3 | 20 | Ag2CO3 | 2 | 130 | 1 | 90 |
| 4 | 30 | Ag2CO3 | 2 | 130 | 1 | 89 |
| 5 | 40 | Ag2CO3 | 2 | 130 | 1 | 91 |
| 6 | 15 | Ag2O | 2 | 130 | 1 | 68 |
| 7 | 15 | AgOAc | 2 | 130 | 1 | 51 |
| 8 | 15 | AgSO3CF3 | 2 | 130 | 1 | 43 |
| 9 | 15 | Ag2CO3 | 2 (Oxone®) | 130 | 1 | 30 |
| 10 | 15 | Ag2CO3 | 2 | 100 | 1 | 45 |
| 11 | 15 | Ag2CO3 | 2 | 110 | 1 | 63 |
| 12 | 15 | Ag2CO3 | 2 | 140 | 1 | 80 |
| 13 | 15 | Ag2CO3 | 2 | 130 | 0.5 | 90 |
| 14 | 15 | Ag2CO3 | 2 | 130 | 0.3 | 63 |

| Entry | Loading of Ag2CO3 (mol %) | Iodine Source (equiv.) | Temperature (°C) | Time (h) | Yield (%) 1 |
|---|---|---|---|---|---|
| 1 | 15 | I2 (2) | 130 | 0.5 | 76 |
| 2 | 20 | I2 (2) | 130 | 0.5 | 80 |
| 3 | 25 | I2 (2) | 130 | 0.5 | 86 |
| 4 | 30 | I2 (2) | 130 | 0.5 | 88 |
| 5 | 25 | KI (2) | 130 | 0.5 | trace |
| 6 | 25 | NaI (2) | 130 | 0.5 | trace |
| 7 2 | 25 | I2 (2) | 130 | 0.5 | 93 |
| 8 2 | 25 | I2 (2) | 140 | 0.5 | 80 |
| 9 2 | 25 | I2 (2) | 150 | 0.5 | 75 |
| 10 2 | 25 | I2 (2) | 130 | 0.75 | 97 |
| 11 2 | 25 | I2 (2) | 130 | 1 | 91 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, K.; Li, Y. Microwave-Assisted Silver-Catalyzed Protodecarboxylation and Decarboxylative Iodination of Aromatic Carboxylic Acids. Catalysts 2017, 7, 314. https://doi.org/10.3390/catal7110314
Zhan K, Li Y. Microwave-Assisted Silver-Catalyzed Protodecarboxylation and Decarboxylative Iodination of Aromatic Carboxylic Acids. Catalysts. 2017; 7(11):314. https://doi.org/10.3390/catal7110314
Chicago/Turabian StyleZhan, Kun, and Yi Li. 2017. "Microwave-Assisted Silver-Catalyzed Protodecarboxylation and Decarboxylative Iodination of Aromatic Carboxylic Acids" Catalysts 7, no. 11: 314. https://doi.org/10.3390/catal7110314




