Study of 8 Types of Glutathione Peroxidase Mimics Based on β-Cyclodextrin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Enzymatic Activity the Eight Types of GPx Mimics
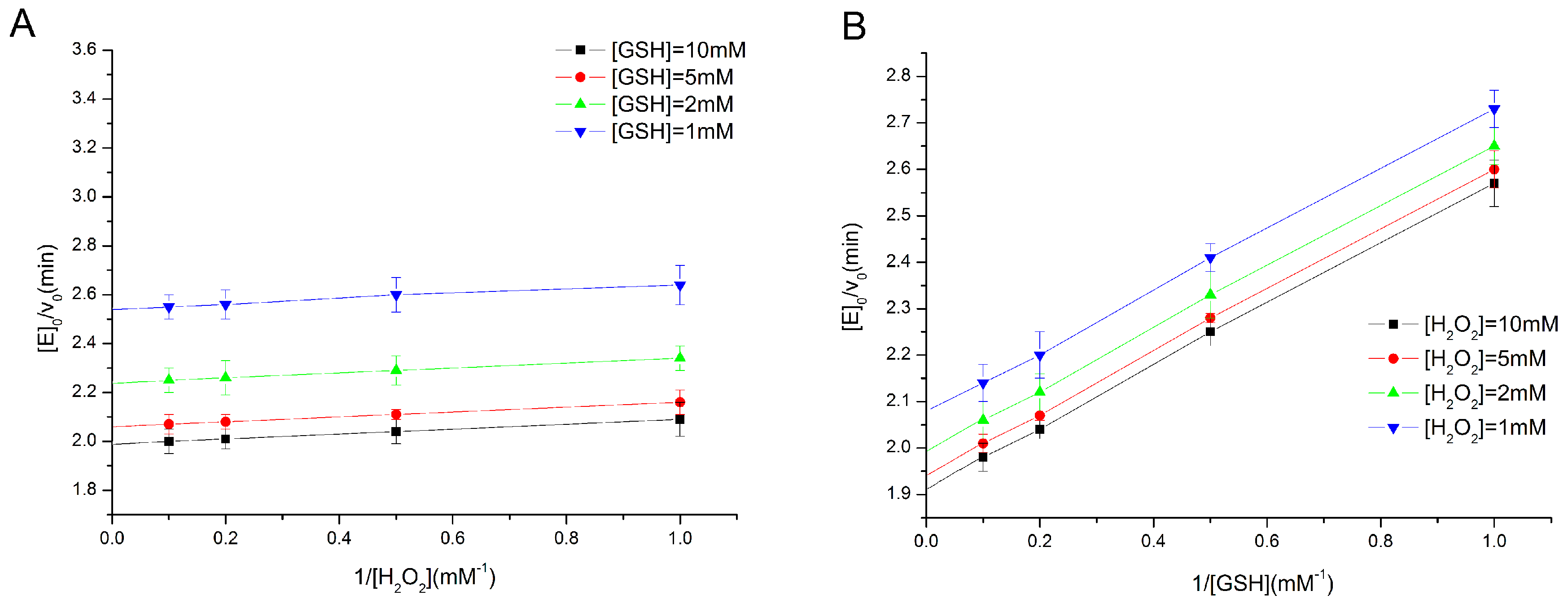
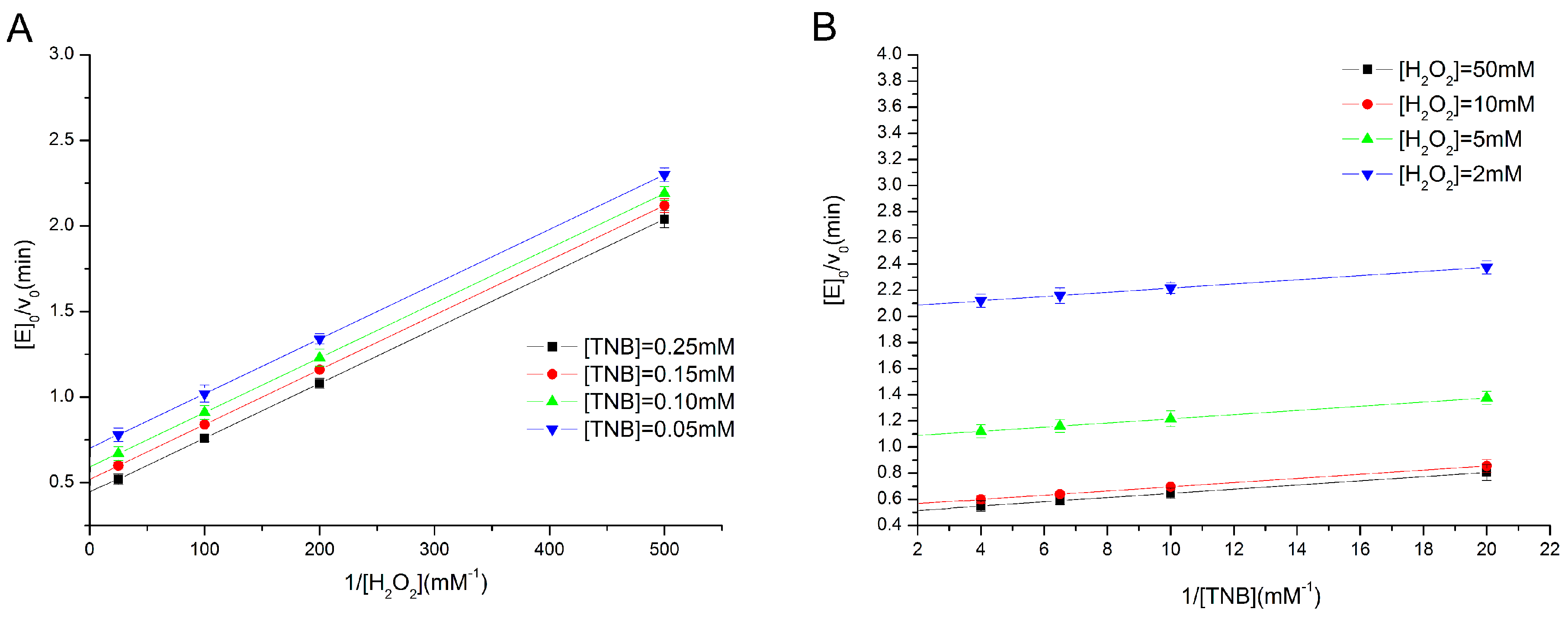
2.2. Determination of Enzyme Kinetics of the Eight Types of GPx Mimics
3. Materials and Methods
3.1. General Procedure
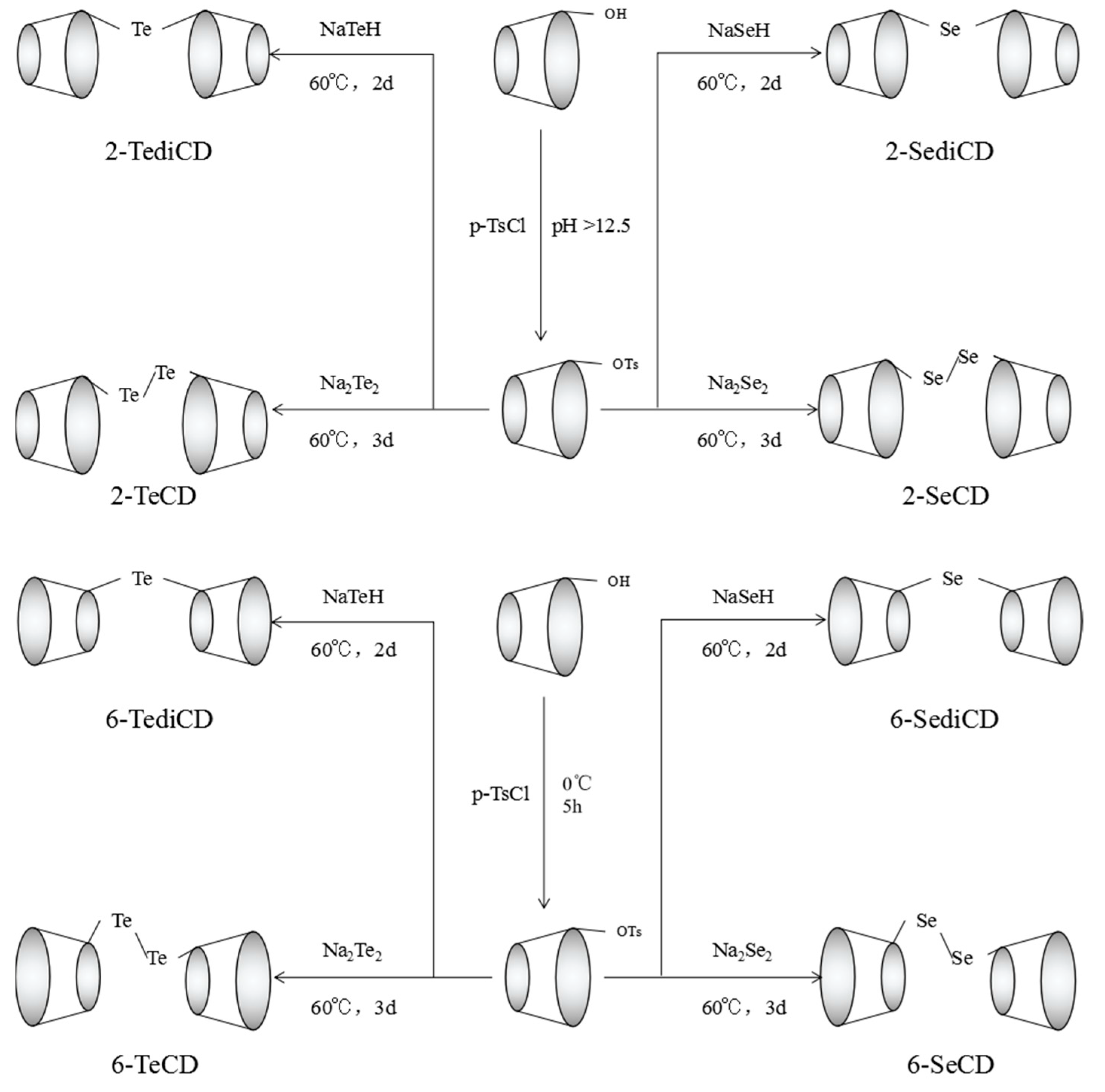
3.2. Synthesis of Eight Types of GPx Mimics
3.3. Determination of Activity and Steady-State Kinetics of GPx Mimics by a Coupled Reductase Assay
3.4. Determination of Activity and Steady-State Kinetics of GPx Mimics by a TNB Reduction Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Flohe, L.; Günzler, W.A.; Schock, H.H. Glutathione peroxidase: A selenoenzyme. FEBS Lett. 1973, 32, 132–134. [Google Scholar] [CrossRef]
- Sies, H. Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free Radic. Biol. Med. 1993, 14, 313–323. [Google Scholar] [CrossRef]
- Flohé, L. The selenoprotein glutathione peroxidase. In Glutathione: Chemical, Biochemical and Medical Aspects; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1989; pp. 644–731. [Google Scholar]
- Kian-Leong, T.A.N.; Philip, G. Purification and characterization of a recombinant human Theta-class glutathione transferase (GSTT2–2). Biochem. J. 1996, 315, 727–732. [Google Scholar]
- Haratake, M.; Matsumoto, S.; Ono, M.; Nakayama, M. Nanoparticulate glutathione peroxidase mimics based on selenocystine—Pullulan conjugates. Bioconjug. Chem. 2008, 19, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.W.; Wang, X.G.; Mu, Y.; Zang, T.Z.; Ji, Y.T.; Liu, J.Q.; Luo, G.M. A novel dicyclodextrinyl diselenide compound with glutathione peroxidase activity. FEBS J. 2007, 274, 3846–3854. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Song, J.; Su, J.; Wei, J.; Yu, Y.; Lv, S.; Nie, G. A new human catalytic antibody Se-scFv-2D8 and its selenium-containing single domains with high GPX activity. J. Mol. Recognit. 2010, 23, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Board, P.G.; Fei, X.; Sun, Y.; Lv, S.; Yan, G.; Luo, G. A novel selenium-containing glutathione transferase zeta1–1, the activity of which surpasses the level of some native glutathione peroxidases. Int. J. Biochem. Cell Boil. 2008, 40, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Ramazani, A.; Ayoubi, S.; Ahmadi, Y.; Ahankar, H.; Aghahosseini, H.; Woo Joo, S. “β-Cyclodextrin Nanoreactor” Catalyzed Synthesis of Coumarin Derivatives from In-Situ Generated Stabilized Phosphorus Ylides in Water. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 2307–2314. [Google Scholar] [CrossRef]
- Aghahosseini, H.; Ramazani, A. General Overview on Cyclodextrin-Based Artificial Enzymes’ Activity. Curr. Org. Chem. 2016, 20, 2817–2836. [Google Scholar] [CrossRef]
- Luo, G.M.; Ren, X.J.; Liu, J.Q.; Mu, Y.; Shen, J.C. Towards more efficient glutathione peroxidase mimics: Substrate recognition and catalytic group assembly. Curr. Med. Chem. 2003, 10, 1151–1183. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Xue, Y.; Liu, J.; Zhang, K.; Zheng, J.; Luo, G.; Shen, J. A Novel Cyclodextrin-Derived Tellurium Compound with Glutathione Peroxidase Activity. ChemBioChem 2002, 3, 356–363. [Google Scholar] [CrossRef]
- Jiao, A.; Yang, N.; Wang, J.; Xu, X.; Jin, Z. Cyclodextrin-derived chalcogenides as glutathione peroxidase mimics and their protection of mitochondria against oxidative damage. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 155–163. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Huang, X.; Mao, S.Z.; Liang, K.; Liu, J.Q.; Luo, G.M.; Shen, J.C. Cyclodextrin-Derived Mimic of Glutathione Peroxidase Exhibiting Enzymatic Specificity and High Catalytic Efficiency. Chem.-A Eur. J. 2006, 12, 3575–3579. [Google Scholar] [CrossRef] [PubMed]
- Jamier, V.; Ba, L.A.; Jacob, C. Selenium-and Tellurium-Containing Multifunctional Redox Agents as Biochemical Redox Modulators with Selective Cytotoxicity. Chem. A Eur. J. 2010, 16, 10920–10928. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, G.; Ren, X.; Mu, Y.; Bai, Y.; Shen, J.C. A bis-cyclodextrin diselenide with glutathione peroxidase-like activity. Biochim. Biophys. Acta 2000, 1481, 222–228. [Google Scholar] [CrossRef]
- Ren, X.; Yang, L.; Liu, J.; Su, D.; You, D.; Liu, C.; Shen, J. A novel glutathione peroxidase mimic with antioxidant activity. Arch. Biochem. Biophys. 2001, 387, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Ribaudo, G.; Bellanda, M.; Menegazzo, I.; Wolters, L.P.; Bortoli, M.; Ferrer-Sueta, G.; Orian, L. Mechanistic Insight into the Oxidation of Organic Phenylselenides by H2O2. Chem. A Eur. J. 2017, 23, 2405–2422. [Google Scholar] [CrossRef] [PubMed]
- Wolters, L.P.; Orian, L. Peroxidase activity of organic selenides: Mechanistic insights from quantum chemistry. Curr. Organ. Chem. 2016, 20, 189–197. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, J.; Mao, S.; Huang, X.; Yang, B.; Ren, X.; Shen, J. Aryl thiol substrate 3-carboxy-4-nitrobenzenethiol strongly stimulating thiol peroxidase activity of glutathione peroxidase mimic 2, 2′-ditellurobis (2-deoxy-β-cyclodextrin). J. Am. Chem. Soc. 2004, 126, 16395–16404. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, X.; Luo, Q.; Liu, J.; Shen, J. Artificial selenoenzymes: Designed and redesigned. Chem. Soc. Rev. 2011, 40, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; He, G.Y.; Lu, R.H. A convenient and economic method for the synthesis of mono-2-tosyl-β-cyclodextrin. Mon. Chem. Chem. Mon. 2008, 139, 1109–1111. [Google Scholar] [CrossRef]
- Matsui, Y.; Okimoto, A. The binding and catalytic properties of a positively charged cyclodextrin. Bull. Chem. Soc. Jpn. 1978, 51, 3030–3034. [Google Scholar] [CrossRef]
- Klayman, D.L.; Griffin, T.S. Reaction of selenium with sodium borohydride in protic solvents. A Facile Method for the introduction of selenium into organic molecules. J. Phys. Chem. B 1973, 95, 197–199. [Google Scholar] [CrossRef]
- Anslyn, E.; Breslow, R. Proton inventory of a bifunctional ribonuclease model. J. Am. Chem. Soc. 1989, 111, 8931–8932. [Google Scholar] [CrossRef]
- Breslow, R.; Dong, S.D. Biomimetic reactions catalyzed by cyclodextrins and their derivatives. Chem. Rev. 1998, 98, 1997–2012. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, B.; Li, L.; Zhang, H.Y. Synthesis of Organoselenium-Modified β-Cyclodextrins Possessing a 1, 2-Benzisoselenazol-3(2H)-one Moiety and Their Enzyme-Mimic Study. Helv. Chim. Acta 2002, 85, 9–18. [Google Scholar] [CrossRef]
- Wilson, S.R.; Zucker, P.A.; Huang, R.R.C.; Spector, A. Development of synthetic compounds with glutathione peroxidase activity. J. Am. Chem. Soc. 1989, 111, 5936–5939. [Google Scholar] [CrossRef]
- Silver, M. Thin-layer chromatography of lipoic acid, lipoamide, and their persulfides. Methods Enzymol. 1979, 62, 135–137. [Google Scholar] [PubMed]
- Bell, I.M.; Hilvert, D. Peroxide dependence of the semisynthetic enzyme selenosubtilisin. Biochemistry 1993, 32, 13969–13973. [Google Scholar] [CrossRef] [PubMed]
| GPx Mimics | Activity (U/μmol) by Coupled Reductase Assay | Activity (U/μmol) by TNB |
|---|---|---|
| β-CD | <0.1 | ≈0 |
| 2-SediCD | 0.1 ± 0.1 | 90 ± 3.2 |
| 2-SeCD | 7.4 ± 0.2 | 250 ± 10.2 |
| 6-SediCD | 0.1 ± 0.1 | 41 ± 3.5 |
| 6-SeCD | 4.2 ± 0.1 | 181 ± 10.1 |
| 2-TediCD | 0.5 ± 0.1 | 502 ± 22.1 |
| 2-TeCD | 46.7 ± 0.4 | 1155 ± 39.4 |
| 6-TediCD | 0.3 ± 0.1 | 304.5 ± 14.1 |
| 6-TeCD | 5.8 ± 0.2 | 858 ± 15.2 |
| GPx Mimics | kmax/KH2O2 (M·min−1) | kmax/KGSH (M·min−1) | kmax/KTNB (M·min−1) | |
|---|---|---|---|---|
| Coupled Reductase Assay | TNB | |||
| 2-SediCD | (6.5 ± 0.7) × 103 | (7.2 ± 0.8) × 102 | (7.3 ± 0.4) × 102 | (9.3 ± 0.8) × 104 |
| 2-SeCD | (5.2 ± 0.6) × 104 | (5.6 ± 0.5) × 103 | (2 ± 0.4) × 103 | (8.4 ± 0.9) × 105 |
| 6-SediCD | (3.3 ± 0.7) × 103 | (3.0 ± 0.6) × 102 | (6.8 ± 0.3) × 102 | (5.2 ± 0.4) × 104 |
| 6-SeCD | (3.3 ± 0.4) × 104 | (4.3 ± 0.3) × 103 | (1.8 ± 0.2) × 103 | (6.1 ± 0.6) × 105 |
| 2-TediCD | (7 ± 0.39) × 103 | (8.7 ± 0.3) × 102 | (1.6 ± 0.4) × 103 | (7 ± 0.4) × 106 |
| 2-TeCD | (8.2 ± 0.9) × 104 | (6 ± 0.5) × 103 | (6.2 ± 0.7) × 104 | (1.1 ± 0.6) × 107 |
| 6-TediCD | (5.4 ± 0.8) × 103 | (8.2 ± 0.2) × 102 | (1.3 ± 0.2) × 103 | (4.8 ± 0.5) × 106 |
| 6-TeCD | (3.7 ± 0.7) × 104 | (1.9 ± 0.3) × 103 | (1.6 ± 0.1) × 104 | (8.6 ± 0.7) × 106 |
| GPx Mimics | KH2O2 (μM-1) | KGSH (μM−1) | KTNB (μM−1) | Kmax (min−1) | ||
|---|---|---|---|---|---|---|
| GSH | TNB | GSH | TNB | |||
| 2-SediCD | 15.64 ± 1.37 | 12.67 ± 1.21 | 139.32 ± 5.97 | 98.95 ± 3.49 | 0.101 ± 0.03 | 91.22 ± 3.59 |
| 2-SeCD | 236.57 ± 12.54 | 46.74 ± 1.57 | 615.89 ± 31.33 | 91.62 ± 5.24 | 12.30 ± 0.98 | 261.76 ± 19.92 |
| 6-SediCD | 30.94 ± 2.49 | 115.31 ± 4.92 | 150.17 ± 7.42 | 65.29 ± 5.13 | 0.102 ± 0.03 | 34.59 ± 2.63 |
| 6-SeCD | 171.7 ± 9.32 | 43.5 ± 2.66 | 314.99 ± 19.65 | 69.85 ± 3.22 | 5.666 ± 1.07 | 189.01 ± 9.53 |
| 2-TediCD | 74.83 ± 4.56 | 136.36 ± 5.33 | 327.4 ± 22.38 | 109.57 ± 6.84 | 0.523 ± 0.15 | 1186.99 ± 43.36 |
| 2-TeCD | 719.2 ± 23.48 | 238.42 ± 15.29 | 951.2 ± 63.12 | 130.04 ± 5.39 | 58.97 ± 2.49 | 1430.52 ± 38.94 |
| 6-TediCD | 57.52 ± 3.42 | 90.74 ± 8.31 | 238.93 ± 15.27 | 100.91 ± 7.27 | 0.310 ± 0.08 | 484.41 ± 21.04 |
| 6-TeCD | 168.12 ± 5.35 | 120.23 ± 7.26 | 388.78 ± 25.58 | 141.7 ± 10.74 | 6.220 ± 1.84 | 1218.68 ± 49.81 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Qu, X.; Xie, Y.; Lv, S. Study of 8 Types of Glutathione Peroxidase Mimics Based on β-Cyclodextrin. Catalysts 2017, 7, 289. https://doi.org/10.3390/catal7100289
Wang L, Qu X, Xie Y, Lv S. Study of 8 Types of Glutathione Peroxidase Mimics Based on β-Cyclodextrin. Catalysts. 2017; 7(10):289. https://doi.org/10.3390/catal7100289
Chicago/Turabian StyleWang, Liwu, Xiaonan Qu, Ying Xie, and Shaowu Lv. 2017. "Study of 8 Types of Glutathione Peroxidase Mimics Based on β-Cyclodextrin" Catalysts 7, no. 10: 289. https://doi.org/10.3390/catal7100289




