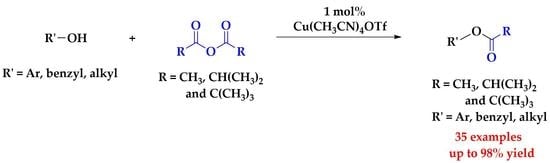
Mild and Highly Efficient Copper(I) Inspired Acylation of Alcohols and Polyols
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Materials and Methods
3.2. Typical Experimental Procedure for O-Acetylation of Alcohols with Acetic Anhydride
3.3. Typical Experimental Procedure for O-Acylation of Alcohols with Isobutyric Anhydride
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hofle, G.; Steglich, W.; Vorbruggen, H. 4-Dialkylaminopyridines as Highly Active Acylation Catalysts. Angew. Chem. Int. Ed. 1978, 17, 569–583. [Google Scholar] [CrossRef]
- Phukan, P. Iodine as an Extremely Powerful Catalyst for the Acetylation of Alcohols under solvent-free conditions. Tetrahedron Lett. 2004, 45, 4785–4787. [Google Scholar] [CrossRef]
- Borah, R.; Deka, N.; Sarma, J.C. Iodine as an Acetyl Transfer Catalyst. J. Chem. Res. 1997, 110–111. [Google Scholar] [CrossRef]
- Kartha, K.P.R.; Field, R.A. Iodine: A Versatile Reagent in Carbohydrate Chemistry IV. Per-O-acetylation, regioselective acylation and acetolysis. Tetrahedron 1997, 53, 11753–11766. [Google Scholar] [CrossRef]
- Khan, A.T.; Choudhury, L.H.; Ghosh, S. Acetonyltriphenylphosphonium Bromide (ATPB): A Versatile Reagent for the Acylation of Alcohols, Phenols, Thiols and Amines and for 1,1-Diacylation of Aldehydes under Solvent-Free Conditions. Eur. J. Org. Chem. 2005, 2005, 2782–2787. [Google Scholar] [CrossRef]
- Bhaskar, P.M.; Loganathan, D. Per-O-acetylation of sugars catalysed by montmorillonite K-10. Tetrahedron Lett. 1998, 39, 2215–2218. [Google Scholar] [CrossRef]
- Dasgupta, F.; Singh, P.P.; Srivastava, H.C. Acetylation of carbohydrates using ferric chloride in acetic anhydride. Carbohydr. Res. 1980, 80, 346–349. [Google Scholar] [CrossRef]
- Miyashita, M.; Shiina, I.; Miyoshi, S.; Mukaiyama, T. A New and Efficient Esterification Reaction via Mixed Anhydrides by the Promotion of a Catalytic Amount of Lewis Acid. Bull. Chem. Soc. Jpn. 1993, 66, 1516–1527. [Google Scholar] [CrossRef]
- Lu, K.C.; Hsieh, S.Y.; Patkar, L.N.; Chen, C.T.; Lin, C.C. Simple and efficient per-O-acetylation of carbohydrates by lithium perchlorate catalyst. Tetrahedron 2004, 60, 8967–8973. [Google Scholar] [CrossRef]
- Heravi, M.M.; Behbahani, F.K.; Zadsirjan, V.; Oskooie, H.A. Copper(II) Sulfate Pentahydrate (CuSO4·5H2O). A Green Catalyst for Solventless Acetylation of Alcohols and Phenols with Acetic Anhydride. J. Braz. Chem. Soc. 2006, 17, 1045–1047. [Google Scholar]
- Procopiou, P.A.; Baugh, S.P.D.; Flack, S.S.; Inglis, G.G.A. An Extremely Powerful Acylation Reaction of Alcohols with Acid Anhydrides Catalyzed by Trimethylsilyl Trifluoromethanesulfonate. J. Org. Chem. 1998, 63, 2342–2347. [Google Scholar] [CrossRef]
- Vedejs, E.; Diver, S.T. Tributylphosphine: A Remarkable Acylation Catalyst. J. Am. Chem. Soc. 1993, 115, 3358–3359. [Google Scholar] [CrossRef]
- Vedejs, E.; Bennett, N.S.; Conn, L.M.; Diver, S.T.; Gingras, M.; Lin, S.; Oliver, P.A.; Peterson, M.J. Tributylphosphine-catalyzed acylations of alcohols: Scope and related reactions. J. Org. Chem. 1993, 58, 7286–7288. [Google Scholar] [CrossRef]
- Reddy, T.S.; Narasimhulu, M.; Suryakiran, N.; Mahesh, K.C.; Ashalatha, K.; Venkateswarlu, Y. A Mild and Efficient Acetylation of Alcohols, Phenols and Amines with Acetic Anhydride Using La(NO3)3·6H2O as a Catalyst Under Solvent-free Conditions. Tetrahedron Lett. 2006, 47, 6825–6829. [Google Scholar] [CrossRef]
- Misra, A.K.; Tiwari, P.; Madhusudan, S.K. HClO4-SiO2 Catalyzed per-O-Acetylation of Carbohydrates. Carbohydr. Res. 2005, 340, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, M.; Barone, G.; Iadonisi, A.; Schiattarella, M. An Easy Approach for the Acetylation of Saccharidic Alcohols. Applicability for Regioselective Protections. Tetrahedron Lett. 2003, 44, 4661–4663. [Google Scholar] [CrossRef]
- Lugemwa, F.N.; Shaikh, K.; Hochstedt, E. Facile and efficient acetylation of primary alcohols and phenols with acetic anhydride aatalyzed by dried sodium bicarbonate. Catalysts 2013, 3, 954–965. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Shivani. Magnesium Bistrifluoromethanesulfonimide as a New and Efficient Acylation Catalyst. J. Org. Chem. 2006, 71, 5785–5788. [Google Scholar] [CrossRef] [PubMed]
- Tale, R.H.; Adude, R.N. A novel 3-nitrobenzeneboronic acid as an extremely mild and environmentally benign catalyst for the acetylation of alcohols under solvent-free conditions. Tetrahedron Lett. 2006, 47, 7263–7265. [Google Scholar] [CrossRef]
- Breton, G.W.; Kurtz, M.J.; Kurtz, S.L. Acetylation of unsymmetrical diols in the presence of Al2O3. Tetrahedron Lett. 1997, 38, 3825–3828. [Google Scholar] [CrossRef]
- Karimi, B.; Seradj, H. N-Bromosuccinimide (NBS), a novel and highly effective catalyst for acetylation of alcohols under mild reaction conditions. Synlett 2001, 2001, 519–520. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Ramachander, T.; Takhi, M. Acylation of alcohols with acetic anhydride catalyzed by TaCl5: Some implications in kinetic resolution. Tetrahedron Lett. 1998, 39, 3263–3266. [Google Scholar] [CrossRef]
- Choudary, B.M.; Kantam, M.L.; Neeraja, V.; Bandyopadhyay, T.; Reddy, P.N. Vanadyl(IV) acetate, a new reusable catalyst for acetylation of alcohols. J. Mol. Catal. A 1999, 140, 25–29. [Google Scholar] [CrossRef]
- Mensah, E.A.; Franscisco, R.R.; Standiford, E.S. Highly Efficient Cationic Palladium Catalyzed Acetylation of Alcohols and Carbohydrate-Derived Polyols. Catalysts 2016, 6, 27. [Google Scholar] [CrossRef]
- Chandra, K.L.; Saravanan, P.; Singh, R.K.; Singh, V.K. Lewis Acid Catalyzed Acylation Reactions: Scope and Limitations. Tetrahedron 2002, 58, 1369–1374. [Google Scholar] [CrossRef]
- Orita, A.; Tanahashi, C.; Kakuda, A.; Otera, J. Highly Powerful and Practical Acylation of Alcohols with Acid Anhydride Catalyzed by Bi(OTf)3. J. Org. Chem. 2001, 66, 8926–8934. [Google Scholar] [CrossRef] [PubMed]
- Bizier, N.P.; Atkins, S.R.; Helland, L.C.; Colvin, S.F.; Twitchell, J.R.; Cloninger, M.J. Indium Triflate Catalyzed Peracetylation of Carbohydrates. Carbohydr. Res. 2008, 343, 1814–1818. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, K.K.; Frost, C.G.; Love, I.; Waite, D. Indium Triflate: An Efficient Catalyst for Acylation reactions. Synlett 1999, 1999, 1743–1744. [Google Scholar] [CrossRef]
- Ishihara, K.; Kubota, M.; Kurihara, H.; Yamamoto, H. Scandium Trifluoromethanesulfonate as an extremely active lewis acid catalyst in acylation of alcohols with acid anhydrides and mixed anhydrides. J. Org. Chem. 1996, 61, 4560–4567. [Google Scholar] [CrossRef] [PubMed]
- Dalpozzo, R.; De Nino, A.; Maiuolo, L.; Procopio, A.; Nardi, M.; Bartoli, M.; Romeo, R. Highly efficient and versatile acetylation of alcohols catalyzed by cerium(III) triflate. Tetrahedron Lett. 2003, 44, 5621–5624. [Google Scholar] [CrossRef]
- Velusamy, S.; Borpuzari, S.; Punniyamurthy, T. Cobalt(II)-Catalyzed Direct Acetylation of Alcohols with Acetic acid. Tetrahedron 2005, 61, 2011–2015. [Google Scholar] [CrossRef]
- Mulla, S.A.R.; Inamdar, S.M.; Pathan, M.Y.; Chavan, S.S. Highly Efficient Cobalt (II) Catalyzed O-Acylation of Alcohols and Phenols under Solvent-Free Conditions. Open J. Synth. Theory Appl. 2012, 1, 31–35. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Movassaghi, M.; Hunt, D.K.; Tjandra, M. Total Synthesis and Absolute Stereochemical Assignment of (+)- and (−)-Galbulimima Alkaloid 13. J. Am. Chem. Soc. 2006, 128, 8126–8127. [Google Scholar] [CrossRef] [PubMed]
- Movassaghi, M.; Tjandra, M.; Qi, J. Total Synthesis of (−)-Himandrine. J. Am. Chem. Soc. 2009, 131, 9648–9650. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, S.H.; Ellman, J.A.; Bergman, R.G. Rhodium-Catalyzed Direct C–H Addition of 3,4-Dihydroquinazolines to Alkenes and Their Use in the Total Synthesis of Vasicoline. J. Org. Chem. 2006, 71, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Minatti, A.; Buchwald, S.L. Synthesis of Indolines via a Domino Cu-Catalyzed Amidation/Cyclization Reaction. Org. Lett. 2008, 10, 2721–2724. [Google Scholar] [CrossRef] [PubMed]
- Greene, T.W.; Wuts, P.M.G. Protective Groups in Organic Synthesis, 3rd ed.; John Wiley and Sons: New York, NY, USA, 1999; pp. 219–229. [Google Scholar]
- Kocienski, P.J. Protecting Groups, 1st ed.; Georg Thieme Verlag: Stuttgart, Germany, 1994; pp. 137–155. [Google Scholar]
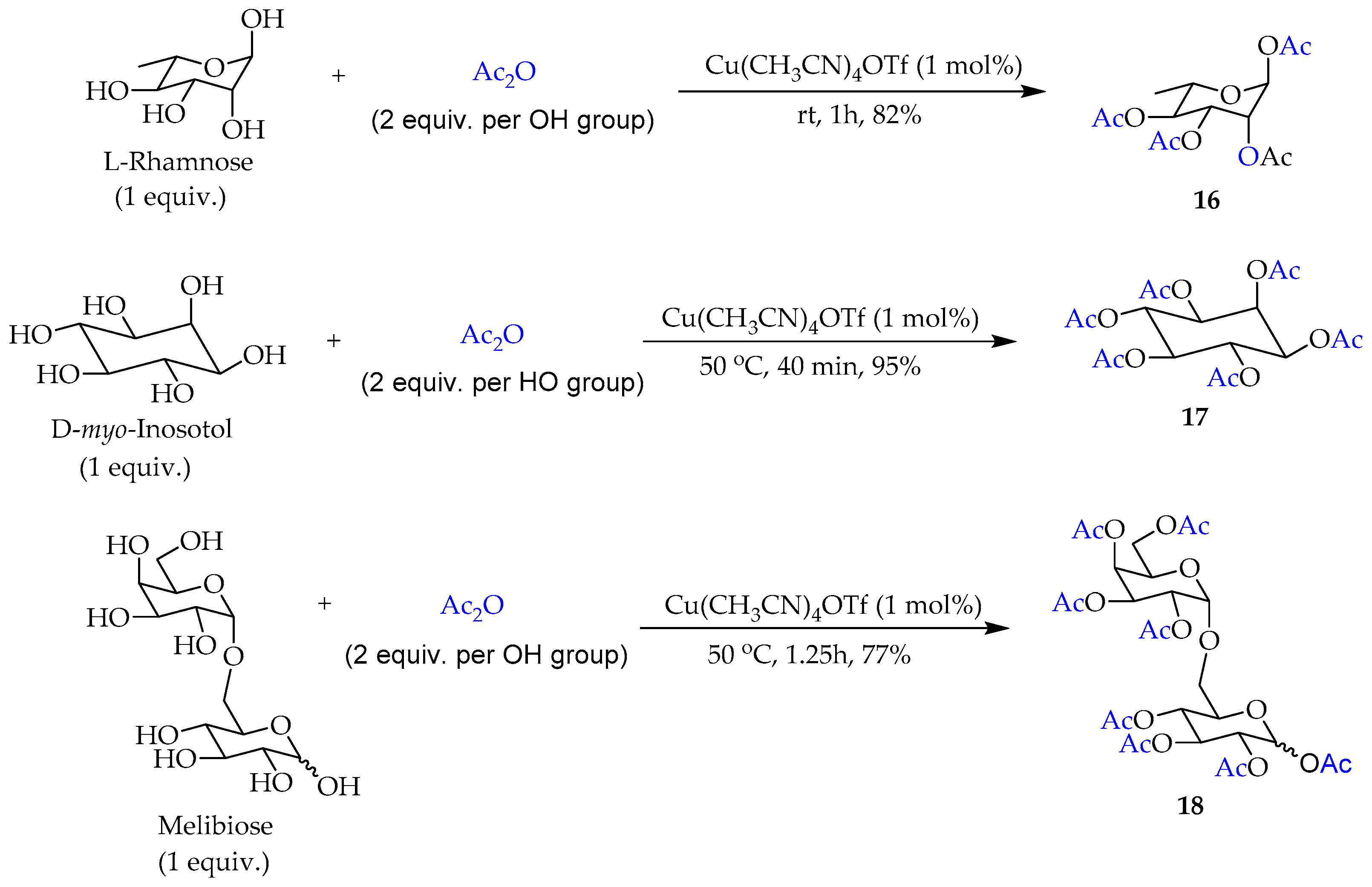
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Product | Time (min) | Yield (%) b |
| 1 |  |  | 3 | 98 |
| 2 |  |  | 20 | 91 |
| 3 |  |  | 30 | 94 |
| 4 |  |  | 30 | 90 |
| 5 |  |  | 45 | 89 |
| 6 |  |  | 22 | 92 |
| 7 |  |  | 30 | 92 |
| 8 |  |  | 55 | 93 |
| 9 |  |  | 60 | 83 |
| 10 |  |  | 60 | 87 |
| 11 |  |  | 45 | 90 |
| 12 |  |  | 35 | 90 |
| 13 |  |  | 60 | 83 |
| 14 |  |  | 45 | 89 |
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Product | Time (min) | Yield (%) b |
| 1 |  |  | 15 | 92 |
| 2 |  |  | 180 | 93 |
| 3 |  |  | 30 | 90 |
| 4 |  |  | 60 | 93 |
| 5 |  |  | 90 | 89 |
| 6 |  |  | 60 | 91 |
| 7 |  |  | 210 | 88 |
| 8 |  |  | 90 | 85 |
| 9 |  |  | 120 | 90 |
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Product | Time (min) | Yield (%) b |
| 1 |  |  | 30 | 90 |
| 2 |  |  | 210 | 90 |
| 3 |  |  | 30 | 93 |
| 4 |  |  | 60 | 90 |
| 5 |  |  | 90 | 86 |
| 6 |  |  | 60 | 93 |
| 7 |  |  | 50 | 86 |
| 8 |  |  | 210 | 89 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensah, E.A.; Earl, L. Mild and Highly Efficient Copper(I) Inspired Acylation of Alcohols and Polyols. Catalysts 2017, 7, 33. https://doi.org/10.3390/catal7010033
Mensah EA, Earl L. Mild and Highly Efficient Copper(I) Inspired Acylation of Alcohols and Polyols. Catalysts. 2017; 7(1):33. https://doi.org/10.3390/catal7010033
Chicago/Turabian StyleMensah, Enoch A., and Lindsey Earl. 2017. "Mild and Highly Efficient Copper(I) Inspired Acylation of Alcohols and Polyols" Catalysts 7, no. 1: 33. https://doi.org/10.3390/catal7010033
APA StyleMensah, E. A., & Earl, L. (2017). Mild and Highly Efficient Copper(I) Inspired Acylation of Alcohols and Polyols. Catalysts, 7(1), 33. https://doi.org/10.3390/catal7010033