Preparation of Fe3O4/TiO2/C Nanocomposites and Their Application in Fenton-Like Catalysis for Dye Decoloration
Abstract
:1. Introduction
2. Results and Discussion
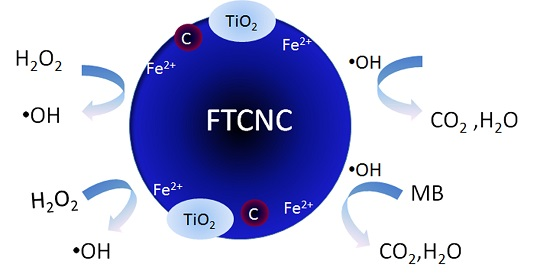
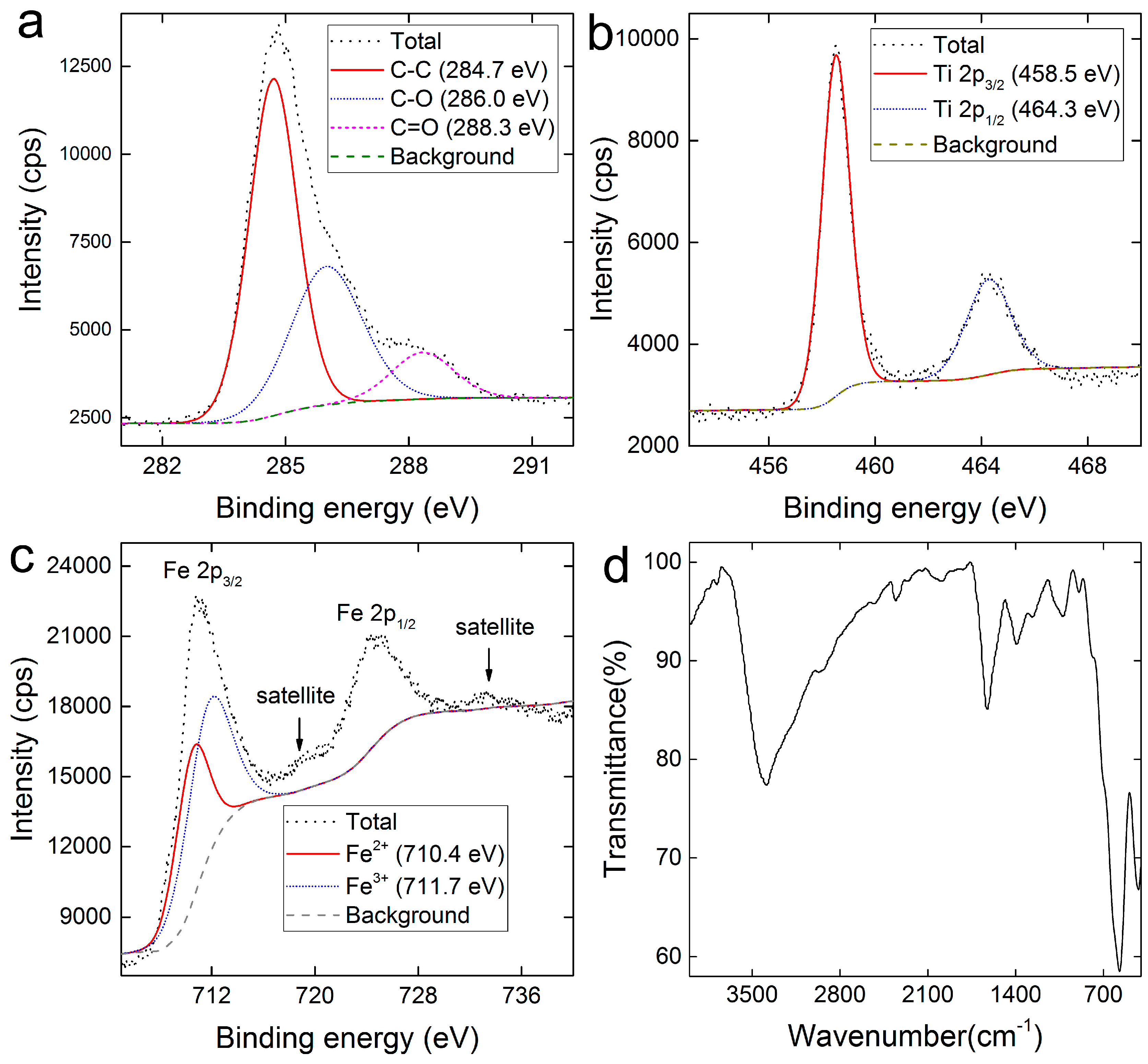
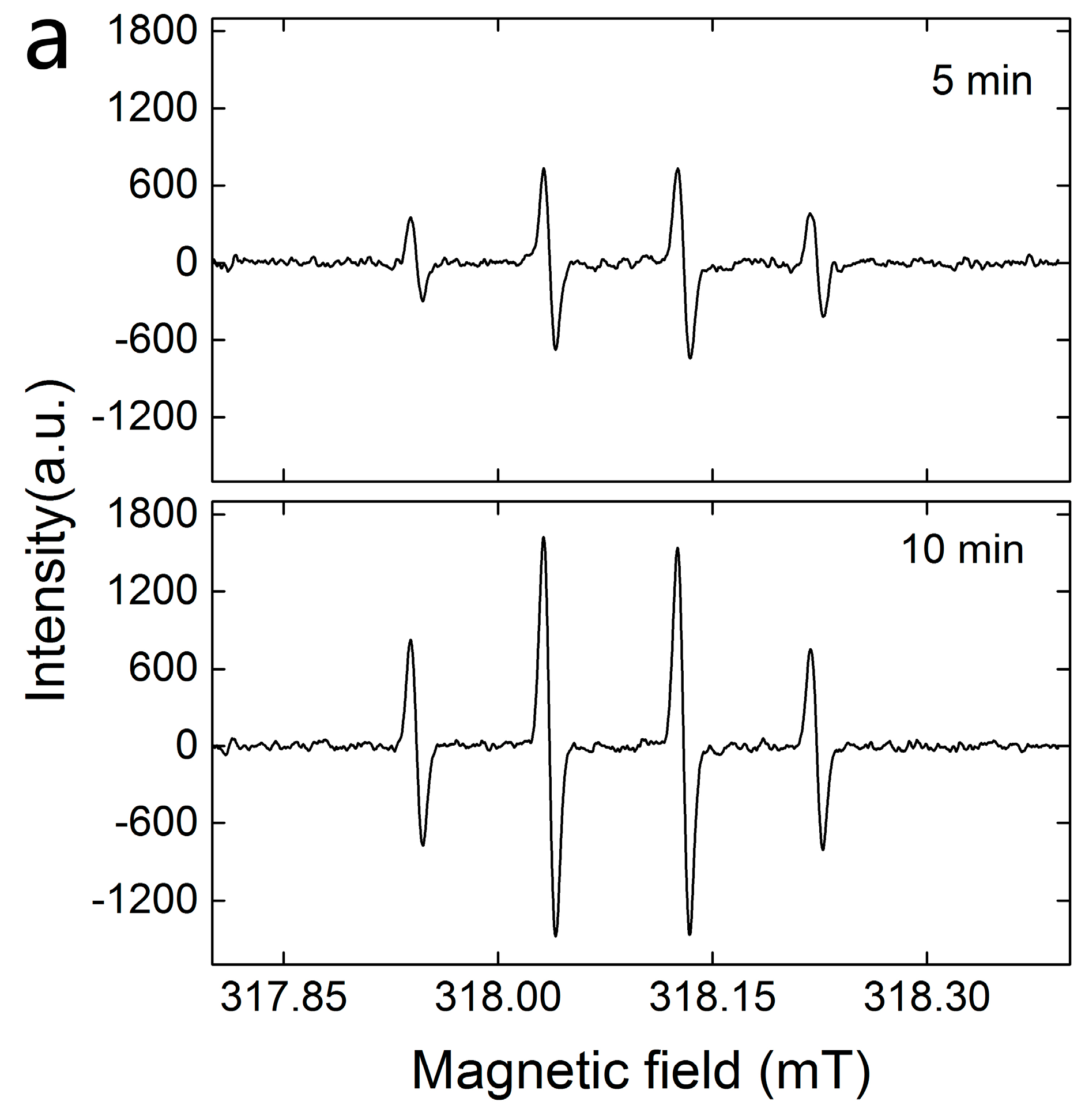
2.1. Characterization of FTCNCs
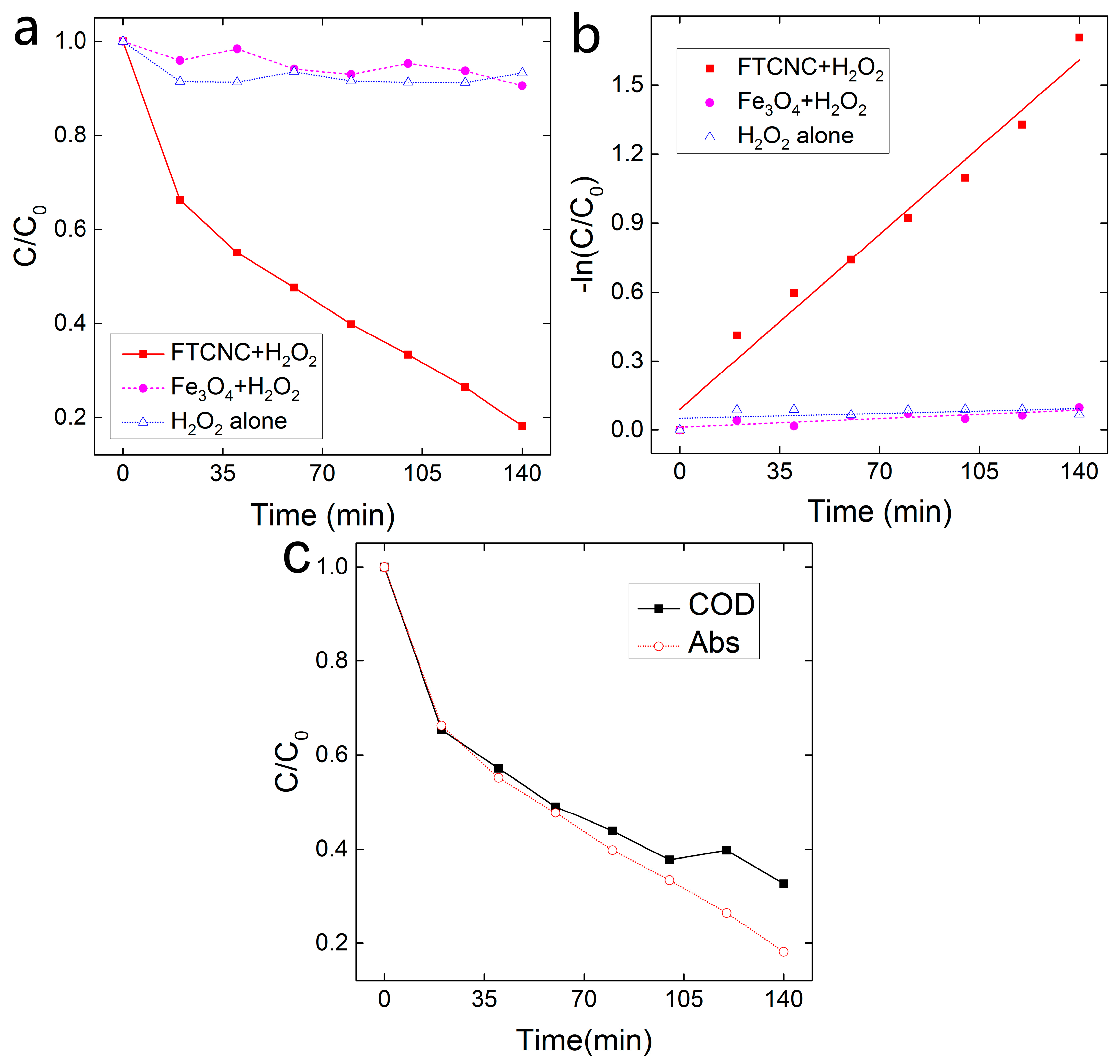
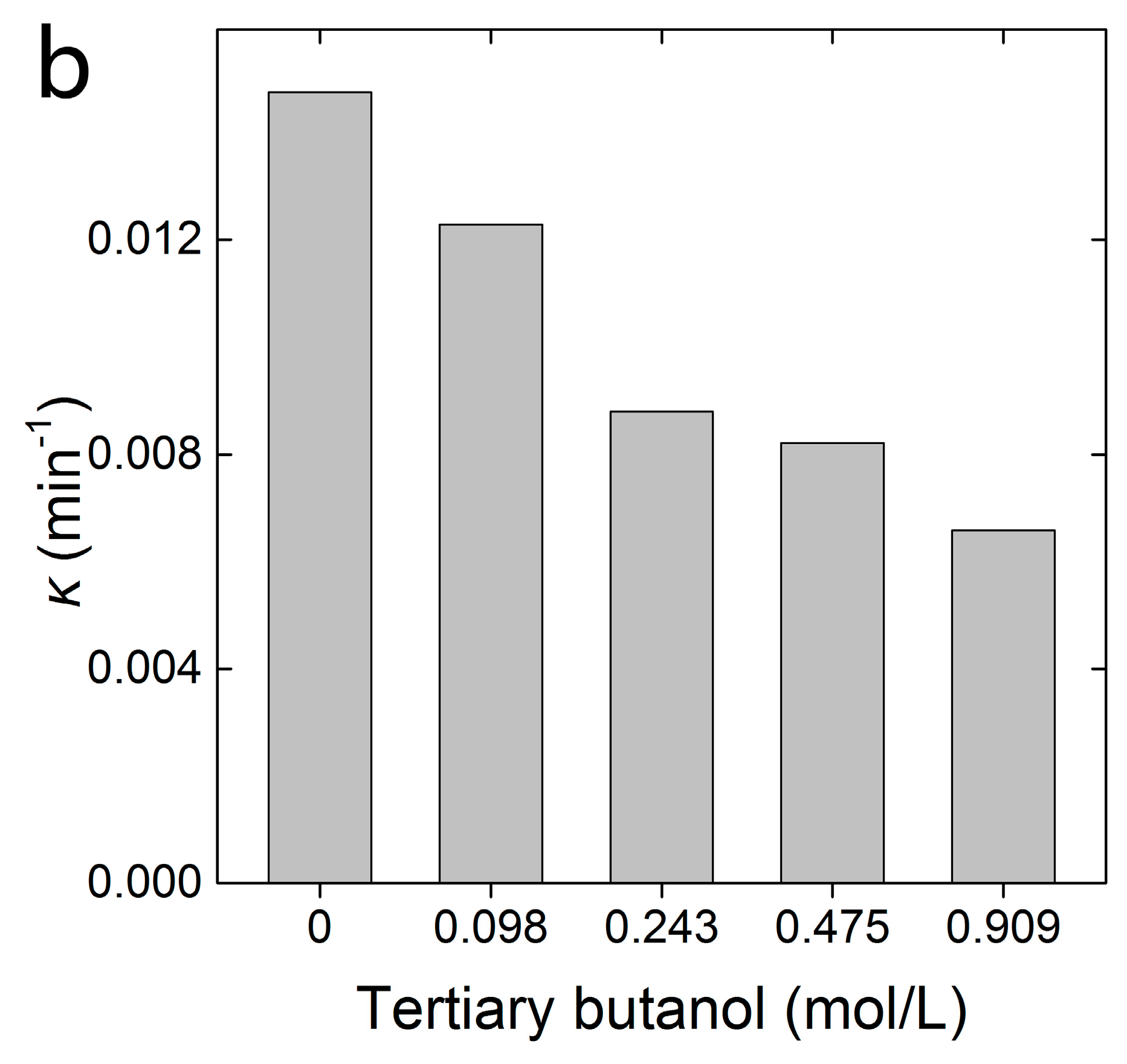
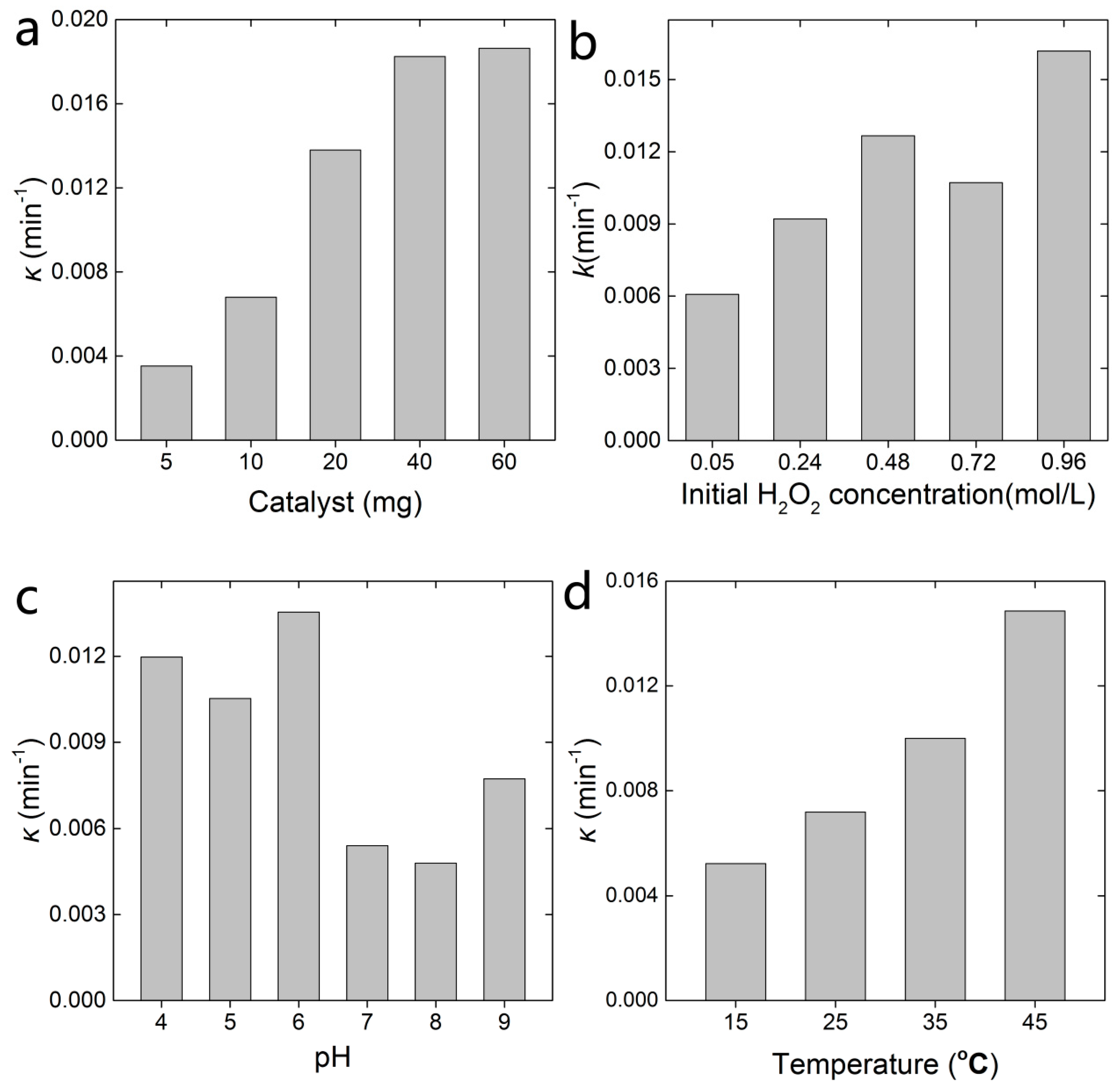
2.2. Decoloration of MB in the FTCNC-H2O2 Fenton-Like System
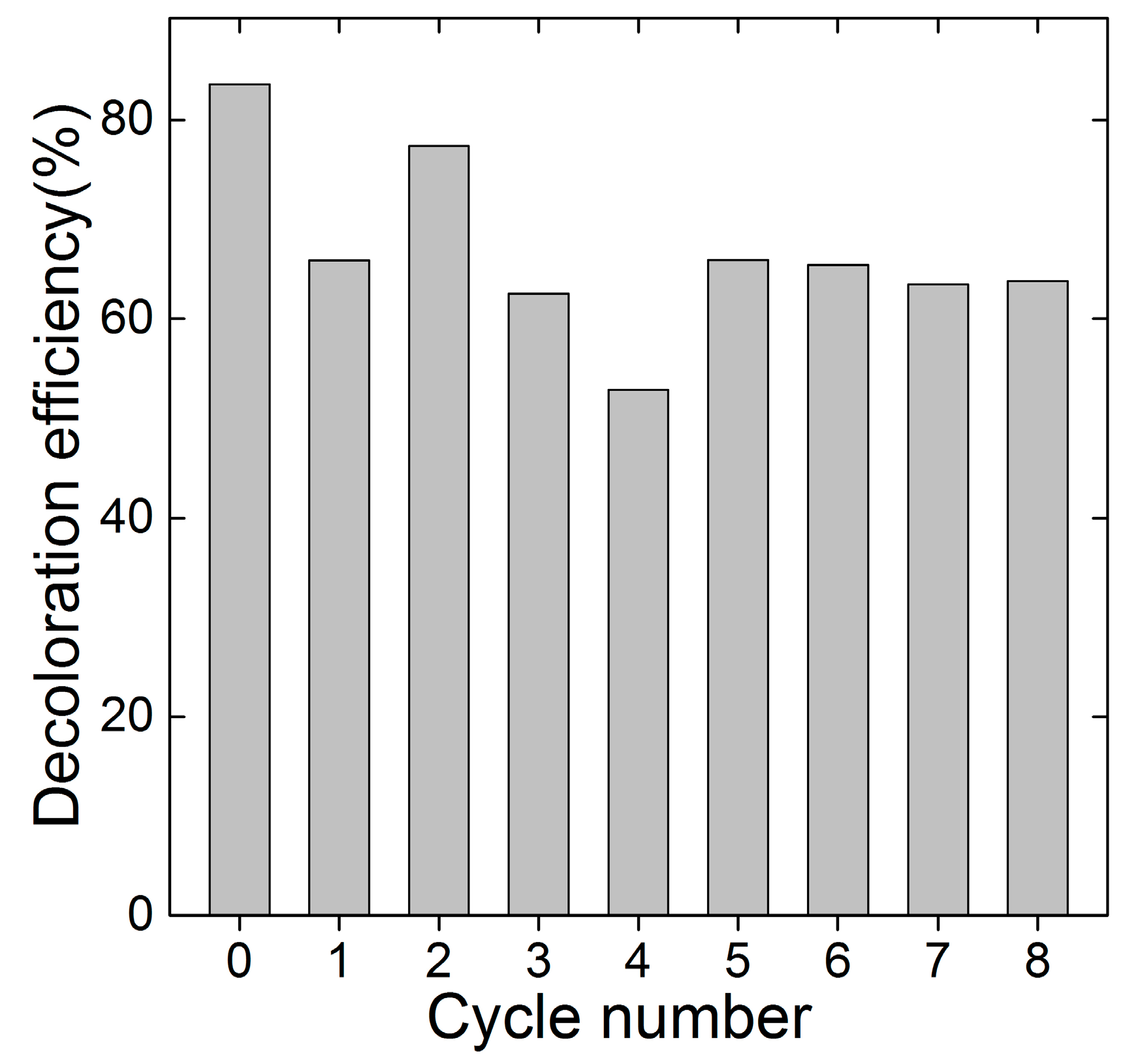
2.3. The Recycle of FTCNCs
3. Materials and Methods
3.1. Preparation of FTCNCs
3.2. Decoloration of MB in FTCNC-H2O2 Fenton-Like System
3.3. Resistance to Radical Scavengers
3.4. Influencing Factors
3.5. Regeneration of FTCNC
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sires, I.; Brillas, E. Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review. Environ. Int. 2012, 40, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; van Hullebusch, E.D.; Rodrigo, M.A.; Esposito, G.; Oturan, M.A. Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes: A review. Chem. Eng. J. 2013, 228, 944–964. [Google Scholar] [CrossRef]
- Naumczyk, J.; Bogacki, J.; Marcinowski, P.; Kowalik, P. Cosmetic waste water treatment by coagulation and advanced oxidation processes. Environ. Technol. 2013, 35, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Xu, H.Y.; Prasad, M.; He, X.L.; Shan, L.W.; Qi, S.Y. Discoloration of Rhodamine B dyeing waste water by schorl-catalyzed Fenton-like reaction. Sci. Chin. Technol. Sci. 2009, 52, 3054–3060. [Google Scholar] [CrossRef]
- Niu, H.Y.; Zhang, D.; Zhang, S.X.; Zhang, X.L.; Meng, Z.F.; Cai, Y.Q. Humic acid coated Fe3O4 magnetic nanoparticles as highly efficient Fenton-like catalyst for complete mineralization of sulfathiazole. J. Hazard. Mater. 2011, 190, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Zhang, W.; Xie, J.R.; Liao, R.; Zhang, X.L.; Yu, B.W.; Wu, R.H.; Liu, X.Y.; Li, H.L.; Guo, Z. Fe3O4@SiO2 nanoparticles as high-performance Fenton-like catalyst in neutral environment. RSC Adv. 2015, 5, 5458–5463. [Google Scholar] [CrossRef]
- Bai, D.; Yan, P. Magnetic nanoscaled Fe3O4 as an efficient and reusable heterogeneous catalyst for degradation of methyl orange in microwave-enhanced Fenton-like system. Appl. Mech. Mater. 2014, 448–453, 830–833. [Google Scholar]
- Xu, L.J.; Wang, J.L. Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-Like heterogeneous catalyst for degradation of 4-chlorophenol. Environ. Sci. Technol. 2012, 46, 10145–10153. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, S.B.; Adhoum, N.; Monser, L. Synthesis of magnetic alginate beads based on Fe3O4 nanoparticles for the removal of 3-methylindole from aqueous solution using Fenton process. J. Hazard. Mater. 2015, 294, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yu, S.R.; Feng, H.X. Fenton-like mineralization of anion surfactant by Fe2O3/attapulgite catalyst. Adv. Mater. Res. 2012, 399–401, 1392–1395. [Google Scholar] [CrossRef]
- Luo, M.S.; Yuan, S.H.; Tong, M.; Liao, P.; Xie, W.J.; Xu, X.F. An integrated catalyst of Pd supported on magnetic Fe3O4 nanoparticles: Simultaneous production of H2O2 and Fe2+ for efficient electro-Fenton degradation of organic contaminants. Water Res. 2014, 48, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.X.; Fang, Z.Q.; Fang, X.B.; Tsang, E.P. Ultrasonic Fenton-like catalytic degradation of bisphenol A by ferroferric oxide (Fe3O4) nanoparticles prepared from steel pickling waste liquor. J. Colloid Interface Sci. 2014, 436, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Shih, Y. Degradation and detoxification of diazinon by sono-Fenton and sono-Fenton-like processes. Sep. Purif. Technol. 2015, 140, 6–12. [Google Scholar] [CrossRef]
- Paola, A.D.; Bellardita, M.; Palmisano, L. Brookite, the least known TiO2 photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Yasuda, M.; Tomo, T.; Hirata, S.; Shiragami, T.; Matsumoto, T. Neighboring hetero-atom assistance of sacrificial amines to hydrogen evolution using Pt-loaded TiO2-photocatalyst. Catalysts 2014, 4, 162–173. [Google Scholar] [CrossRef]
- Gil, S.; Garcia-Vargas, J.M.; Liotta, L.F.; Pantaleo, G.; Ousmane, M.; Retailleau, L.; Giroir-Fendler, A. Catalytic oxidation of propene over Pd catalysts supported on CeO2, TiO2, Al2O3 and M/Al2O3 oxides (M = Ce, Ti, Fe, Mn). Catalysts 2015, 5, 671–689. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Yang, X.; Shao, E.; Deng, X.; Liu, N.; Wu, M. Facile synthesis of three-dimensional Mn3O4 hierarchical microstructures and their application in the degradation of methylene blue. J. Mater. Chem. A 2015, 3, 2934–2941. [Google Scholar] [CrossRef]
- Yang, S.T.; Yang, L.J.; Liu, X.Y.; Xie, J.R.; Zhang, X.L.; Yu, B.W.; Wu, R.H.; Li, H.L.; Chen, L.Y.; Liu, J.H. TiO2-doped Fe3O4 nanoparticles as high-performance Fenton-like catalyst for dye decoloration. Sci. Chin. Technol. Sci. 2015, 58, 858–863. [Google Scholar] [CrossRef]
- Hsieh, S.; Lin, P.Y. FePt nanoparticles as heterogeneous Fenton-like catalysts for hydrogen peroxide decomposition and the decolorization of methylene blue. J. Nanopart. Res. 2012, 14, 956–965. [Google Scholar] [CrossRef]
- Wang, R.J.; Liu, X.Y.; Wu, R.H.; Yu, B.W.; Li, H.L.; Zhang, X.L.; Xie, J.R.; Yang, S.T. Fe3O4/SiO2/C nanocomposite as a high-performance Fenton-like catalyst in a neutral environment. RSC Adv. 2016, 6, 8594–8600. [Google Scholar] [CrossRef]
- Zhou, Q.; Gong, W.Q.; Yang, D.J.; Xie, C.X.; Li, Y.B.; Liu, X.F.; Bai, C.P.; Wang, R. Assessment of the biosorption characteristics of a spent cottonseed husk substrate for the decolorization of methylene blue. Clean Soil Air Water 2011, 39, 1087–1094. [Google Scholar] [CrossRef]
- Low, L.W.; Teng, T.T.; Alkarkhi, A.F.; Ahmad, A.; Morad, N. Optimization of the adsorption conditions for the decolorization and COD reduction of methylene blue aqueous solution using low-cost adsorbent. Water Air Soil Pollut. 2011, 214, 185–195. [Google Scholar] [CrossRef]
- Min, Y.L.; Zhang, K.; Zhao, W.; Zheng, F.C.; Chen, Y.C.; Zhang, Y.G. Enhanced chemical interaction between TiO2 and graphene oxide for photocatalytic decolorization of methylene blue. Chem. Eng. J. 2012, 193–194, 203–210. [Google Scholar] [CrossRef]
- Zhang, C.F.; Qiu, L.G.; Ke, F.; Zhu, Y.J.; Yuan, Y.P.; Xu, G.S.; Jiang, X. A novel magnetic recyclable photocatalyst based on metal-organic framework Fe3O4@MIL-100(Fe) core-shell for the decolorization of methylene blue dye. J. Mater. Chem. A 2013, 1, 14329–14334. [Google Scholar] [CrossRef]
- Rachmilovich-Calis, S.; Masarwa, A.; Meyerstein, N.; Meyerstein, D.; van Eldik, R. New mechanistic aspects of the Fenton reaction. Chem. Eur. J. 2009, 15, 8303–8309. [Google Scholar] [CrossRef] [PubMed]
- Bertus, L.M.; Carcel, R.A. Prediction of TiO2 and WO3 nanopowders surface charge by the evaluation of point of zero charge (PZC). Environ. Eng. Manag. J. 2011, 10, 1021–1026. [Google Scholar]
- Zhang, X.L.; He, M.L.; Liu, J.H.; Liao, R.; Zhao, L.Q.; Xie, J.R.; Wang, R.J.; Yang, S.T.; Wang, H.F.; Liu, Y.F. Fe3O4@C nanoparticles as high-performance Fenton-like catalyst for dye decoloration. Chin. Sci. Bull. 2014, 59, 3406–3412. [Google Scholar] [CrossRef]
- Zubir, N.A.; Yacou, C.; Motuzas, J.; Zhang, X.; Zhao, X.; da Costa, J.C.D. The sacrificial role of graphene oxide in stabilising a Fenton-like catalyst GO-Fe3O4. Chem. Commun. 2015, 51, 9291–9293. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, Q.; Yu, B.; Wu, R.; Mai, J.; Wang, R.; Chen, L.; Yang, S.-T. Preparation of Fe3O4/TiO2/C Nanocomposites and Their Application in Fenton-Like Catalysis for Dye Decoloration. Catalysts 2016, 6, 146. https://doi.org/10.3390/catal6090146
Liu X, Zhang Q, Yu B, Wu R, Mai J, Wang R, Chen L, Yang S-T. Preparation of Fe3O4/TiO2/C Nanocomposites and Their Application in Fenton-Like Catalysis for Dye Decoloration. Catalysts. 2016; 6(9):146. https://doi.org/10.3390/catal6090146
Chicago/Turabian StyleLiu, Xiaoyang, Qian Zhang, Baowei Yu, Ruihan Wu, Jinxia Mai, Ruijue Wang, Lingyun Chen, and Sheng-Tao Yang. 2016. "Preparation of Fe3O4/TiO2/C Nanocomposites and Their Application in Fenton-Like Catalysis for Dye Decoloration" Catalysts 6, no. 9: 146. https://doi.org/10.3390/catal6090146