Hydrothermal Method Using DMF as a Reducing Agent for the Fabrication of PdAg Nanochain Catalysts towards Ethanol Electrooxidation
Abstract
:1. Introduction
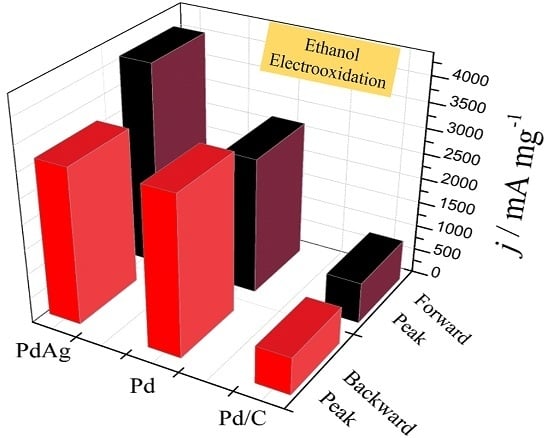
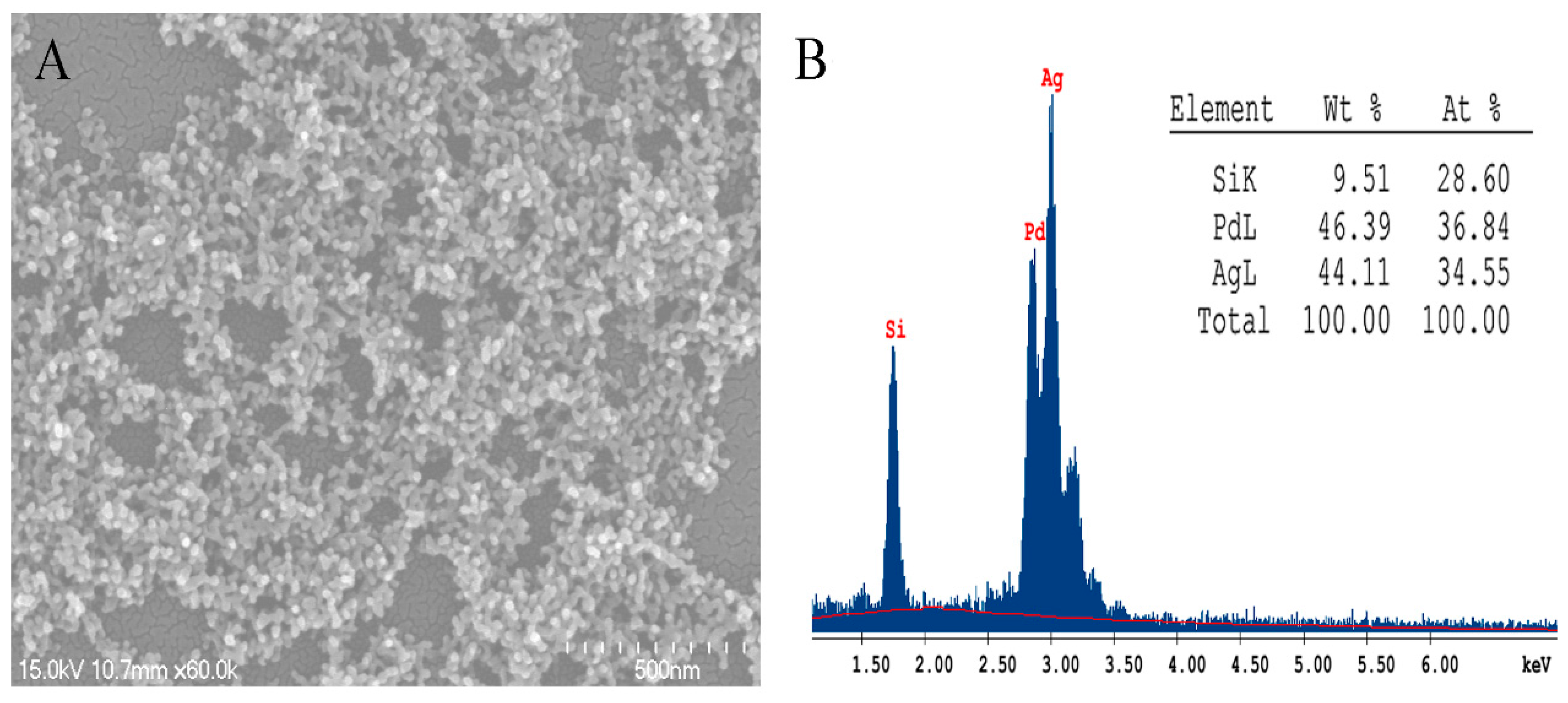
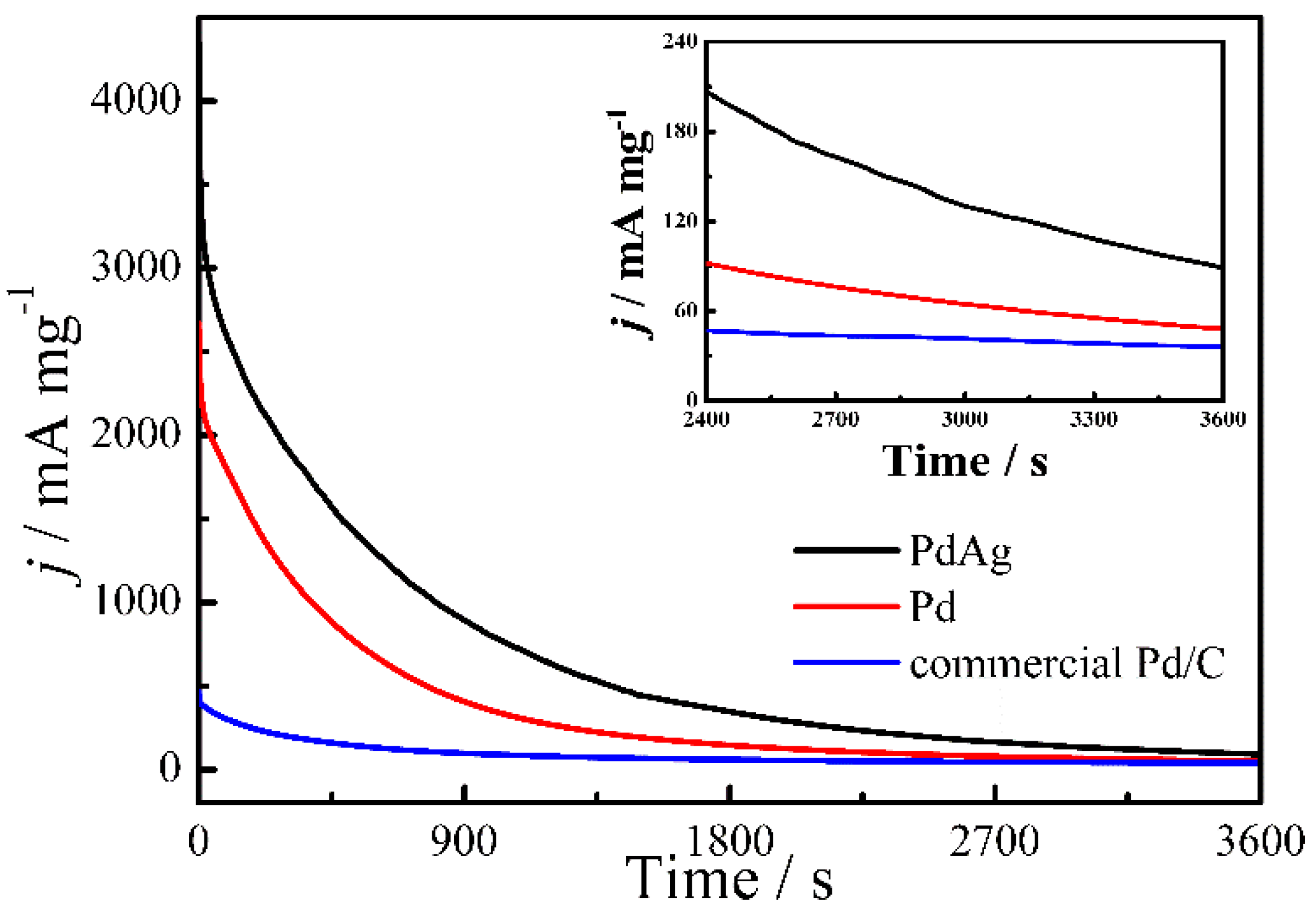
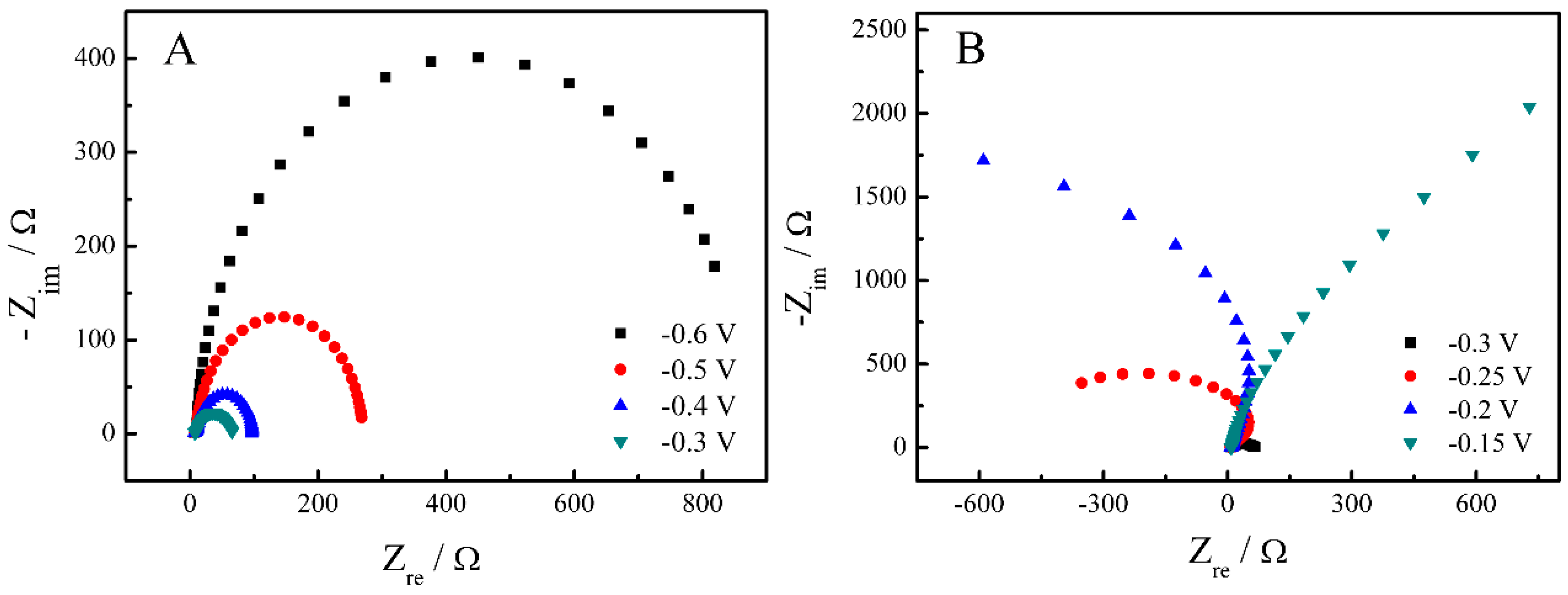
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis of Nanochain PdAg Catalyst
3.3. Preparation of Pd and PdAg Modified Electrodes
3.4. Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guo, Y.; Zheng, Y.; Huang, M. Enhanced activity of PtSn/C anodic electrocatalyst prepared by formic acid reduction for direct ethanol fuel cells. Electrochim. Acta 2008, 53, 3102–3108. [Google Scholar] [CrossRef]
- Li, Y.S.; Zhao, T.S.; Liang, Z.X. Performance of alkaline electrolyte-membrane-based direct ethanol fuel cells. J. Power Sources 2009, 187, 387–392. [Google Scholar] [CrossRef]
- Antolini, E. Catalysts for direct ethanol fuel cells. J. Power Sources 2007, 170, 1–12. [Google Scholar] [CrossRef]
- Bambagioni, V.; Bianchini, C.; Marchionni, A.; Filippi, J.; Vizza, F.; Teddy, J.; Serp, P.; Zhiani, M. Pd and Pt–Ru anode electrocatalysts supported on multi-walled carbon nanotubes and their use in passive and active direct alcohol fuel cells with an anion-exchange membrane (alcohol = methanol, ethanol, glycerol). J. Power Sources 2009, 190, 241–251. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, S.; Dong, S. PdM (M = Pt, Au) Bimetallic Alloy Nanowires with Enhanced Electrocatalytic Activity for Electro-oxidation of Small Molecules. Adv. Mater. 2012, 24, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Hu, Y.; Liu, M.; Zheng, Y. Hollow raspberry-like PdAg alloy nanospheres: High electrocatalytic activity for ethanol oxidation in alkaline media. J. Power Sources 2015, 278, 69–75. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, C.; Bin, D.; Zhai, C.; Ren, F.; Yang, P.; Du, Y. One-pot Synthesis of PtSn Bimetallic Composites and Their Application as Highly Active Catalysts for Ethanol Electrooxidation. ChemPlusChem 2016, 81, 93–99. [Google Scholar] [CrossRef]
- Cheng, F.; Dai, X.; Wang, H.; Jiang, S.P.; Zhang, M.; Xu, C. Synergistic effect of Pd–Au bimetallic surfaces in Au-covered Pd nanowires studied for ethanol oxidation. Electrochim. Acta 2010, 55, 2295–2298. [Google Scholar] [CrossRef]
- Soundararajan, D.; Park, J.H.; Kim, K.H.; Ko, J.M. Pt–Ni alloy nanoparticles supported on CNF as catalyst for direct ethanol fuel cells. Current Appl. Phys. 2012, 12, 854–859. [Google Scholar] [CrossRef]
- Xu, C.; Tian, Z.; Shen, P.; Jiang, S.P. Oxide (CeO2, NiO, Co3O4 and Mn3O4)-promoted Pd/C electrocatalysts for alcohol electrooxidation in alkaline media. Electrochim. Acta 2008, 53, 2610–2618. [Google Scholar] [CrossRef]
- Shen, P.K.; Xu, C. Alcohol oxidation on nanocrystalline oxide Pd/C promoted electrocatalysts. Electrochem. Commun. 2006, 8, 184–188. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, F.; Shen, P.K. Carbonized porous anodic alumina as electrocatalyst support for alcohol oxidation. Electrochem. Commun. 2006, 8, 1764–1768. [Google Scholar] [CrossRef]
- Hong, W.; Wang, J.; Wang, E. Synthesis of porous PdAg nanoparticles with enhanced electrocatalytic activity. Electrochem. Commun. 2014, 40, 63–66. [Google Scholar] [CrossRef]
- Hong, J.W.; Kim, D.; Lee, Y.W.; Kim, M.; Kang, S.W.; Han, S.W. Atomic-distribution-dependent electrocatalytic activity of Au-Pd bimetallic nanocrystals. Angew. Chem. 2011, 50, 8876–8880. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Bin, D.; Feng, Y.; Zhang, K.; Wang, J.; Wang, C.; Guo, J.; Yang, P.; Du, Y. Synthesis and high electrocatalytic activity of Au-decorated Pd heterogeneous nanocube catalysts for ethanol electro-oxidation in alkaline media. Catal. Sci. Technol. 2016, 6, 5397–5404. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, S.; Dong, S. Rapid, general synthesis of PdPt bimetallic alloy nanosponges and their enhanced catalytic performance for ethanol/methanol electrooxidation in an alkaline medium. Chem. Eur. J. 2013, 19, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zeng, Y.; Guo, Y. Copper@palladium–copper core–shell nanospheres as a highly effective electrocatalyst for ethanol electro-oxidation in alkaline media. J. Power Sources 2014, 270, 257–261. [Google Scholar] [CrossRef]
- Li, G.; Jiang, L.; Jiang, Q.; Wang, S.; Sun, G. Preparation and characterization of PdxAgy/C electrocatalysts for ethanol electrooxidation reaction in alkaline media. Electrochim. Acta 2011, 56, 7703–7711. [Google Scholar] [CrossRef]
- Zhu, L.D.; Zhao, T.S.; Xu, J.B.; Liang, Z.X. Preparation and characterization of carbon-supported sub-monolayer palladium decorated gold nanoparticles for the electro-oxidation of ethanol in alkaline media. J. Power Sources 2009, 187, 80–84. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Liz-Marza´n, L.M. Synthesis of Silver Nanoprisms in DMF. Nano Lett. 2002, 2, 903–905. [Google Scholar] [CrossRef]
- Wiley, B.J.; Xiong, Y.; Li, Z.Y.; Yin, Y.; Xia, Y. Right Bipyramids of Silver: A New Shape Derived from Single Twinned Seeds. Nano Letters 2006, 6, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qiao, X.; Chen, J.; Wang, X.; Ding, S. Mechanisms of PVP in the preparation of silver nanoparticles. Mater. Chem. Phys. 2005, 94, 449–453. [Google Scholar] [CrossRef]
- Lan, F.; Wang, D.; Lu, S.; Zhang, J.; Liang, D.; Peng, S.; Liu, Y.; Xiang, Y. Ultra-low loading Pt decorated coral-like Pd nanochain networks with enhanced activity and stability towards formic acid electrooxidation. J. Mater. Chem. A 2013, 1, 1548–1552. [Google Scholar] [CrossRef]
- Jana, R.; Subbarao, U.; Peter, S.C. Ultrafast synthesis of flower-like ordered Pd3Pb nanocrystals with superior electrocatalytic activities towards oxidation of formic acid and ethanol. J. Power Sources 2016, 301, 160–169. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, W.; Wang, J.; Wexler, D.; Poynton, S.D.; Slade, R.C.; Liu, H.; Winther-Jensen, B.; Kerr, R.; Shi, D.; Chen, J. PdNi hollow nanoparticles for improved electrocatalytic oxygen reduction in alkaline environments. ACS Appl. Mater. Inter. 2013, 5, 12708–12715. [Google Scholar] [CrossRef] [PubMed]
- Colmati, F.; Antolini, E.; Gonzalez, E.R. Ethanol oxidation on a carbon-supported Pt75Sn25 electrocatalyst prepared by reduction with formic acid: Effect of thermal treatment. Appl. Catal. 2007, 73, 106–115. [Google Scholar] [CrossRef]
- Bin, D.; Ren, F.; Wang, Y.; Zhai, C.; Wang, C.; Guo, J.; Yang, P.; Du, Y. Pd-nanoparticle-supported, PDDA-functionalized graphene as a promising catalyst for alcohol oxidation. Chem. Asian J. 2015, 10, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xie, Y.; Wang, R.; Jiang, B.; Tian, C.; Mu, G.; Yin, J.; Wang, B.; Fu, H. Synergistic effect of tungsten carbide and palladium on graphene for promoted ethanol electrooxidation. ACS Appl. Mater. Inter. 2013, 5, 6571–6579. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Yue, R.; Zhai, C.; Jiang, F.; Wang, H.; Du, Y.; Wang, C.; Yang, P. Electrochemical layer-by-layer fabrication of a novel three-dimensional Pt/graphene/carbon fiber electrode and its improved catalytic performance for methanol electrooxidation in alkaline medium. Int. J. Hydrogen Energy 2013, 38, 6368–6376. [Google Scholar] [CrossRef]
- Bai, L.; Zhu, H.; Thrasher, J.S.; Street, S.C. Synthesis and electrocatalytic activity of photoreduced platinum nanoparticles in a poly(ethylenimine) matrix. ACS Appl. Mater. Inter. 2009, 1, 2304–2311. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Cui, X. Platinum/Carbon Nanotube Nanocomposite Synthesized in Supercritical Fluid as Electrocatalysts for Low-Temperature Fuel Cells. J. Phys. Chem. B 2005, 109, 14410–14415. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Mahapatra, S.S.; Datta, J. High performance PtPdAu nano-catalyst for ethanol oxidation in alkaline media for fuel cell applications. Int. J. Hydrogen Energy 2011, 36, 14898–14906. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Zhai, C.; Ren, F.; Zhu, M.; Yang, P.; Du, Y. Three-dimensional Au0.5/reduced graphene oxide/Au0.5/reduced graphene oxide/carbon fiber electrode and its high catalytic performance toward ethanol electrooxidation in alkaline media. J. Mater. Chem. A 2015, 3, 4389–4398. [Google Scholar] [CrossRef]
- Zhou, W.; Du, Y.; Ren, F.; Wang, C.; Xu, J.; Yang, P. High efficient electrocatalytic oxidation of methanol on Pt/polyindoles composite catalysts. Int. J. Hydrogen Energy 2010, 35, 3270–3279. [Google Scholar] [CrossRef]




| Catalysts | ECSA (Electrochemical Surface Area) (cm2·mg−1) | If (mA·mg−1) | Ib (mA·mg−1) | If/Ib |
|---|---|---|---|---|
| PdAg | 402.1 | 4098.2 | 3137.7 | 1.3 |
| Pd | 328.2 | 2735.3 | 3207.8 | 0.9 |
| Commercial Pd/C | 241.8 | 827.2 | 756.9 | 1.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Zhang, K.; Yan, B.; Li, S.; Du, Y. Hydrothermal Method Using DMF as a Reducing Agent for the Fabrication of PdAg Nanochain Catalysts towards Ethanol Electrooxidation. Catalysts 2016, 6, 103. https://doi.org/10.3390/catal6070103
Feng Y, Zhang K, Yan B, Li S, Du Y. Hydrothermal Method Using DMF as a Reducing Agent for the Fabrication of PdAg Nanochain Catalysts towards Ethanol Electrooxidation. Catalysts. 2016; 6(7):103. https://doi.org/10.3390/catal6070103
Chicago/Turabian StyleFeng, Yue, Ke Zhang, Bo Yan, Shumin Li, and Yukou Du. 2016. "Hydrothermal Method Using DMF as a Reducing Agent for the Fabrication of PdAg Nanochain Catalysts towards Ethanol Electrooxidation" Catalysts 6, no. 7: 103. https://doi.org/10.3390/catal6070103
APA StyleFeng, Y., Zhang, K., Yan, B., Li, S., & Du, Y. (2016). Hydrothermal Method Using DMF as a Reducing Agent for the Fabrication of PdAg Nanochain Catalysts towards Ethanol Electrooxidation. Catalysts, 6(7), 103. https://doi.org/10.3390/catal6070103