The Use of Biobased Surfactant Obtained by Enzymatic Syntheses for Wax Deposition Inhibition and Drag Reduction in Crude Oil Pipelines
Abstract
:1. Introduction
2. Results and Discussion
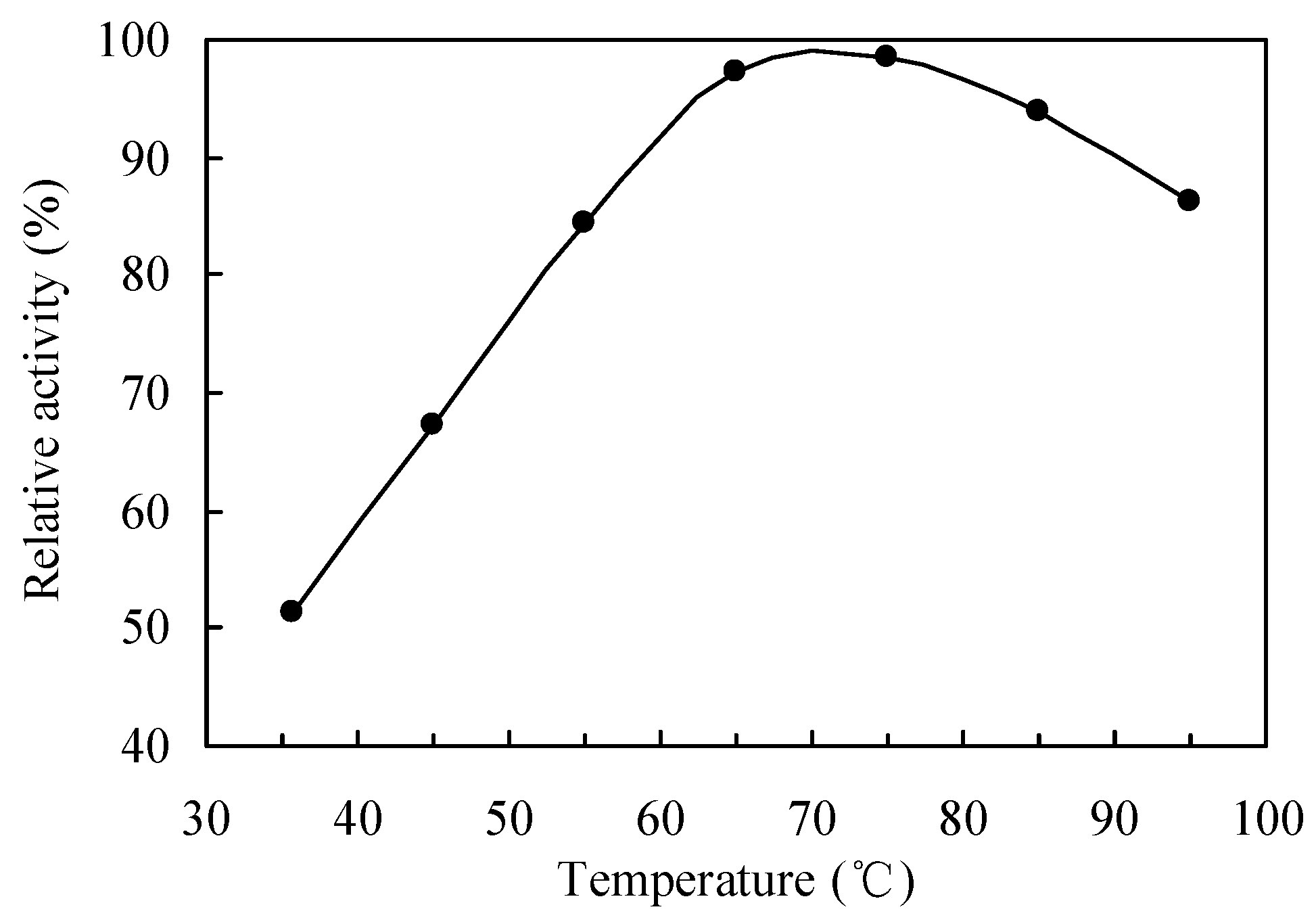
2.1. Selection and Reaction Conditions Optimization of Catalysts during the Drag-Reducing Surfactant Preparation
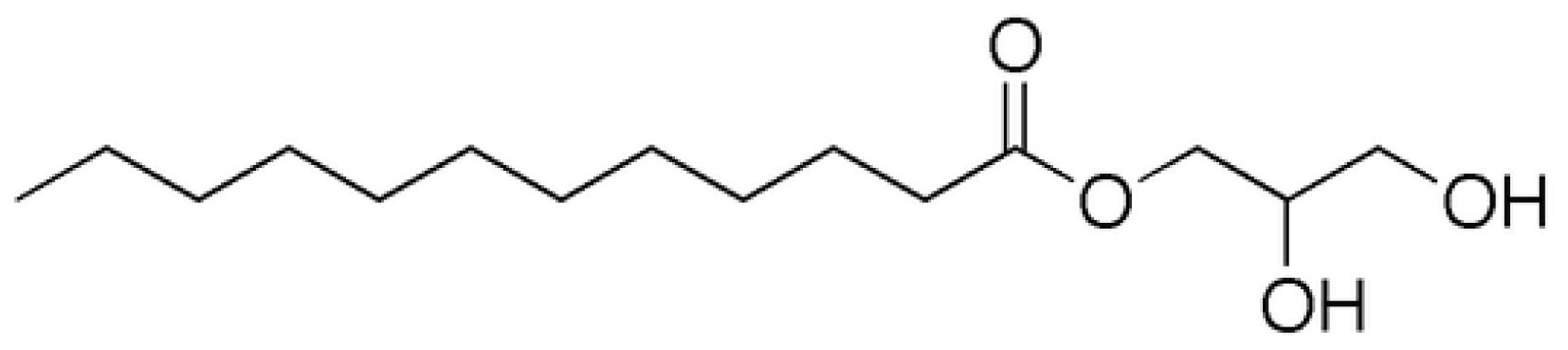
2.2. Enzymatic Process in the Drag-Reducing Surfactant Preparation
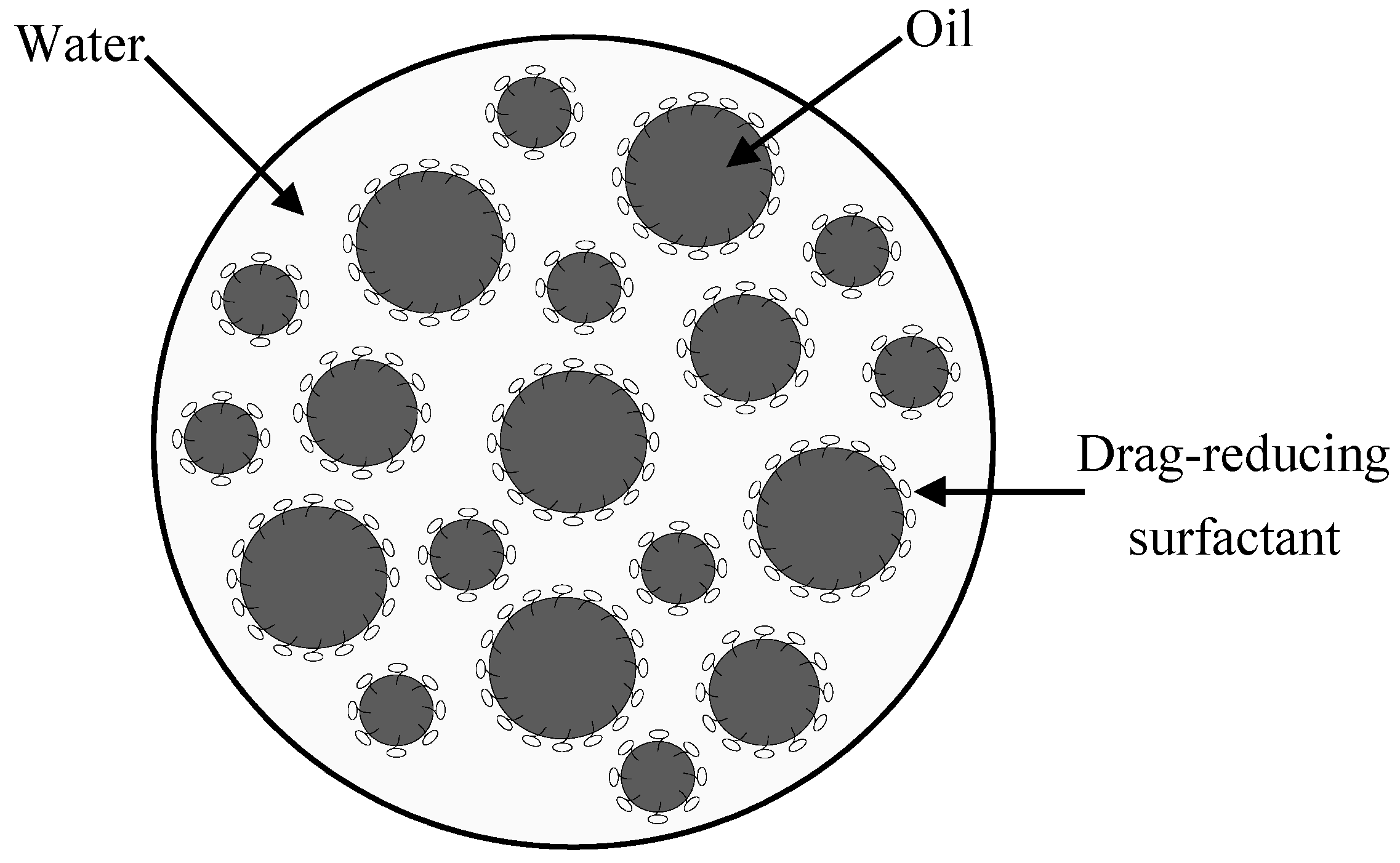
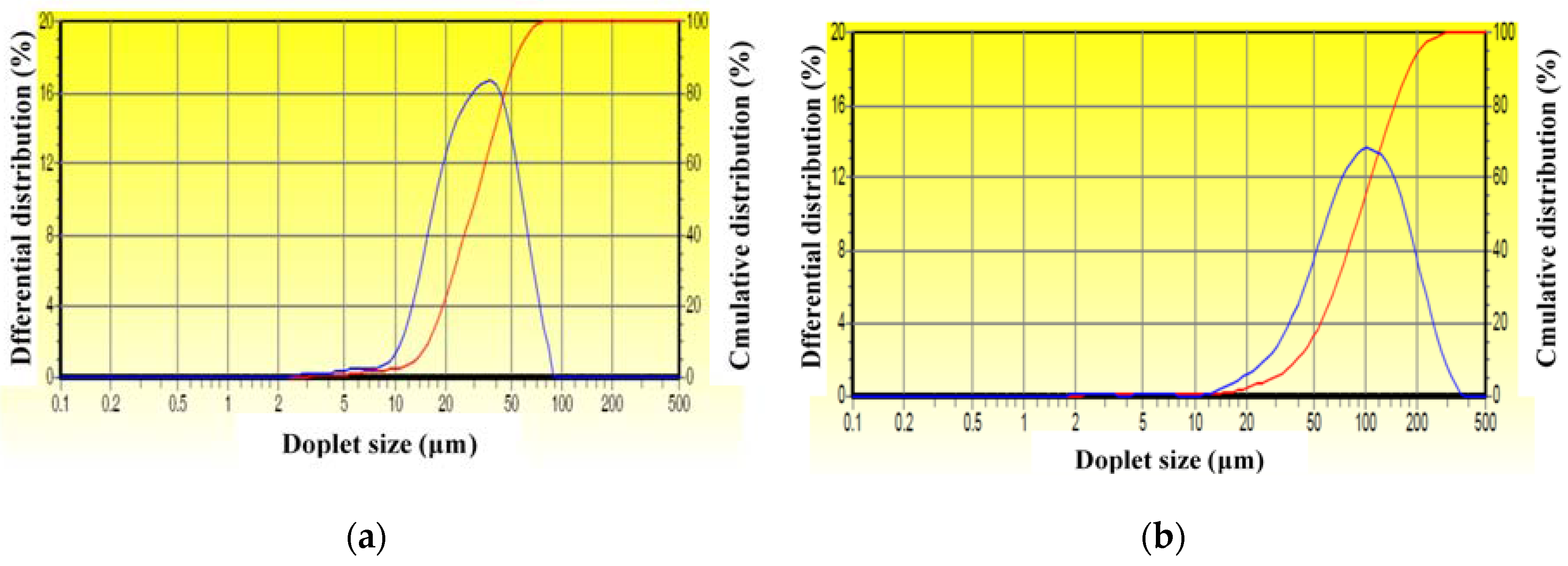
2.3. Availability of the Drag-Reducing Surfactant for Heavy Crude Emulsification
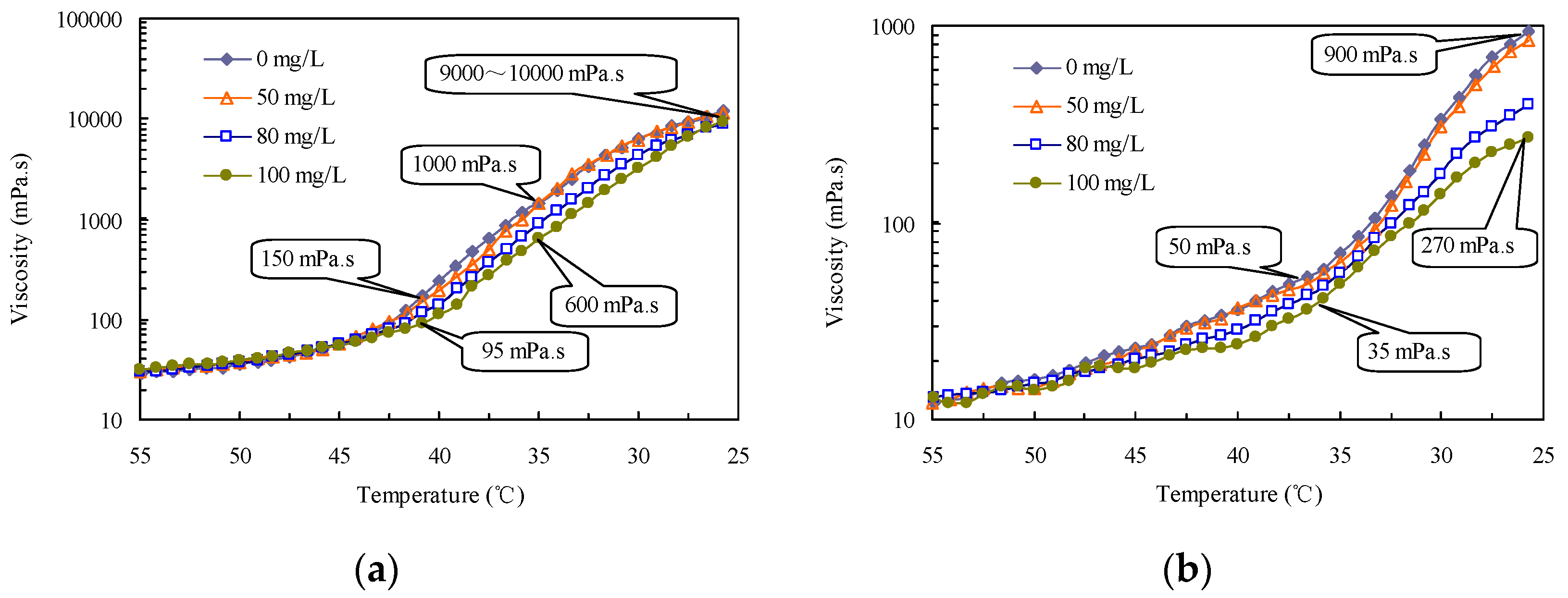
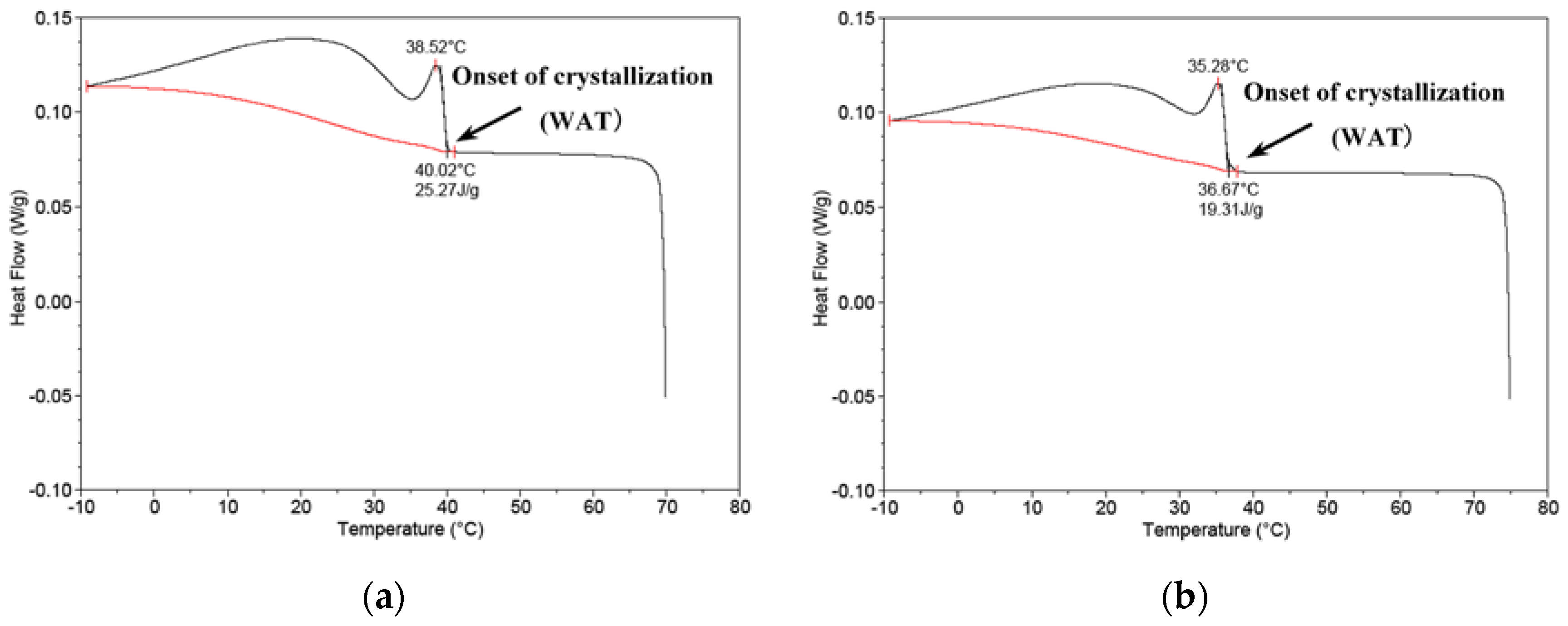
2.4. Viscosity and Pour Point Reduction with the Drag-Reducing Surfactant
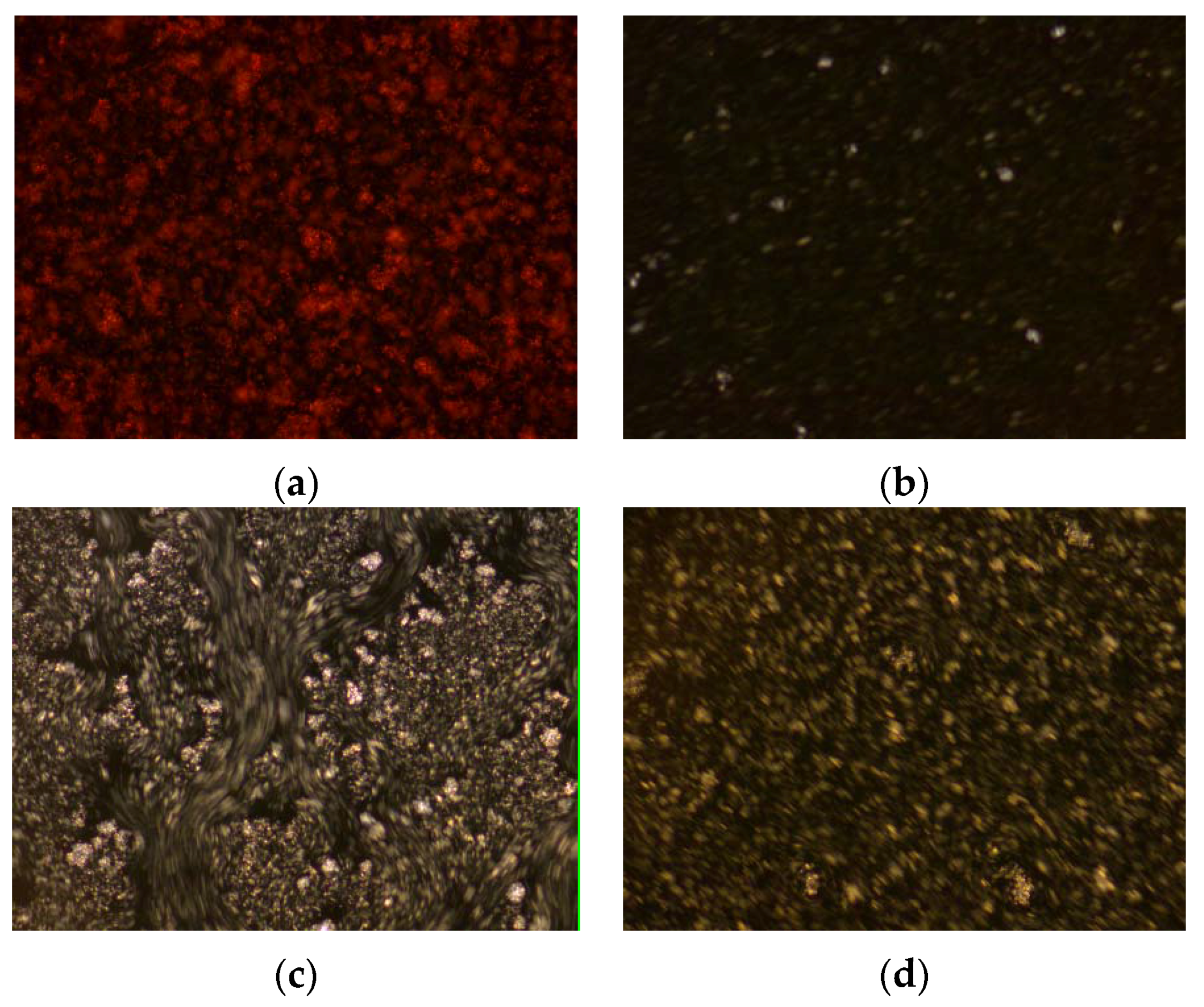
2.5. Effect of the Drag-Reducing Surfactant on Wax Crystals’ Agglomeration Structure
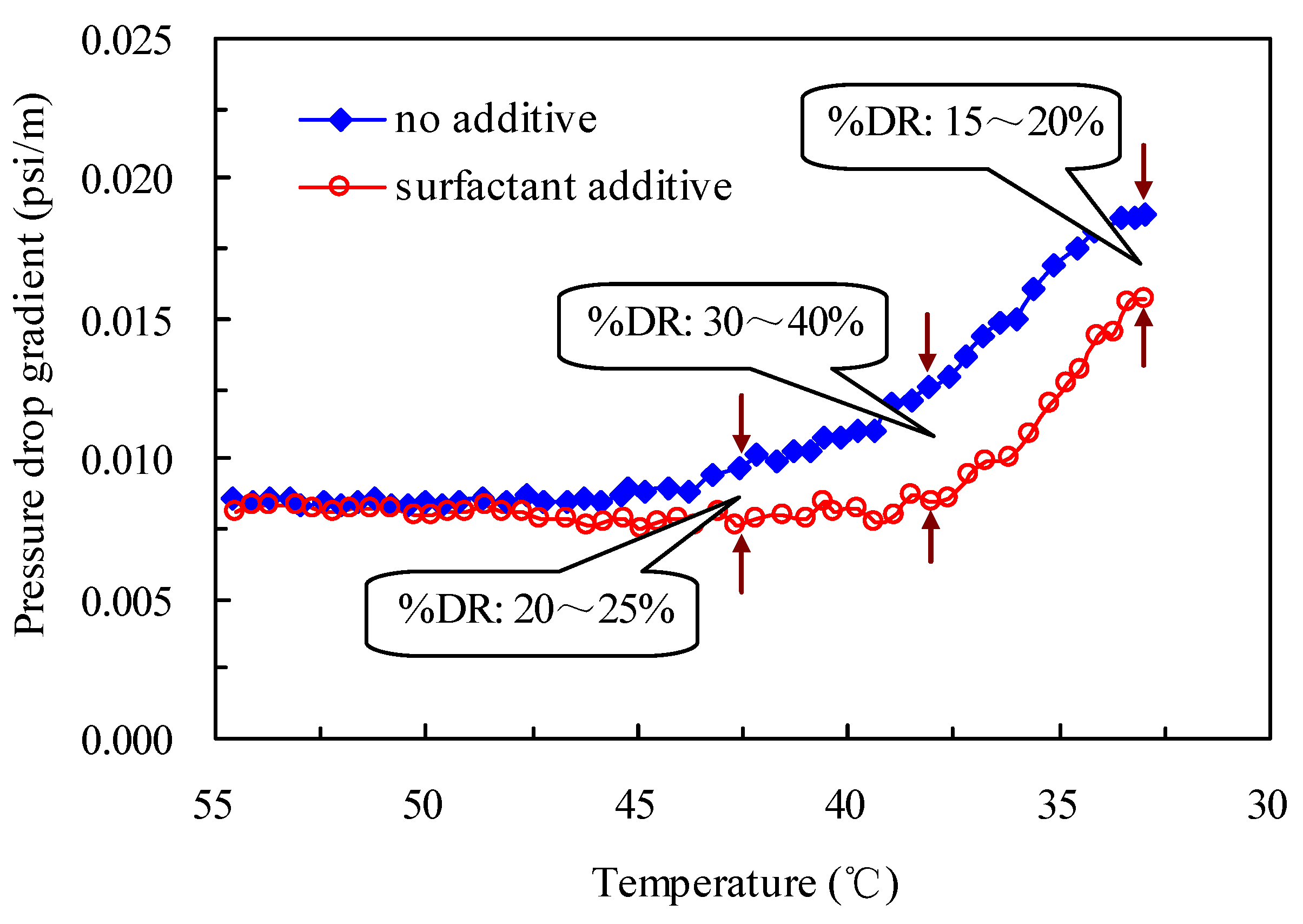
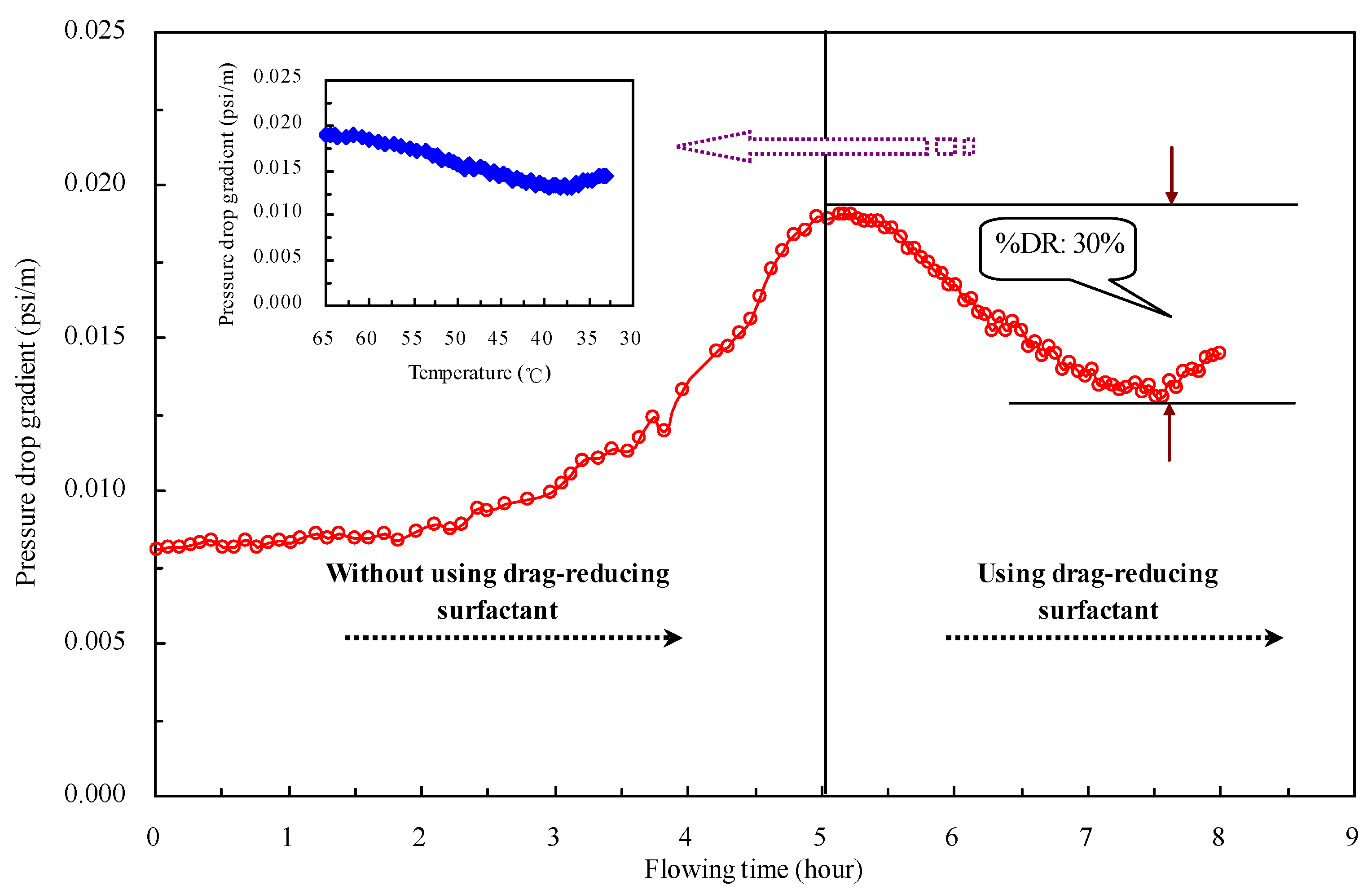
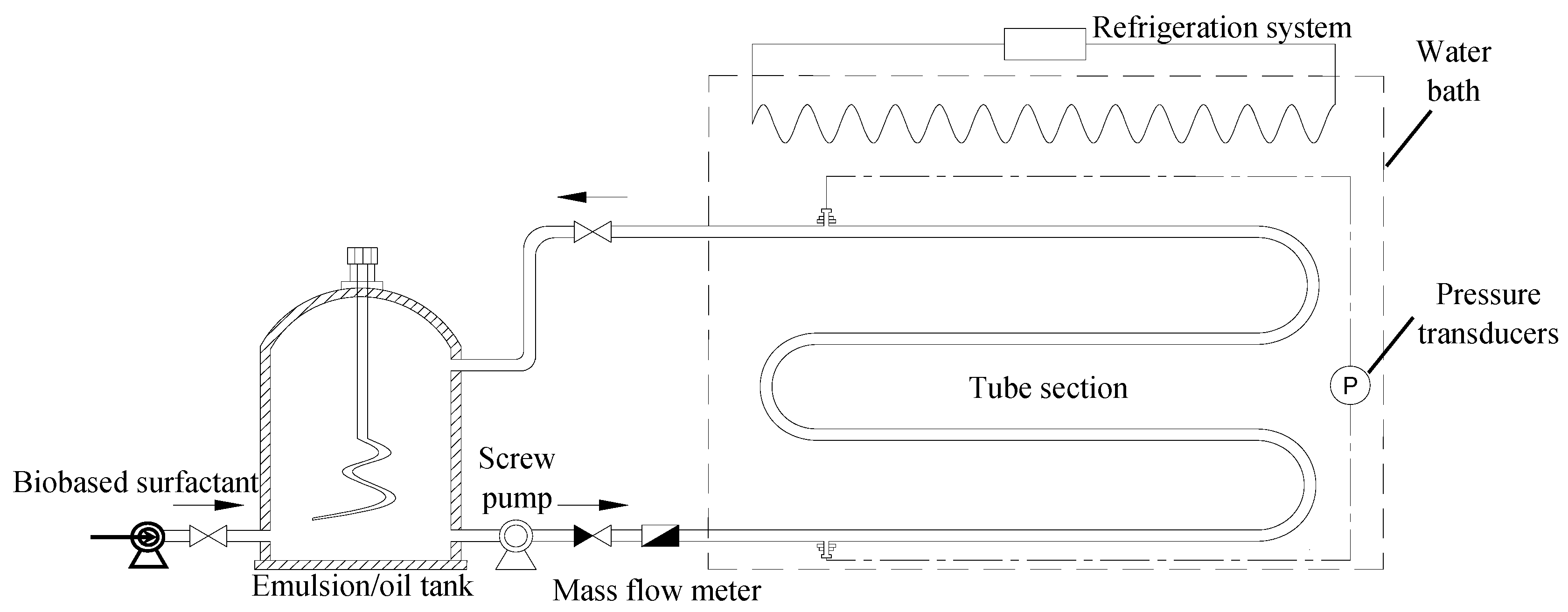
2.6. Pressure Drop Reduction in Oil Pipelines by the Drag-Reducing Surfactant
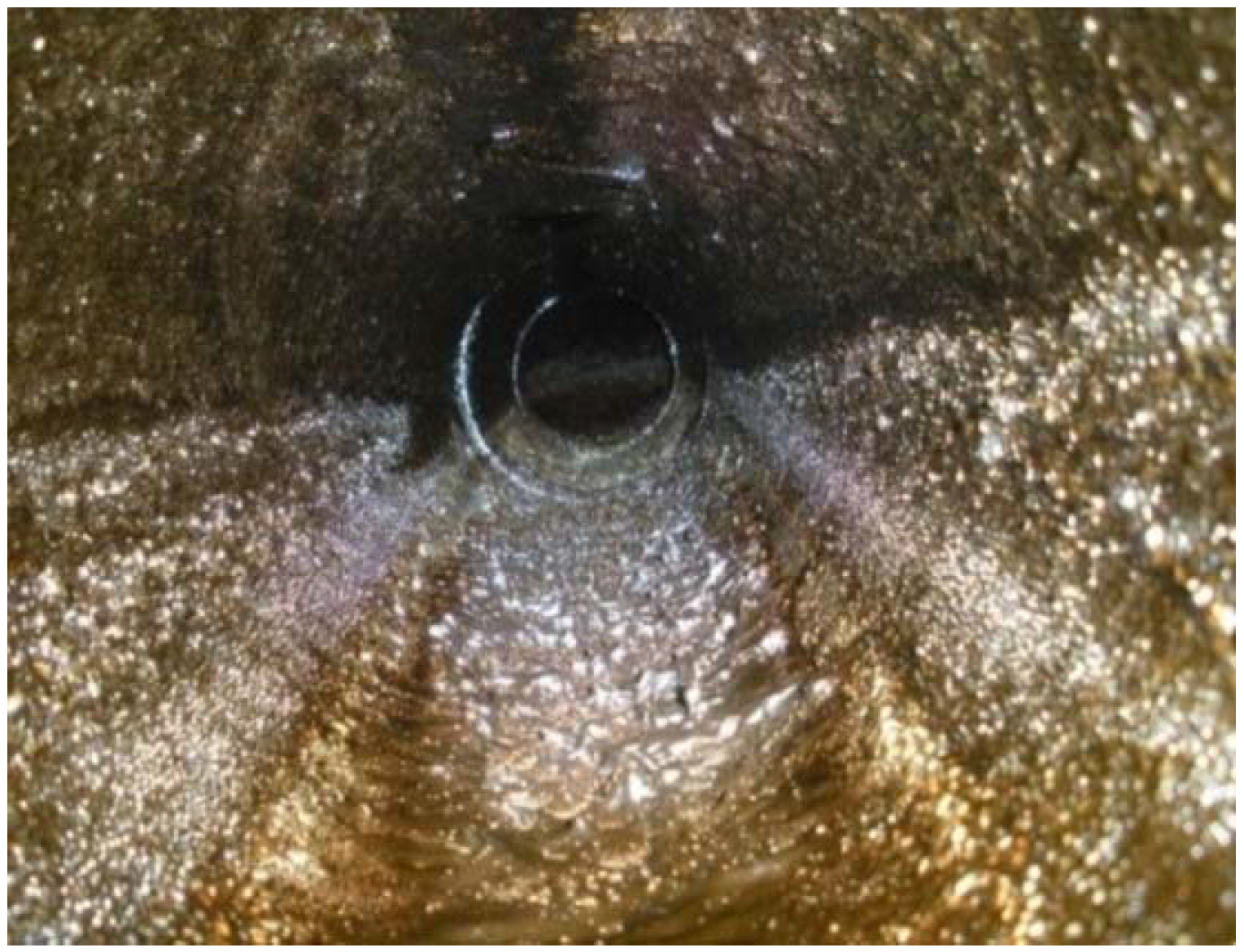
2.7. Field Application Effect of the Drag-Reducing Surfactant
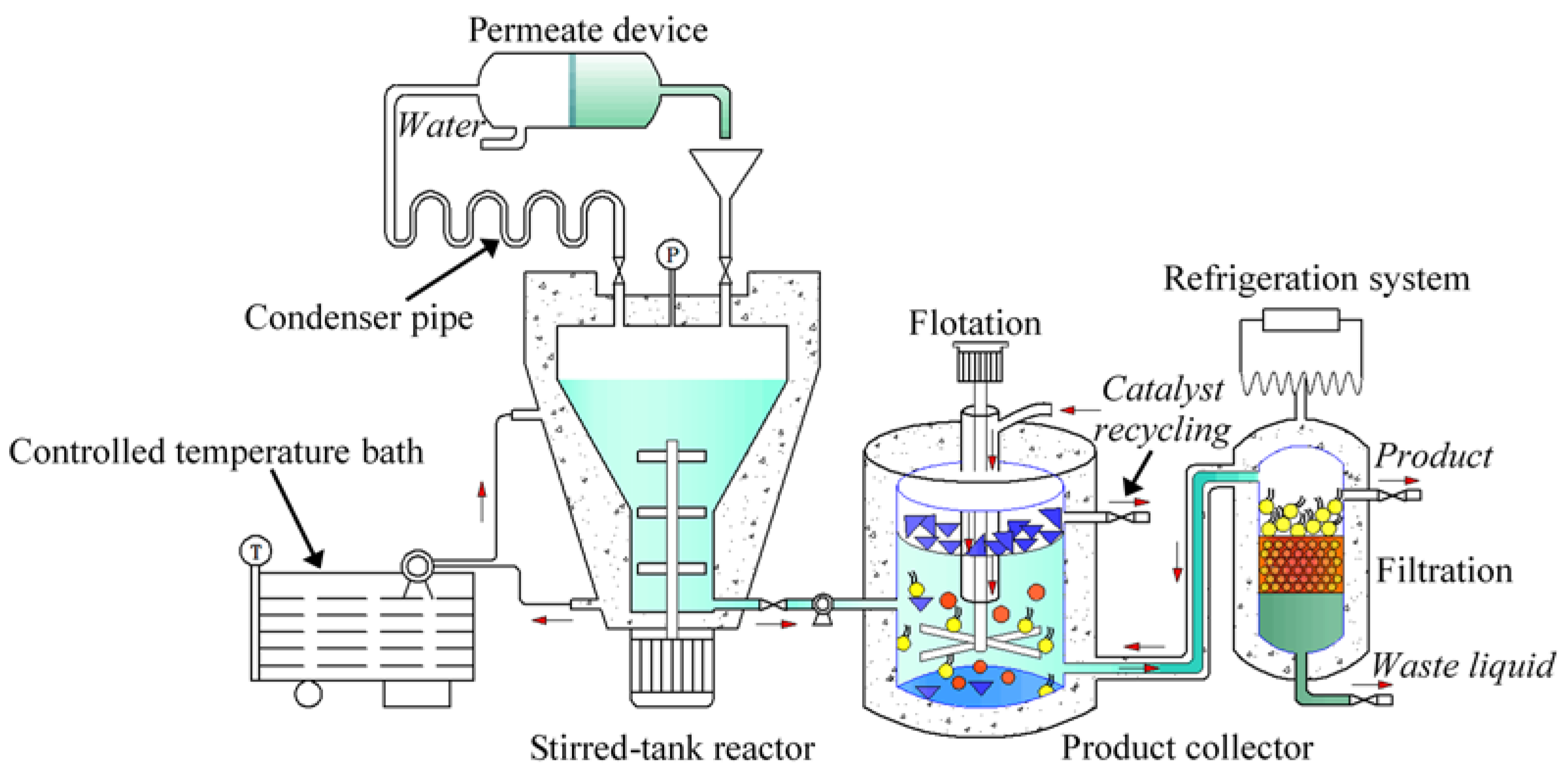
3. Experimental Section
3.1. Materials
3.2. Enzymatic Preparation of Biobased Surfactant
3.3. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hasan, S.W.; Ghannam, M.T.; Esmail, N. Heavy crude oil viscosity reduction and rheology for pipeline transportation. Fuel 2010, 89, 1095–1100. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.H.; Zhuge, X.L.; Zhao, S.B.; Pang, R.S. Study of deposition behavior in small-diameter gathering pipelines for water-cut oil. Chem. Technol. Fuels Oils 2012, 48, 386–392. [Google Scholar] [CrossRef]
- Lafakis, C. A call for crude oil exports and pipelines. Pipeline & Gas J. 2015, 242, 2. [Google Scholar]
- Hart, A. A review of technologies for transporting heavy crude oil and bitumen via pipelines. J. Pet. Explor. Prod. Technol. 2014, 4, 327–336. [Google Scholar] [CrossRef]
- Plegue, T.H.; Frank, S.G.; Fruman, D.H.; Zakln, J.L. Studies of water-continuous emulsions of heavy crude oils prepared by alkali treatment. SPE Prod. Eng. 1989, 4, 181–183. [Google Scholar] [CrossRef]
- Eskin, D.; Ratuloswski, J.; Akbarzadeh, K.; Pan, S. Modelling asphaltene deposition in turbulent pipeline flows. Ca. J. Chem. Eng. 2011, 89, 421–441. [Google Scholar] [CrossRef]
- Martinez-Palou, R.; de Lourdes Mosqueira, M.; Zapata-Rendón, B.; Mar-Juárez, E.; Bernal-Huicochea, C.; de la Cruz Clavel-López, J.; Aburto, J. Transportation of heavy and extra-heavy crude oil by pipeline: A review. J. Pet. Sci. Eng. 2011, 75, 274–282. [Google Scholar]
- Zaman, M.; Bjorndalen, N.; Islam, M.R. Detection of precipitation in pipelines. Pet. Sci. Technol. 2004, 22, 1119–1141. [Google Scholar] [CrossRef]
- Abdurahman, N.H.; Rosli, Y.M.; Azhari, N.H.; Hayder, B.A. Pipeline transportation of viscous crudes as concentrated oil-in-water emulsions. J. Pet. Sci. Eng. 2012, 90–91, 139–144. [Google Scholar] [CrossRef]
- Ahmed, N.S.; Zaki, N.N.; Gharieb, H.K.; Nassar, A.M. Formation of fluid heavy oil-in-water emulsions for pipeline transportation. Fuel 1999, 78, 593–600. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Rønningsen, H.P. Influence of wax inhibitors on wax appearance temperature, pour point, and viscosity of waxy crude oils. Energy Fuels 2003, 17, 321–328. [Google Scholar] [CrossRef]
- Bilderback, C.A.; McDougall, L.A. Complete paraffin control in petroleum production. J. Pet. Technol. 1969, 21, 1151–1156. [Google Scholar] [CrossRef]
- Azevedo, L.F.A.; Teixeira, A.M. A critical review of the modeling of wax deposition mechanisms. Pet. Sci. Technol. 2003, 21, 393–408. [Google Scholar] [CrossRef]
- Patton, C.C. Paraffin deposition from refined wax-solvent systems. Soc. Pet. Eng. J. 1970, 10, 17–24. [Google Scholar] [CrossRef]
- Karami, H.R.; Mowla, D. Investigation of the effects of various parameters on pressure drop reduction in crude oil pipelines by drag reducing agents. J. Non-Newton. Fluid Mech. 2012, 177–178, 37–45. [Google Scholar] [CrossRef]
- Kelland, M.A. Production Chemicals for the Oil and Gas Industry, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 259–286. [Google Scholar]
- Burger, E.D.; Munk, W.R.; Wahl, H.A. Flow increase in trans Alaska pipeline through use of polymeric drag-reducing additive. J. Pet. Technol. 1982, 34, 377–386. [Google Scholar] [CrossRef]
- Jubran, B.A.; Zurigat, Y.H.; Al-Shukri, M.S.; Al-Busaidi, H.H. The use of drag reduction agent and a detergent for drag reduction in circulatory vertical pipe flow. Polym.-Plast. Technol. Eng. 2006, 45, 533–538. [Google Scholar] [CrossRef]
- Lakshmi, D.S.; Krishna, M.R.; Rao, M.V.; Rao, M.B.; Purohit, R.C.; Srivastava, S.P.; Nautiyal, S.P. Low temperature flow characteristics of some waxy crude oils in relation to their composition: Part I with and without pour point depressant additives. Pet. Sci. Technol. 1997, 15, 495–502. [Google Scholar] [CrossRef]
- Jang, Y.H.; Blanco, M.; Creek, J.; Tang, Y.; Goddard, W.A. Wax inhibition by comb-like polymers: Support of the incorporation-perturbation mechanism from molecular dynamics simulations. J. Phys. Chem. B 2007, 111, 13173–13179. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.S.; Wu, C.H.; Creek, J.L.; Shuler, P.J.; Tang, Y. Evaluation of effects of Selected wax inhibitors on paraffin deposition. Pet. Sci. Technol. 2003, 21, 369–379. [Google Scholar] [CrossRef]
- Bello, O.O.; Fasesan, S.O.; Teodoriu, C.; Reinicke, K.M. An evaluation of the performance of selected wax inhibitors on paraffin deposition of Nigerian crude oils. Pet. Sci. Technol. 2006, 24, 195–206. [Google Scholar] [CrossRef]
- Aburto, J.; Mar-Juarez, E.; Juarez-Soto, C. Transportation of heavy and extra-heavy crude oil by pipeline: A patent review for technological options. Recent Pat. Chem. Eng. 2009, 2, 86–97. [Google Scholar] [CrossRef]
- Nakata, T.; Inaba, H.; Horibe, A.; Haruki, N.; Sato, K. Surfactant development as a flow drag reduction agent in piping. Jpn. J. Trib. 2004, 49, 1–10. [Google Scholar]
- Gunstone, F.D. Phospholipid Technology and Applications, 1st ed.; Oily Press: Bridgwater, UK, 2008; pp. 1–19. [Google Scholar]
- Oguntimein, G.B.; Erdmann, H.; Schmid, R.D. Lipase catalyzed synthesis of sugar ester in organic solvents. Biotechnol. Lett. 1993, 15, 175–180. [Google Scholar] [CrossRef]
- Hayes, D.G. Using enzymes to prepare biobased surfactants. Int. News Fats Oils Relat. Mater. 2012, 23, 477. [Google Scholar]
- Abbate, V.; Brandstadt, K.F.; Taylor, P.G.; Bassindale, A.R. Enzyme-catalyzed transetherification of alkoxysilanes. Catalysts 2013, 3, 27–35. [Google Scholar] [CrossRef]
- Wang, Z.H.; Liu, Y.; Pang, R.S.; Qiu, X.D. A performance evaluation of glycolipid biosurfactant synthesized by the enzyme-catalyzed method. Pet. Sci. Technol. 2013, 31, 2371–2377. [Google Scholar] [CrossRef]
- Alkhatib, M.H.; Hayes, D.G.; Urban, V.S. Characterization of microemulsion systems formed by a mixed 1,3-dioxolane ethoxylate/octyl glucoside surfactant system. J. Surfactants Deterg. 2009, 12, 277–283. [Google Scholar] [CrossRef]
- Partha, K.; Akanksha, A.; Haaris, M.; Indra, M.M. Stability of oil-in-water macro-emulsion with anionic surfactant: Effect of electrolytes and temperature. Chem. Eng. Sci. 2013, 102, 176–185. [Google Scholar]
- Akbota, O.A.; Kainzhamal, I.O.; Ainamkoz, K.; Kuanyshbek, B.M. Nonionic surfactants based on polyoxyalkylated copolymers used as demulsifying agents. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 433–438. [Google Scholar]
- Başoğul, Y.; Keçebaş, A. Economic and environmental impacts of insulation in district heating pipelines. Energy 2011, 36, 6156–6164. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhang, J.J.; Yan, D.F. Correlations between the pour point/gel point and the amount of precipitated wax for waxy crudes. Pet. Sci. Technol. 2005, 23, 1313–1322. [Google Scholar] [CrossRef]
- García, M.C.; Urbina, A. Effect of crude oil composition and blending on flowing properties. Pet. Sci. Technol. 2005, 21, 863–878. [Google Scholar] [CrossRef]
- Machado, A.L.C.; Lucas, E.F. Influence of ethylene-co-vinyl acetate copolymers on the flow properties of wax synthetic systems. J. Appl. Polym. Sci. 2002, 85, 1337–1348. [Google Scholar] [CrossRef]
- Nakamura, K.; Watanabe, T.; Katayama, K.; Amano, T. Some aspects of nonisothermal crystallization of polymers. J. Appl. Polym. Sci. 1972, 16, 1077–1091. [Google Scholar] [CrossRef]
- Toms, B.A. On the early experiments on drag reduction by polymers. Phys. Fluids 1977, 20, 3–5. [Google Scholar] [CrossRef]
- Metzner, A.B.; Reed, J.C. Flow of non-newtonian fluids-correlation of the laminar, transition, and turbulent-flow regions. Am. Inst. Chem. Eng. 1955, 1, 434–440. [Google Scholar] [CrossRef]
- Zhang, R.L.; Yin, X.L.; Winterfeld, P.H.; Wu, Y.S. A fully coupled thermal-hydrological-mechanical-chemical model for CO2 geological sequestration. J. Nat. Gas Sci. Eng. 2016, 28, 280–304. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, J.; Wang, Z.; Zhang, Z.; Zheng, F.; Zhu, Y.; Han, S. Full and partial emulsification of crude oil-water systems as a function of shear intensity, water fraction, and temperature. Ind. Eng. Chem. Res. 2014, 53, 9513–9520. [Google Scholar] [CrossRef]
- Zhang, R.L. Numerical Simulation of Thermal Hydrological Mechanical Chemical Processes during CO2 Geological Sequestration. Ph.D. Thesis, Colorado School of Mines, Goden, CO, USA, 2013. [Google Scholar]
- Wang, Z.H.; Pang, R.S.; Le, X.P.; Peng, Z.G.; Hu, Z.W.; Wang, X.T. Survey on injection-production status and optimized surface process of ASP flooding in industrial pilot area. J. Pet. Sci. Eng. 2013, 111, 178–183. [Google Scholar] [CrossRef]

| Crudes | Primary Pour Point (°C) | Adding Additives | Depression Rate of Pour Point (%) | |||
|---|---|---|---|---|---|---|
| Concentration (mg/L) | Pour Point Reduction (°C) | Drag-Reducing Surfactant | Contrast Additive | |||
| Drag-Reducing Surfactant | Contrast Additive | |||||
| Highly viscous | 30.2 | 50 | 28.0 | 30.0 | 7.3 | 0.66 |
| 100 | 25.5 | 28.5 | 15.6 | 5.63 | ||
| Low viscous | 25.4 | 50 | 24.0 | 25.0 | 5.5 | 1.57 |
| 100 | 22.0 | 25.0 | 13.4 | 1.57 | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yu, X.; Li, J.; Wang, J.; Zhang, L. The Use of Biobased Surfactant Obtained by Enzymatic Syntheses for Wax Deposition Inhibition and Drag Reduction in Crude Oil Pipelines. Catalysts 2016, 6, 61. https://doi.org/10.3390/catal6050061
Wang Z, Yu X, Li J, Wang J, Zhang L. The Use of Biobased Surfactant Obtained by Enzymatic Syntheses for Wax Deposition Inhibition and Drag Reduction in Crude Oil Pipelines. Catalysts. 2016; 6(5):61. https://doi.org/10.3390/catal6050061
Chicago/Turabian StyleWang, Zhihua, Xueying Yu, Jiaxu Li, Jigang Wang, and Lei Zhang. 2016. "The Use of Biobased Surfactant Obtained by Enzymatic Syntheses for Wax Deposition Inhibition and Drag Reduction in Crude Oil Pipelines" Catalysts 6, no. 5: 61. https://doi.org/10.3390/catal6050061






