Practical Pd(TFA)2-Catalyzed Aerobic [4+1] Annulation for the Synthesis of Pyrroles via “One-Pot” Cascade Reactions
Abstract
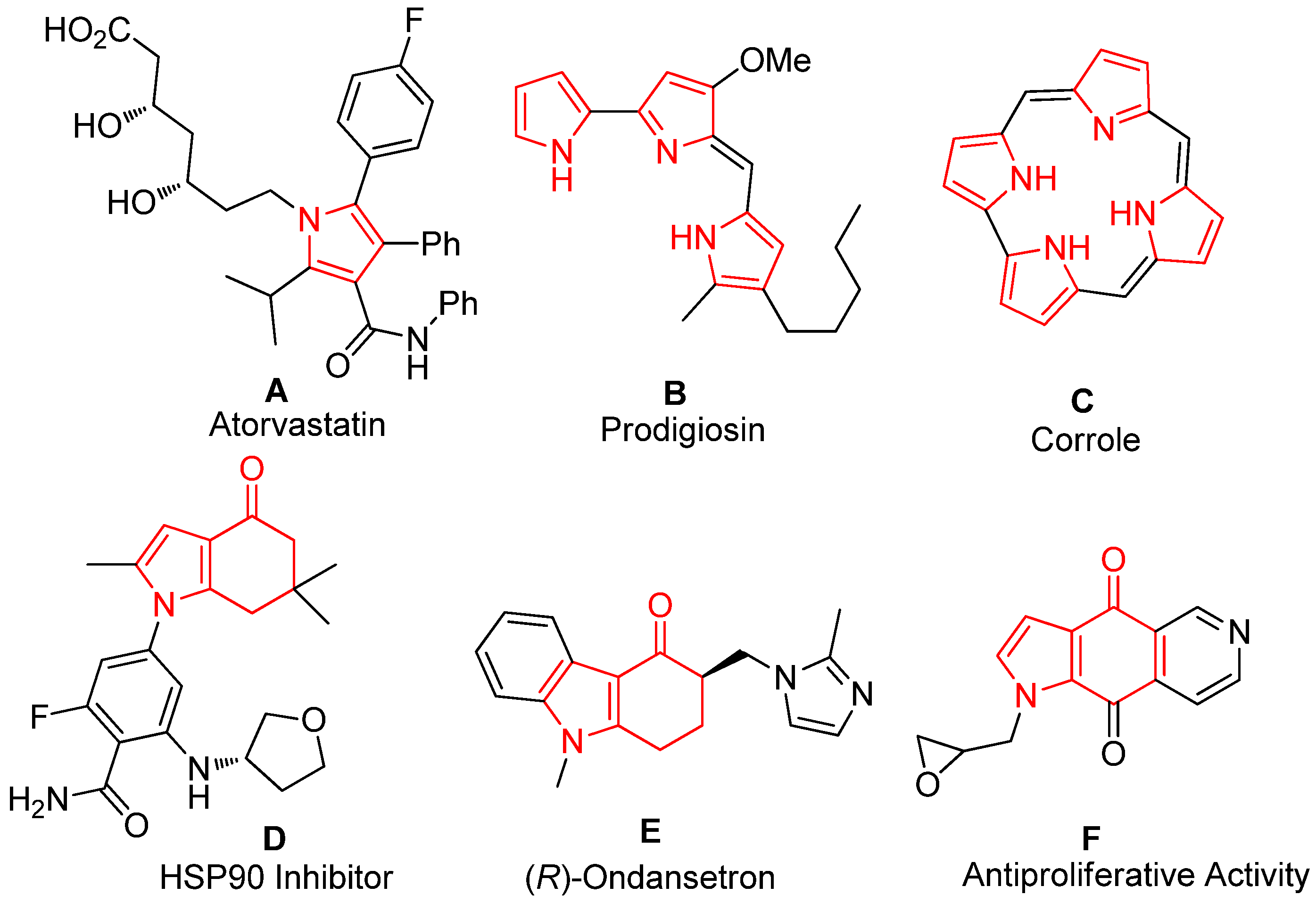
:1. Introduction
2. Results and Discussion
2.1. Optimized Synthesis of 4a
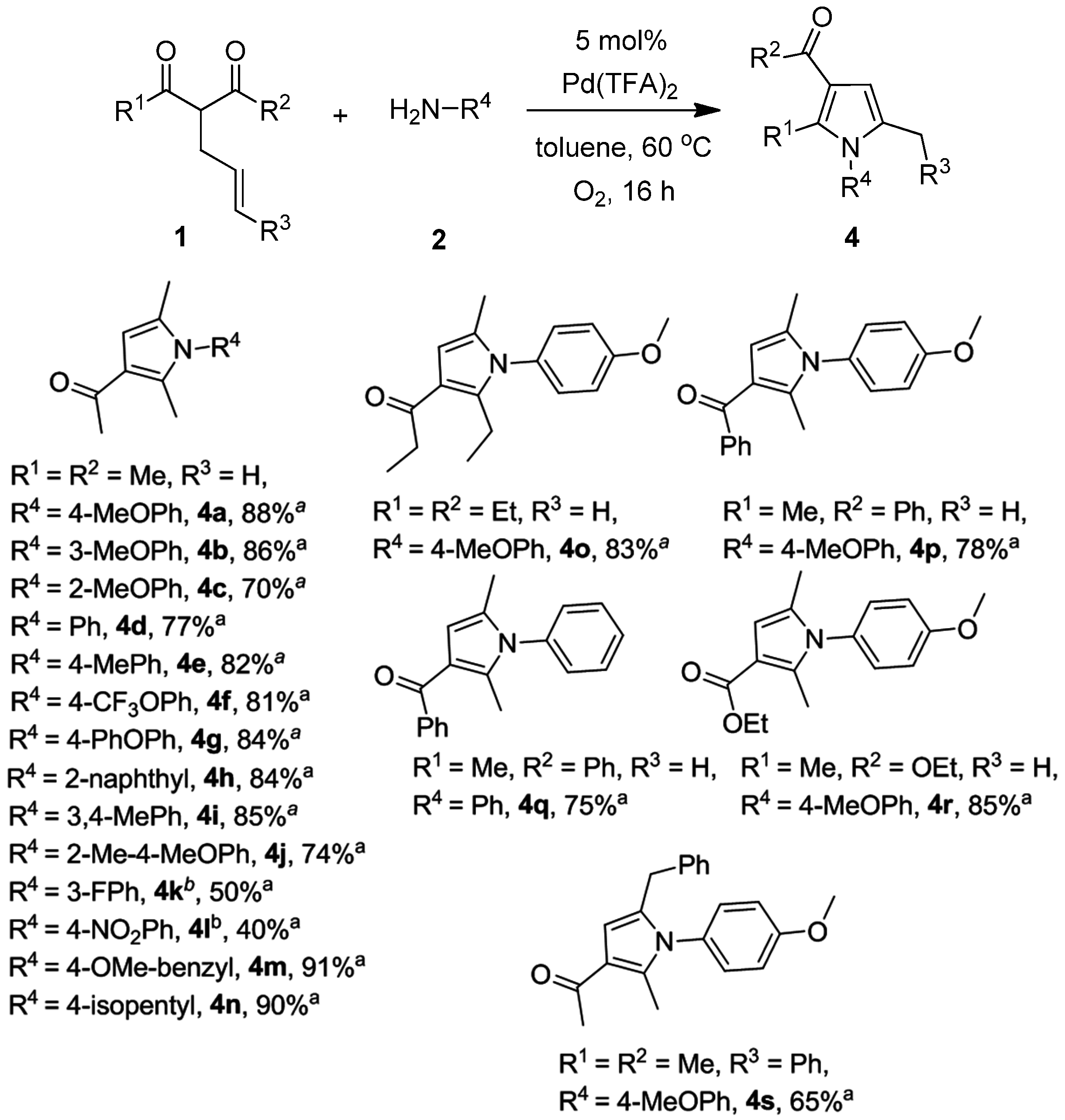
2.2. One-Pot Synthesis of 4
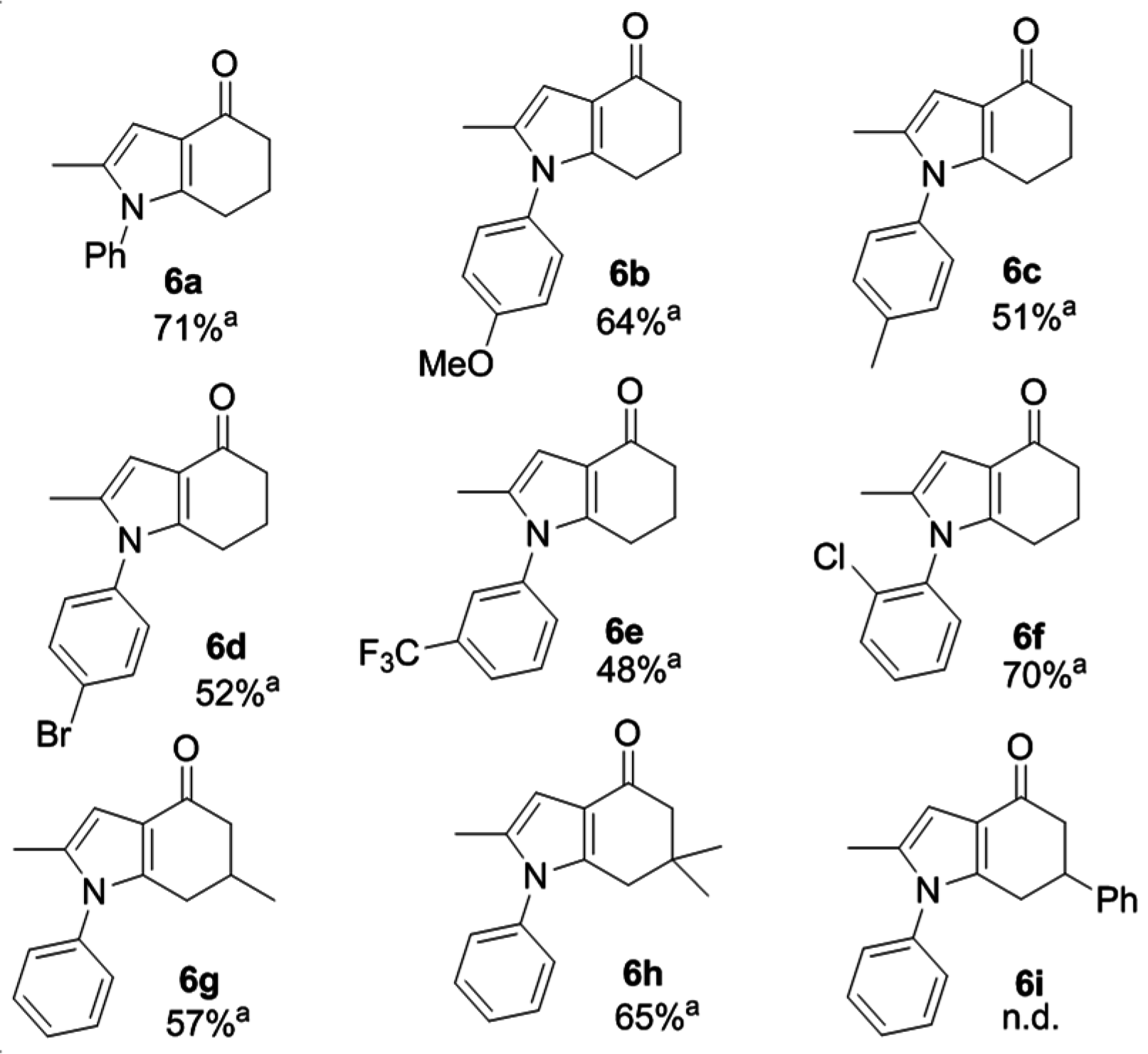
2.3. One-Pot Synthesis of 6
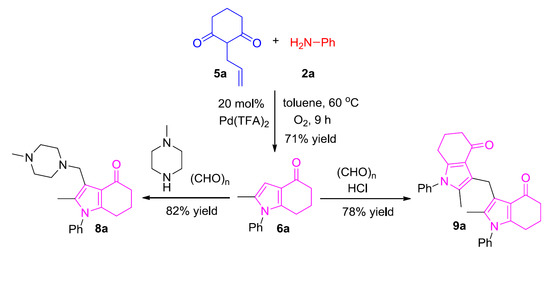
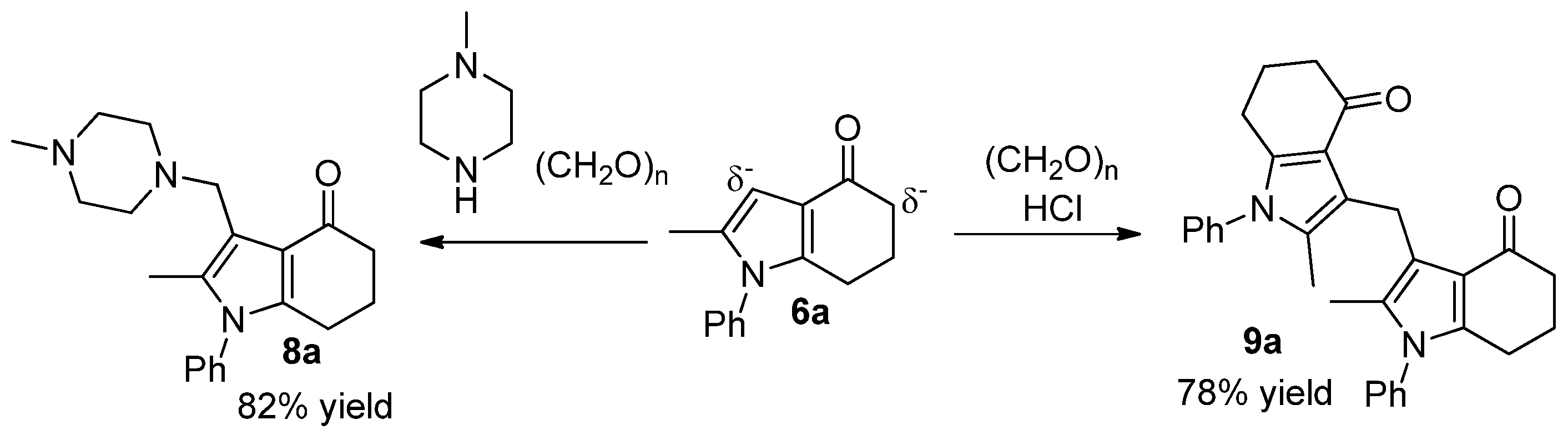
2.4. Synthesis of Aminomethylated and Di(1H-Pyrrol-3-yl)methane Products
3. Experimental Section
3.1. One-Pot Synthesis of 4
3.2. Synthesis of 6
3.3. Synthesis of 8a and 9a
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bellina, F.; Rossi, R. Synthesis and biological activity of pyrrole, pyrroline and pyrrolidine derivatives with two aryl groups on adjacent positions. Tetrahedron 2006, 62, 7213–7256. [Google Scholar] [CrossRef]
- Syamaiah, K.; Mallikarjuna Reddy, G.; Padmavathi, V.; Padaja, A. Synthesis and antimicrobial activity of some new amido/sulfonamido-linked 3,4-disubstituted pyrroles. Med. Chem. Res. 2014, 23, 3287–3297. [Google Scholar] [CrossRef]
- Yoshimura, H.; Kikuchi, K.; Hibi, S.; Tagami, K.; Satoh, T.; Yamauchi, T.; Ishibahi, A.; Tai, K.; Hida, T.; Tokuhara, N.; et al. Discovery of novel and potent retinoic acid receptor α agonists: Syntheses and evaluation of benzofuranyl-pyrrole and benzothiophenyl-pyrrole derivatives. J. Med. Chem. 2000, 43, 2929–2937. [Google Scholar] [CrossRef] [PubMed]
- Clive, D.L.J.; Cheng, P. The marinopyrroles. Tetrahedron 2013, 69, 5067–5078. [Google Scholar] [CrossRef]
- Rudi, A.; Evan, T.; Aknin, M.; Kashman, Y. Polycitone B and Prepolycitrin A: Two novel alkaloids from the Marine Ascidian Polycitor africanus. J. Nat. Prod. 2000, 63, 832–833. [Google Scholar] [CrossRef] [PubMed]
- Facompré, M.; Tardy, C.; Bal-Mahieu, C.; Colson, P.; Perez, C.; Manzanares, I.; Cuevas, C.; Bailly, C. Lamellarin D: A novel potent inhibitor of topoisomerase I. Cancer Res. 2003, 63, 7392–7399. [Google Scholar] [PubMed]
- Gryko, D.T.; Gryko, D.; Lee, C.H. 5-Substituted dipyrranes: Synthesis and reactivity. Chem. Soc. Rev. 2012, 41, 3780–3789. [Google Scholar] [CrossRef] [PubMed]
- Sadki, S.; Schottland, P.; Brodiec, N.; Sabouraud, G. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar]
- Haketa, Y.; Tamura, Y.; Yasuda, N.; Maeda, H. Dipyrrolylpyrimidines as anion-responsiveπ-electronic systems. Org. Biomol. Chem. 2016, 14, 8035–8038. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xiong, F.; Chen, W.; He, Q.; Chen, F. Asymmetric synthesis of the HMG-CoA reductase inhibitor atorvastatin calcium: An organocatalytic anhydride desymmetrization and cyanide-free side chain elongation approach. J. Org. Chem. 2014, 79, 2723–2728. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.D.; Gao, X.; Strohmeier, M.; Wang, W.; Bai, S.; Dybowski, C. Solid-state NMR studies of form I of atorvastatin calcium. J. Phys. Chem. B 2012, 116, 3641–3649. [Google Scholar] [CrossRef] [PubMed]
- Papireddy, K.; Smilkstein, M.; Kelly, J.X.; Salem, S.M.; Alhamadsheh, M.; Haynes, S.W.; Challis, G.L.; Reynolds, K.A. Antimalarial Activity of Natural and Synthetic Prodiginines. J. Med. Chem. 2011, 54, 5296–5306. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.X.; Withall, M.D.; Challis, G.L.; Thomson, R.J. Structure, chemical synthesis, and biosynthesis of Prodiginine natural products. Chem. Rev. 2016, 116, 7818–7853. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.E.; Thompson, A. Advances in the chemistry of dipyrrins and their complexes. Chem. Rev. 2007, 107, 1831–1861. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhu, W.H.; Xie, Y. Development of ion chemosensors based on porphyrin analogues. Chem. Rev. ASAP 2016. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.H.; Veal, J.M.; Fadden, R.P.; Rice, J.W.; Eaves, J.; Strachan, J.; Barabasz, A.F.; Foley, B.E.; Barta, T.E.; Ma, W.; et al. Discovery of novel 2-aminobenzamide inhibitors of heat shock protein 90 as potent, selective and orally active antitumor agents. J. Med. Chem. 2009, 52, 4288–4305. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Sun, Z.; Mo, M.; Peng, F.; Shao, Z. Catalytic asymmetric assembly of C3-monosubstituted chiral carbazolones and concise formal synthesis of (−)-Aspidofractinine: Application of enantioselective Pd-catalyzed decarboxylative protonation of carbazolones. Org. Lett. 2014, 16, 4178–4181. [Google Scholar] [CrossRef] [PubMed]
- Shanab, K.; Schirmera, E.; Wulza, E.; Weissenbachera, B.; Lassniga, S.; Slanza, R.; Fösleitnera, G.; Holzera, W.; Spreitzer, H.; Peter Schmidt, B.A.; et al. Synthesis and antiproliferative activity of new cytotoxic azanaphthoquinone pyrrolo-annelated derivatives: Part II. Bioorg. Med. Chem. Lett. 2011, 21, 3117–3121. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.H.R.; Zard, S.Z. A new synthesis of pyrroles from nitroalkenes. J. Chem. Soc. Chem. Commun. 1985, 16, 1098–1100. [Google Scholar] [CrossRef]
- Amarnath, V.; Anthony, D.C.; Amarnath, K.; Valentine, W.M.; Wetterau, L.A.; Graham, D.G. Intermediates in the Paal-Knorr synthesis of pyrroles. J. Org. Chem. 1991, 56, 6924–6931. [Google Scholar] [CrossRef]
- Minetto, G.; Raveglia, L.F.; Taddei, M. Microwave-assisted Paal—Knorr reaction. A rapid approach to substituted pyrroles and furans. Org. Lett. 2004, 6, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Moss, T.A.; Nowak, T. Synthesis of 2,3-dicarbonylated pyrroles and furans via the three-component Hantzsch reaction. Tetrahedron Lett. 2012, 53, 3056–3060. [Google Scholar] [CrossRef]
- Herath, A.; Cosford, N.D.P. One-step continuous flow synthesis of highly substituted pyrrole-3-carboxylic acid derivatives via in situ hydrolysis of tert-butyl esters. Org. Lett. 2010, 12, 5182–5185. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Huang, L.; Qi, C.; Wu, W.; Jiang, H. An efficient synthesis of polysubstituted pyrroles via copper-catalyzed coupling of oxime acetates with dialkyl acetylenedicarboxylates under aerobic conditions. Chem. Commun. 2013, 49, 9597–9599. [Google Scholar] [CrossRef] [PubMed]
- Estévez, V.; Villacampa, M.; Menéndez, J.C. Recent advances in the synthesis of pyrroles by multicomponent reactions. Chem. Soc. Rev. 2014, 43, 4633–4657. [Google Scholar] [CrossRef] [PubMed]
- Estévez, V.; Villacampa, M.; Menéndez, J.C. Multicomponent reactions for the synthesis of pyrroles. Chem. Soc. Rev. 2010, 39, 4402–4421. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F.; Neuman, H.; Beller, M. Synthesis of heterocycles via palladium-catalyzed carbonylations. Chem. Rev. 2013, 113, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F.; Neumann, H. Zinc-catalyzed organic synthesis: C–C, C–N, C–O bond formation reactions. Adv. Synth. Catal. 2012, 354, 3141–3160. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Pearce, B.M.; Thompson, A. Chiral molecules containing the pyrrole framework. Chem. Commun. 2010, 46, 1797–1812. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Shen, T.; Cui, Y.; Jiao, N. 2,4- vs. 3,4-disubsituted pyrrole synthesis switched by copper and nickel catalysts. Org. Lett. 2012, 14, 4926–4929. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, B.; Keith, A.J.; Armstrong, D.; Odom, A.L. Pyrrole syntheses based on titanium-catalyzed hydroamination of diynes. Org. Lett. 2004, 6, 2957–2960. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ackermann, L. Versatile pyrrole synthesis through ruthenium(II)-catalyzed alkene C–H bond functionalization on enamines. Org. Lett. 2013, 15, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Xu, X.; Park, C.M. Facile synthesis of 2-alkyl/aryloxy-2H-azirines and their application in the synthesis of pyrroles. Chem. Commun. 2012, 48, 3996–3998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, X.; Shao, Q.; Pan, L.; Liu, Q. Tandem Michael addition/isocyanide insertion into the C–C bond: a novel access to 2-acylpyrroles and medium-ring fused pyrroles. Org. Biomol. Chem. 2013, 11, 7393–7399. [Google Scholar] [CrossRef] [PubMed]
- Lonzi, G.; López, L.A. Regioselective synthesis of functionalized pyrrolesvia gold(I)-catalyzed [3+2] cycloaddition of stabilized vinyl diazo derivatives and nitriles. Adv. Synth. Catal. 2013, 355, 1948–1954. [Google Scholar] [CrossRef]
- Louillat, M.L.; Patureau, F.W. Oxidative C–H amination reactions. Chem. Soc. Rev. 2014, 43, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Kim, J.Y.; Kwak, J.; Chang, S. Recent advances in the transition metal-catalyzed twofold oxidative C–H bond activation strategy for C–C and C–N bond formation. Chem. Soc. Rev. 2011, 40, 5068–5083. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.C.P.; Zheng, N.; Buchwald, S.L. Combined C–H functionalization/C–N bond formation route to carbazoles. J. Am. Chem. Soc. 2005, 127, 14560–14561. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Shen, C.; Zhang, W. An asymmetric aerobic Aza-Wacker-type cyclization: Synthesis of isoindolinones bearing tetrasubstituted carbon stereocenters. Angew. Chem. Int. Ed. 2012, 51, 9141–9145. [Google Scholar] [CrossRef] [PubMed]
- Nadres, E.T.; Daugulis, O. Heterocycle synthesis via direct C–H/N–H coupling. J. Am. Chem. Soc. 2012, 134, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Obora, Y.; Ishii, Y. Palladium-catalyzed intermolecular oxidative amination of alkenes with amines, using molecular oxygen as terminal oxidant. Catalysts 2013, 3, 794–810. [Google Scholar] [CrossRef]
- Louillat, M.L.; Patureau, F.W. Toward polynuclear Ru-Cu catalytic dehydrogenative C–N bond formation on the reactivity of carbazoles. Org. Lett. 2013, 15, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Suri, M.; Glorius, F. RhIII/CuII-cocatalyzed synthesis of 1H-Indazoles through C–H amidation and N–N Bond formation. J. Am. Chem. Soc. 2013, 135, 8802–8805. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Kwak, J.; Shin, K.; Lee, D.; Chang, S. Ir(III)-catalyzed mild C–H amidation of arenes and alkenes: An efficient usage of acyl azides as the nitrogen source. J. Am. Chem. Soc. 2013, 135, 12861–12868. [Google Scholar] [CrossRef] [PubMed]
- Wendlandt, A.E.; Suess, A.M.; Stahl, S.S. Copper-catalyzed aerobic oxidative C–H functionalizations: Trends and mechanistic insights. Angew. Chem. Int. Ed. 2011, 50, 11062–11087. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, A.B.; Schuman, D.P.; Tan, Z.X.; Stahl, S.S. Synthesis of vicinal aminoalcohols by stereoselective aza-Wacker cyclizations: Access to (−)-Acosamine by redox relay. Angew. Chem. Int. Ed. 2013, 52, 11867–11870. [Google Scholar] [CrossRef] [PubMed]
- Redford, J.E.; McDonald, R.I.; Rigsby, M.L.; Wiensch, J.D.; Stahl, S.S. Stereoselective synthesis of cis-2,5-disubstituted pyrrolidines via Wacker-type aerobic oxidative cyclization of alkenes with tert-butanesulfinamide nucleophiles. Org. Lett. 2012, 14, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Tan, J.; Wang, Z. A facile access to pyrroles from amino acids via an Aza-Wacker cyclization. J. Org. Chem. 2008, 73, 5180–5182. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.T.; Yang, M.; Law, K.L.; Zhu, N.Y.; Yang, D. Pd(II)-catalyzed enantioselective oxidative tandem cyclization reactions. Synthesis of indolines through C–N and C–C bond formation. J. Am. Chem. Soc. 2006, 128, 3130–3131. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, W. Regioselective Pd-catalyzed aerobic Aza-Wacker cyclization for preparation of isoindolinones and isoquinolin-1(2H)-ones. Org. Lett. 2012, 14, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, T.A.; Zhao, B.; Shi, Y. Recent advances in transition metal-catalyzed sp3 C–H amination adjacent to double bonds and carbonyl groups. Chem. Soc. Rev. 2012, 41, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.N.; Zhang, W. Synthesis of heterocycles via palladium catalyzed Wacker-type oxidative cyclization reactions of hydroxy- and amino-alkenes. In Topics in Heterocyclic Chemistry; Wolfe, J.P., Ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Obora, Y.; Shimizu, Y.; Ishii, Y. Intermolecular oxidative amination of olefins with amines catalyzed by the Pd(II)/NPMoV/O2 system. Org. Lett. 2009, 11, 5058–5061. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, Y.; Yasuda, K.; Obora, Y. Palladium-catalyzed Z-selective oxidative amination of ortho-substituted primary anilines with olefins under an open air atmosphere. J. Org. Chem. 2013, 78, 6332–6337. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F.; Neumann, H.; Beller, M. Palladium-catalyzed oxidative carbonylation reactions. ChemSusChem 2013, 6, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Mohanta, S.C.; Patil, M.L.; Rao, C.V.L.; Takizawa, S.; Suzuki, T.; Sasai, H. Enantioselective Wacker-type cyclization of 2-alkenyl-1,3-diketones promoted by Pd-SPRIX catalyst. Org. Lett. 2010, 12, 3480–3483. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Akita, M.; Tanigaki, Y.; Takizawa, S.; Sasai, H. Enantioselective cyclization of 4-alkenoic acids via an oxidative allylic C-H esterification. Org. Lett. 2011, 13, 3506–3509. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Huang, L.; Liu, H.; Wang, W.; Li, H. Efficient synthesis of highly substituted pyrroles through a Pd(OCOCF3)2-catalyzed cascade reaction of 2-alkenal-1,3-dicarbonyl compounds with primary amines. Chem. Commun. 2013, 49, 4667–4669. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, Y.; Liu, A.; Xie, H.; Li, H.; Wang, W. Ligand-free Cu-catalyzed [3+2] cyclization for the synthesis of pyrrolo[1,2-a]quinolines with ambient air as a terminal oxidant. Org. Biomol. Chem. 2016, 14, 7455–7458. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, H.; Liao, Z.; Lou, K.; Xie, H.; Li, H.; Wang, W. Divergent synthesis of imidazoles and quinazolines via Pd(OAc)2-catalyzed annulation of N-allylamidines. Org. Lett. 2015, 17, 3434–3537. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, W.; Liu, H.; Wang, W.; Li, H. FeCl3 promoted highly regioselective [3+2] cycloaddition of dimethyl 2-vinyl and aryl cyclopropane-1,1-dicarboxylates with aryl isothiocyanates. Org. Biomol. Chem. 2012, 10, 5032–5035. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, J.; Wang, J.; Li, H.; Wang, W.; Guo, Y. Phosphine-catalyzed Aza-MBH reactions of vinylpyridines: Efficient and rapid access to 2,3,5-triarylsubstituted 3-pyrrolines. Org. Lett. 2015, 17, 2214–2217. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, Y.; Chen, X.; Zhu, H.; Du, P.; Liu, G.; Lou, L.; Li, H.; Wang, W. FeCl3-catalyzed selective acylation of amines with 1,3-diketones via C–C bond cleavage. Tetrahedron Lett. 2015, 56, 3093–3096. [Google Scholar] [CrossRef]
- Zhang, X.S.; Song, X.X.; Li, H.; Zhang, S.L.; Chen, X.B.; Yu, X.H.; Wang, W. An organocatalytic cascade approach toward polysubstituted quinolines and chiral 1,4-dihydroquinolines–unanticipated effect of N-protecting groups. Angew. Chem. Int. Ed. 2012, 51, 7282–7286. [Google Scholar] [CrossRef] [PubMed]
- Zu, L.; Xie, H.; Li, H.; Wang, J.; Yu, X.H.; Wang, W. Chiral amine-catalyzed enantioselective cascade Aza–Ene-type cyclization reactions. Chem. Eur. J. 2008, 14, 6333–6335. [Google Scholar] [CrossRef] [PubMed]
- Barraja, P.; Diana, P.; Lauria, A.; Montalbano, A.; Almerico, A.M.; Dattolo, G.; Cirrincione, G.; Violab, G.; Dall’Acqua, F. Pyrrolo[2,3-h]quinolinones: Synthesis and photochemotherapic activity. Bioorg. Med. Chem. Lett. 2003, 13, 2809–2811. [Google Scholar] [CrossRef]
- Chacón-García, L.; Martínez, R. Synthesis and in vitro cytotoxic activity of pyrrolo[2,3-e]indole derivatives and a dihydro benzoindole analogue. Eur. J. Med. Chem. 2002, 37, 261–266. [Google Scholar] [CrossRef]
- Martínez, R.; Ávila, J.G.; Ramírez, M.T.; Pérez, A.; Martínez, Á. Tetrahydropyrrolo[3,2-c]azepin-4-ones as a new class of cytotoxic compounds. Bioorg. Med. Chem. 2006, 14, 4007–4016. [Google Scholar] [CrossRef] [PubMed]



| Entry | Solvent | Catalyst | Oxidant | Yield b (%) |
|---|---|---|---|---|
| 1 | toluene | Pd(OAc)2 | air | 45 |
| 2 | DMA | Pd(OAc)2 | air | 18 |
| 3 | DMSO | Pd(OAc)2 | air | 12 |
| 4 | DMF | Pd(OAc)2 | air | 32 |
| 5 | DCE | Pd(OAc)2 | air | 40 |
| 6 | CH3CN | Pd(OAc)2 | air | 23 |
| 7 | THF | Pd(OAc)2 | air | 25 |
| 8 | xylenes | Pd(OAc)2 | air | 50 |
| 9 | xylenes | Pd(OAc)2 | Cu(OAc)2 | 21 |
| 10 | xylenes | Pd(OAc)2 | AgOAc | trace |
| 11 | xylenes | Pd(OAc)2 | O2 | 58 |
| 12 | xylenes | Pd(TFA)2 | O2 | 82 |
| 13 | xylenes | PdCl2 | O2 | 28 |
| 14 | xylenes | PdCl2(PPh3)2 | O2 | 14 |
| 15 | xylenes | PdCl2(CH3CN)2 | O2 | trace |
| 16 c | xylenes | Pd(PPh3)4 | O2 | 17 |
| 17 d | toluene | Pd(TFA)2 | O2 | 86 |
| 18 e | toluene | Pd(TFA)2 | O2 | 85 |
| 19 f | toluene | Pd(TFA)2 | O2 | 88 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Mang, Z.; Yang, W.; Li, H.; Wang, W. Practical Pd(TFA)2-Catalyzed Aerobic [4+1] Annulation for the Synthesis of Pyrroles via “One-Pot” Cascade Reactions. Catalysts 2016, 6, 169. https://doi.org/10.3390/catal6110169
Yu Y, Mang Z, Yang W, Li H, Wang W. Practical Pd(TFA)2-Catalyzed Aerobic [4+1] Annulation for the Synthesis of Pyrroles via “One-Pot” Cascade Reactions. Catalysts. 2016; 6(11):169. https://doi.org/10.3390/catal6110169
Chicago/Turabian StyleYu, Yang, Zhiguo Mang, Wei Yang, Hao Li, and Wei Wang. 2016. "Practical Pd(TFA)2-Catalyzed Aerobic [4+1] Annulation for the Synthesis of Pyrroles via “One-Pot” Cascade Reactions" Catalysts 6, no. 11: 169. https://doi.org/10.3390/catal6110169