Co-Pyrolysis Behaviors of the Cotton Straw/PP Mixtures and Catalysis Hydrodeoxygenation of Co-Pyrolysis Products over Ni-Mo/Al2O3 Catalyst
Abstract
:1. Introduction
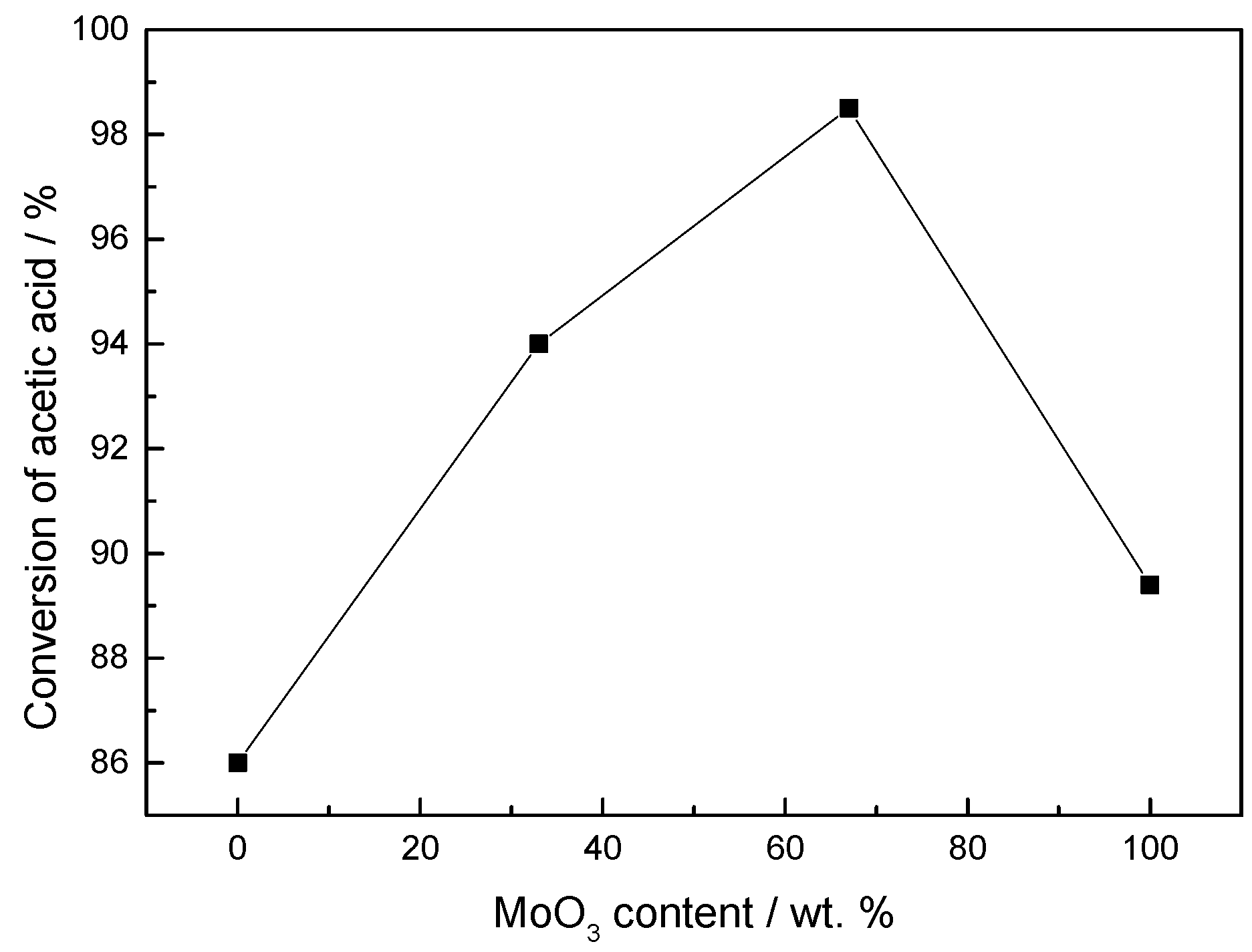
2. Results and Discussion
2.1. Preparation of Crude Oil and Thermal Degradation
| PP wt. % | 0 | 20 | 33 | 50 | 67 | 80 | 100 |
|---|---|---|---|---|---|---|---|
| 1 | 27.1 | 29.4 | 35.8 | 39.1 | 43.6 | - | |
| 2 | 20.0 | 22.5 | 25.2 | 27.3 | 29.5 | 32.3 | 35.4 |
| Materials | C/% | H/% | O/% | N/% | S/% | Calorific Value/MJ·kg−1 |
|---|---|---|---|---|---|---|
| Cotton straw | 50.8 | 8.78 | 39.8 | 0.57 | 0.23 | 15.5 |
| PP/cotton straw | 82.2 | 11.3 | 6.4 | 0.04 | 0.06 | 46.9 |
| PP | 84.7 | 14.6 | 0.7 | 0.00 | 0.00 | 49.5 |
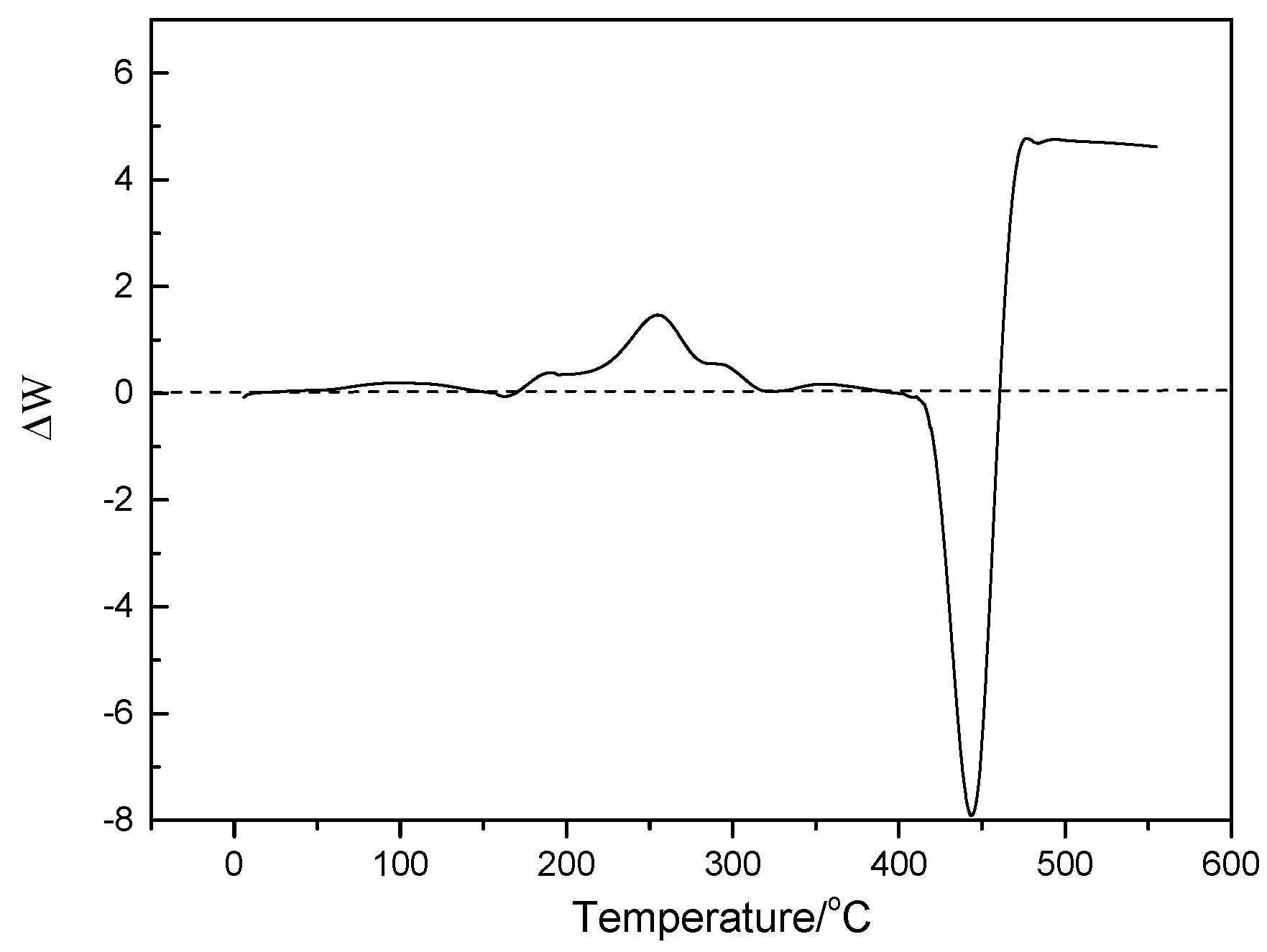
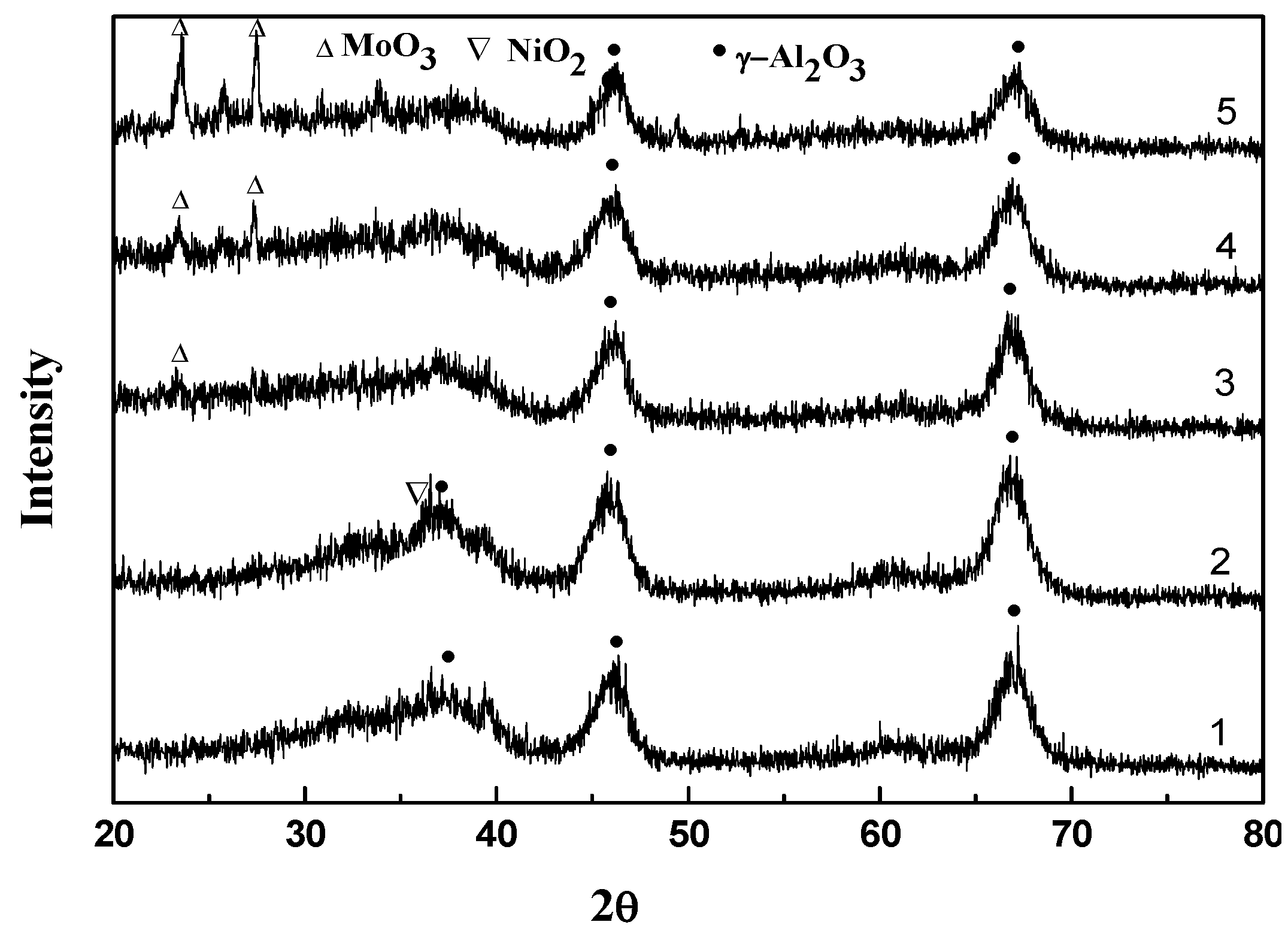
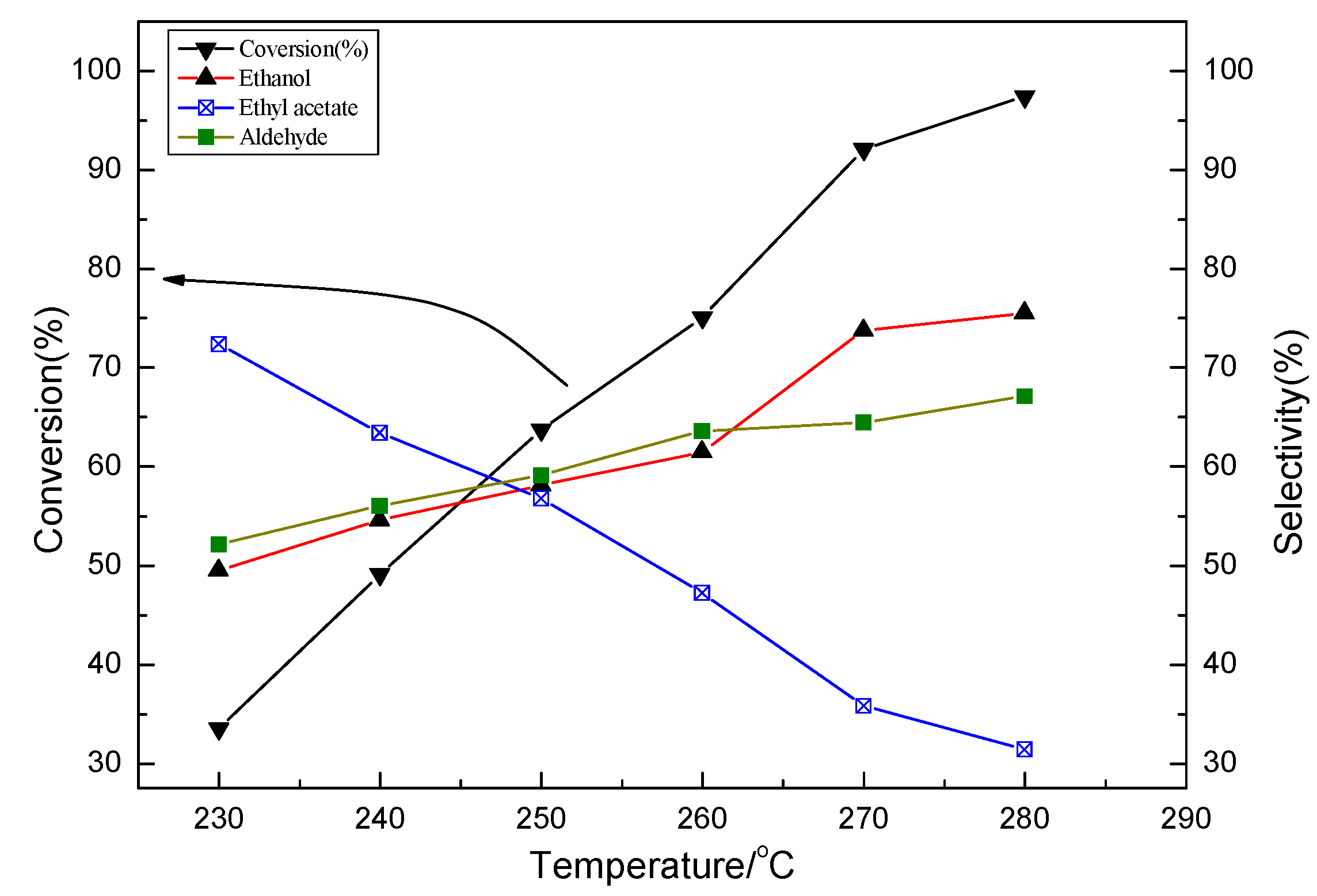
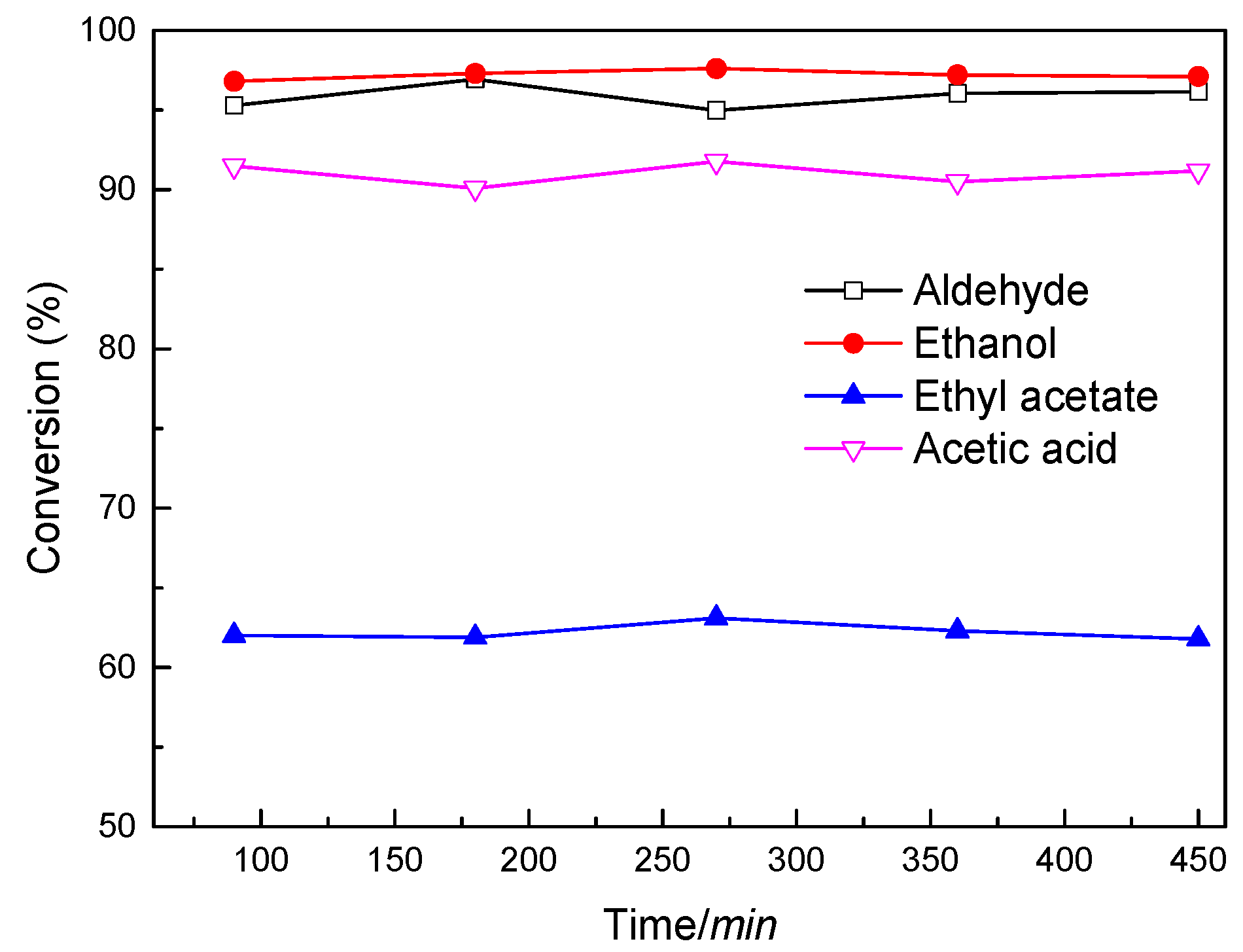
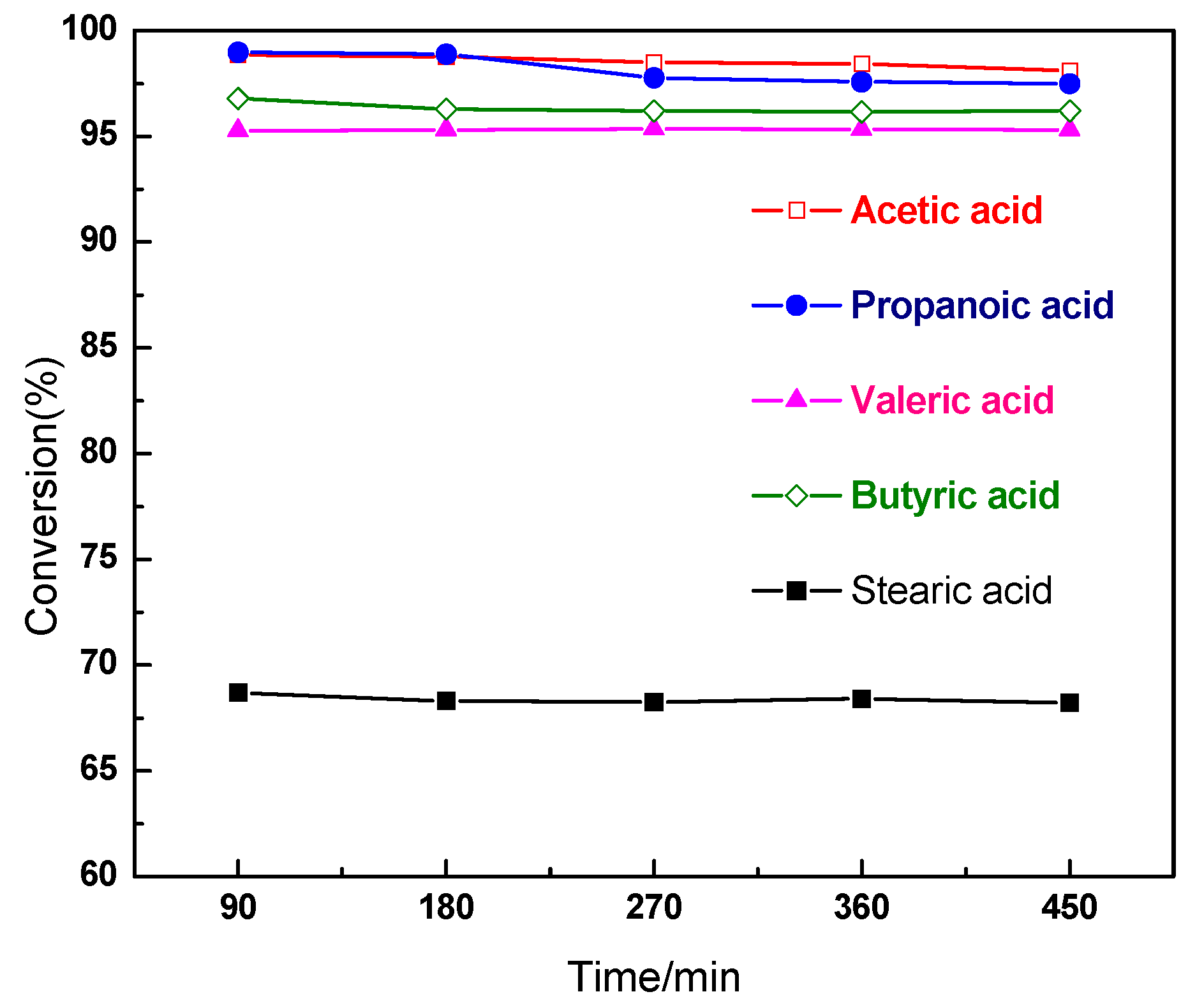
2.2. Characterization and Test of Ni-Mo/Al2O3 Catalyst

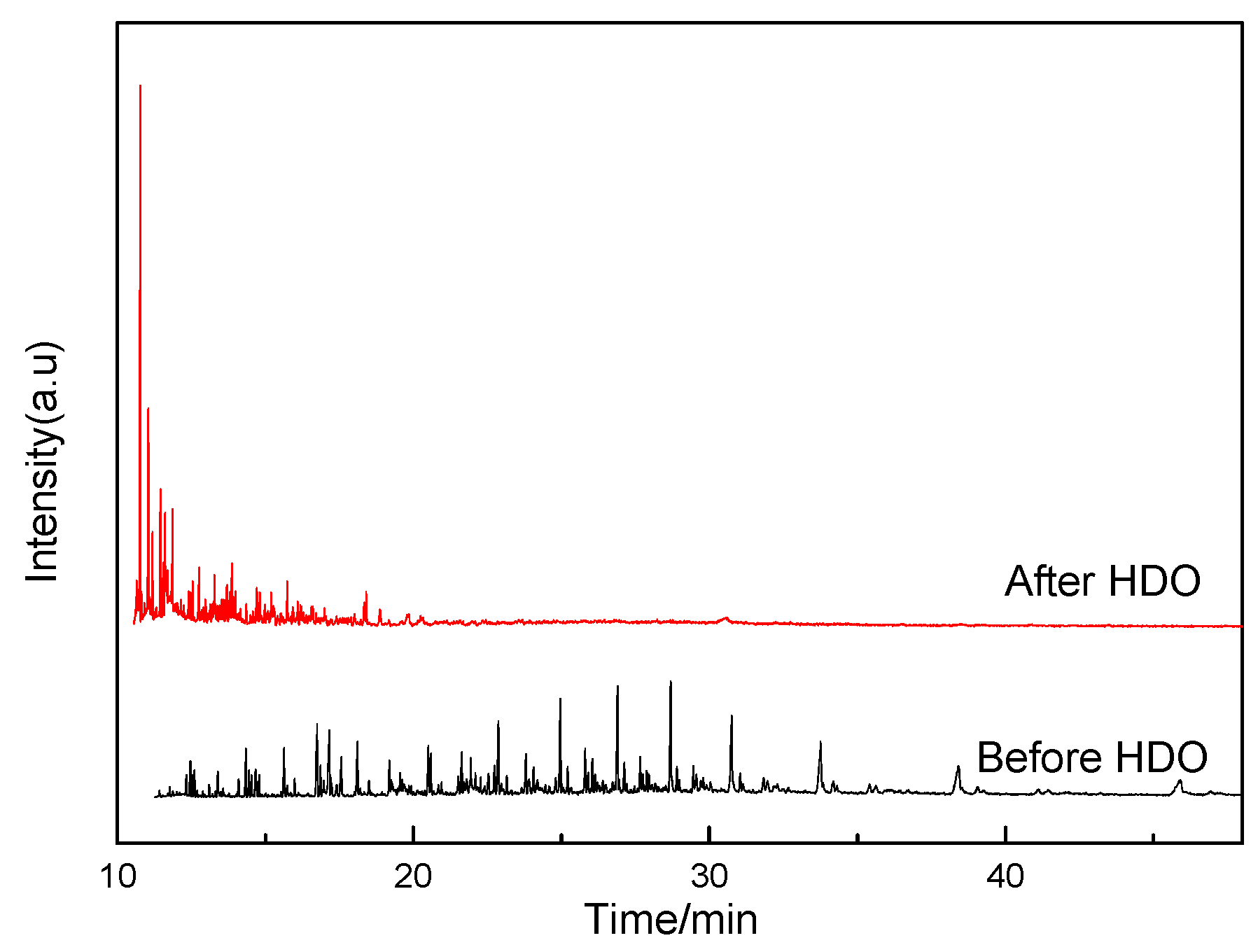
| No. | Before HDO | After HDO | ||||
|---|---|---|---|---|---|---|
| Time/min | Area% | Compositions | Time/min | Area% | Compositions | |
| 1 | 12.4 | 2.8 | 4-methyl-undecane | 10.7 | 15.3 | 3-methyldecane |
| 2 | 14.3 | 3.7 | 2-butyl-1-octanol | 11.0 | 12.7 | 4,5-diethyloctane |
| 3 | 15.6 | 3.2 | Pentatonic acid, 10-undecenyl ester | 11.1 | 2.6 | 4-ethyldecane |
| 4 | 16.7 | 4.8 | 2-hexyl-1-octanol | 11.4 | 4.8 | 2-methylundecane |
| 5 | 17.1 | 7.6 | Z-11-Tetradecen-1-ol propionate | 11.6 | 9.2 | dodecane |
| 6 | 18.1 | 4.2 | 2-hexyl-1-dodecanol | 11.8 | 8.8 | 2,4-dimethylundecane |
| 7 | 19.2 | 4.0 | 5-octadecene | 12.4 | 2.7 | 2,4-dimethyldodecane |
| 8 | 20.5 | 4.4 | 2-methyl-1-decanol | 12.5 | 5.5 | 2,6,11-trimethyldodecane |
| 9 | 21.6 | 3.3 | Z-8-dodecene-1-ol acetate | 12.7 | 2.8 | 3-methyltridecane |
| 10 | 22.8 | 3.7 | 2-hexyl-1-decanol | 13.2 | 4.3 | 2,4-dimethylpentadecane |
| 11 | 24.9 | 8.4 | 1,21-dococadiene | 13.8 | 7.7 | 2,6,10-trimethyltetradecane |
| 12 | 26.9 | 8.2 | 3,7,11,15-tetramethyl-2-hexadecane-1-ol | 14.7 | 5.3 | 2-hexadecanol |
| 13 | 28.6 | 8.1 | 2-methyl hexadecane-1-ol | 15.7 | 3.4 | Hexadecane |
| 14 | 30.7 | 7.3 | 1,16-hexadecanediol | 18.4 | 3.7 | 2-methyloctadecane |
| 15 | 33.7 | 6.4 | E-3-methyl-8-tridecene-2-ol, acetate | - | - | - |
| 16 | 38.4 | 5.9 | 1,19-eicosadiene | - | - | - |
| 17 | 45.9 | 4.7 | Acetic acid octadecylester | - | - | - |
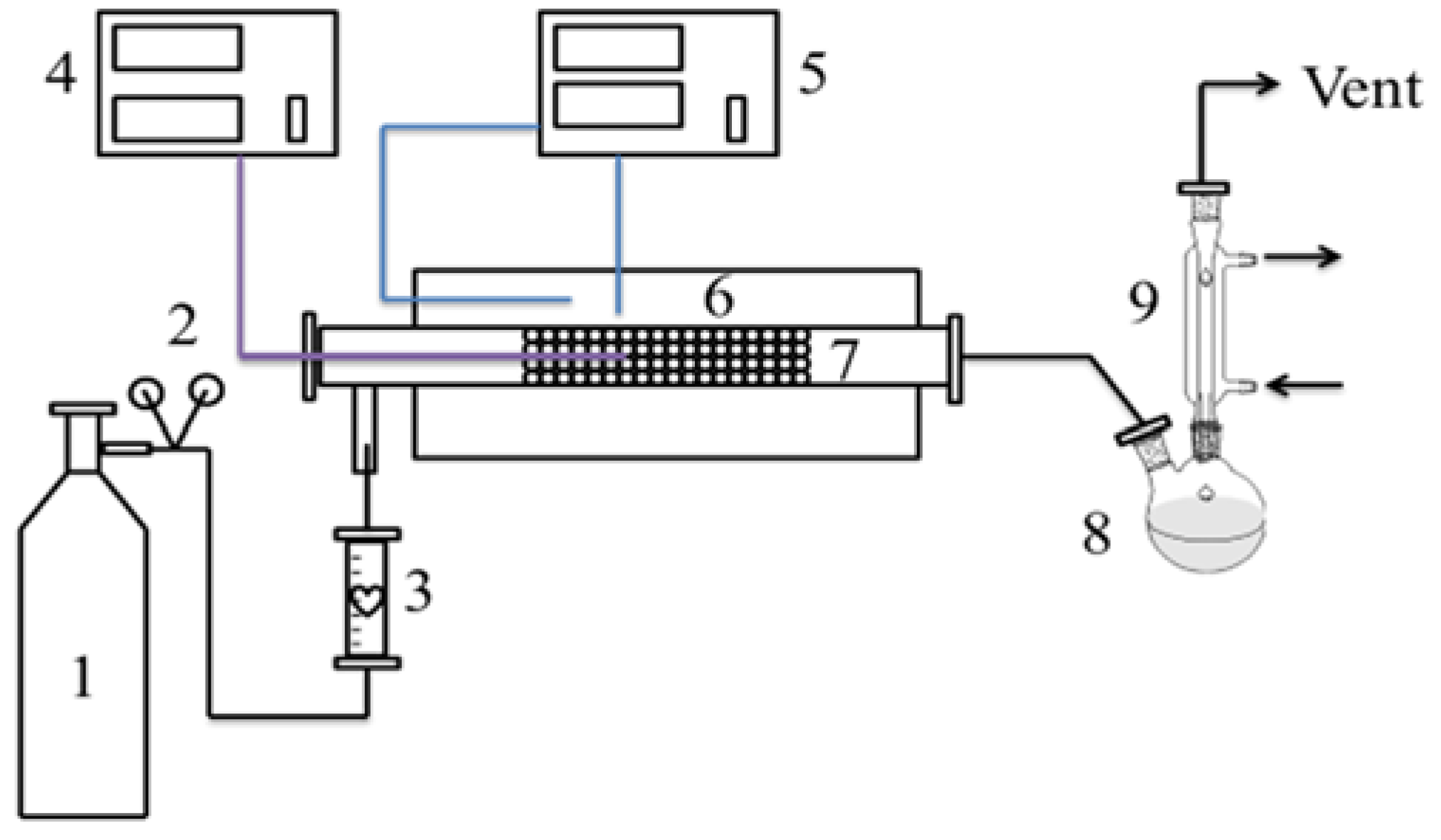
3. Experimental Section
| Sample | C/% | H/% | O/% | N/% | S/% |
|---|---|---|---|---|---|
| PP | 83.58 | 13.82 | 0 | - | 2.60 |
| Cotton straw | 42.78 | 6.01 | 40.35 | 1.51 | - |
| Ratio of Ni to Mo | Texture of Catalyst | |||
|---|---|---|---|---|
| DPore (nm) | VPore (cm3/g) | SBet (m2/g) | ||
| Support | - | 8.1 | 0.43 | 209 |
| Catalyst | 7:3 | 7.7 | 0.41 | 200 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Demirbas, M.F.; Balat, M. Biomass pyrolysis for liquid fuels and chemicals: A review. J. Sci. Ind. Res. 2007, 66, 797–804. [Google Scholar]
- Yaman, S. Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2004, 45, 651–671. [Google Scholar] [CrossRef]
- Stephanidis, S.; Nitsos, C.; Kalogiannis, K.; Iliopoulou, E.F.; Lappas, A.A.; Triantafyllidis, K.S. Catalytic upgrading of lignocellulosic biomass pyrolysis vapours: Effect of hydrothermal pre-treatment of biomass. Catal. Today 2011, 167, 37–45. [Google Scholar] [CrossRef]
- Carlson, T.R.; Vispute, T.P.; Huber, G.W. Green Gasoline by Catalytic Fast Pyrolysis of Solid Biomass Derived Compounds. ChemSusChem 2008, 1, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, S.; Wang, Q.; Guo, Z.; Luo, Z. Properties of Bio-oil from Fast Pyrolysis of Rice Husk. Chin. J. Chem. Eng. 2011, 19, 116–121. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Sharypov, V.I.; Beregovtsova, N.G.; Kuznetsov, B.N.; Membrado, L.; Cebolla, V.L.; Marin, N.; Weber, J.V. Co-pyrolysis of wood biomass and synthetic polymers mixtures. Part III: Characterisation of heavy products. J. Anal. Appl. Pyrolysis 2003, 67, 325–340. [Google Scholar] [CrossRef]
- Marin, N.; Collura, S.; Sharypov, V.I.; Beregovtsova, N.G.; Baryshnikov, S.V.; Kutnetzov, B.N.; Cebolla, V.; Weber, J.V. Copyrolysis of wood biomass and synthetic polymers mixtures. Part II: characterisation of the liquid phases. J. Anal. Appl. Pyrolysis 2002, 65, 41–55. [Google Scholar] [CrossRef]
- Jeon, M.J.; Choi, S.; Yoo, K.S.; Ryu, C.; Park, S.; Lee, J.; Jeon, J.K.; Park, Y.K.; Kim, S. Copyrolysis of block polypropylene with waste wood chip. Korean J. Chem. Eng. 2011, 28, 497–501. [Google Scholar] [CrossRef]
- Ateş, F. Energy Sources, co-pyrolytic Behaviors of Agricultural Wastes. Part A: Recovery, Utilization, and Environmental Effects; Taylor & Francis: London, UK, 2011; Volume 34, pp. 111–121. [Google Scholar]
- Sharypov, V.I.; Beregovtsova, N.G.; Kuznetsov, B.N.; Baryshnikov, S.V.; Cebolla, V.L.; Weber, J.V.; Collura, S.; Finqueneisel, G.; Zimny, T.J. Co-pyrolysis of wood biomass and synthetic polymers mixtures. Part IV: Catalytic pyrolysis of pine wood and polyolefinic polymers mixtures in hydrogen atmosphere. Anal. Appl. Pyrolysis 2006, 76, 265–270. [Google Scholar] [CrossRef]
- Jakab, E.; Várhegyi, G.; Faix, O. Thermal decomposition of polypropylene in the presence of wood-derived materials. J. Anal. Appl. Pyrolysis 2000, 56, 273–285. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Steele, P.H.; Hassan, E.B.M.; Mitchell, B.; Ingram, L.; Pittman, C.U., Jr. Wood/plastic copyrolysis in an auger reactor: Chemical and physical analysis of the products. Fuel 2009, 88, 1251–1260. [Google Scholar] [CrossRef]
- Brebu, M.; Ucar, S.; Vasile, C.; Yanik, J. Co-pyrolysis of pine cone with synthetic polymers. Fuel 2010, 89, 1911–1918. [Google Scholar] [CrossRef]
- Cornelissen, T.; Jans, M.; Yperman, J.; Reggers, G.; Schreurs, S.; Carleer, R. Flash co-pyrolysis of biomass with polyhydroxybutyrate. Part 1. Influence on bio-oil yield, water content, heating value and the production of chemicals. Fuel 2008, 87, 2523–2532. [Google Scholar] [CrossRef]
- Mercader, F.D.M.; Groeneveld, M.J.; Kersten, S.R.A.; Venderbosch, R.H.; Hogendoorn, J.A. Pyrolysis oil upgrading by high pressure thermal treatment. Fuel 2010, 89, 2829–2837. [Google Scholar] [CrossRef]
- Lappas, A.A.; Bezergianni, S.; Vasalos, I.A. Production of biofuels via co-processing in conventional refining processes. Catal. Today 2009, 145, 55–62. [Google Scholar] [CrossRef]
- Huber, G.W.; Corma, A. Synergies between Bio- and Oil Refineries for the Production of Fuels from Biomass. Angew. Chem. Int. Ed. 2007, 46, 7184–7201. [Google Scholar] [CrossRef] [PubMed]
- Taarning, E.; Osmundsen, C.M.; Yang, X.; Voss, B.; Andersen, S.I.; Christensen, C.H. Zeolite-catalyzed biomass conversion to fuels and chemicals. Energy Environ. Sci. 2011, 4, 793–804. [Google Scholar] [CrossRef]
- Wan, H.; Chaudhari, R.; Subramaniam, B. Catalytic Hydroprocessing of p-Cresol: Metal, Solvent and Mass-Transfer Effects. Top. Catal. 2012, 55, 129–139. [Google Scholar] [CrossRef]
- Wildschut, J.; Mahfud, F.H.; Venderbosch, R.H.; Heeres, H.J. Hydrotreatment of Fast Pyrolysis Oil Using Heterogeneous Noble-Metal Catalysts. Ind. Eng. Chem. Res. 2009, 48, 10324–10334. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Ma, L.; Zhang, Q.; Jiang, T. Hydrotreatment of bio-oil over Ni-based catalyst. Bioresour. Technol. 2013, 127, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, H.; Li, S.; Dai, W.; Jiang, J.; Yan, Y. Bio-Oil Upgrading Over Bi-Functional Catalyst in CO/H2O System. Asian J. Chem. 2013, 25, 3789–3792. [Google Scholar]
- Crombie, K.; Mašek, O. Investigating the potential for a self-sustaining slow pyrolysis system under varying operating conditions. Bioresour. Technol. 2014, 162, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.; Shi, D.; Resasco, D.E. Evaluating strategies for catalytic upgrading of pyrolysis oil in liquid phase. Appl. Catal. B 2014, 145, 10–23. [Google Scholar] [CrossRef]
- Saidi, M.; Samimi, F.; Karimipourfard, D.; Nimmanwudipong, T.; Gates, B.C.; Rahimpour, M.R. Upgrading of lignin-derived bio-oils by catalytic hydrodeoxygenation. Energy Environ. Sci. 2014, 7, 103–129. [Google Scholar] [CrossRef]
- Zhang, X.; Long, J.; Kong, W.; Zhang, Q.; Chen, L.; Wang, T.; Ma, L.; Li, Y. Catalytic Upgrading of Bio-oil over Ni-Based Catalysts Supported on Mixed Oxides. Energy Fuels 2014, 28, 2562–2570. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Gardini, D.; de Carvalho, H.W.P.; Damsgaard, C.D.; Grunwaldt, J.D.; Jensen, P.A.; Wagner, J.B.; Jensen, A.D. Stability and resistance of nickel catalysts for hydrodeoxygenation: carbon deposition and effects of sulfur, potassium, and chlorine in the feed. Catal. Sci. Technol. 2014, 4, 3672–3686. [Google Scholar] [CrossRef] [Green Version]
- Kandel, K.; Anderegg, J.W.; Nelson, N.C.; Chaudhary, U.; Slowing, I.I. Supported iron nanoparticles for the hydrodeoxygenation of microalgal oil to green diesel. J. Catal. 2014, 314, 142–148. [Google Scholar] [CrossRef]
- Hensen, E.J.M.; Brans, H.J.A.; Lardinois, G.M.H.J.; de Beer, V.H.J.; van Veen, J.A.R.; van Santen, R.A. Periodic Trends in Hydrotreating Catalysis: Thiophene Hydrodesulfurization over Carbon-Supported 4d Transition Metal Sulfides. J. Catal. 2000, 192, 98–107. [Google Scholar] [CrossRef]
- Eijsbouts, S.; de Beer, V.H.J.; Prins, R. Periodic trends in the hydrodenitrogenation activity of carbon-supported transition metal sulfide catalysts. J. Catal. 1988, 109, 217–220. [Google Scholar] [CrossRef]
- Elkasabi, Y.; Mullen, C.A.; Pighinelli, A.L.M.T.; Boateng, A.A. Hydrodeoxygenation of fast-pyrolysis bio-oils from various feedstocks using carbon-supported catalysts. Fuel Proc. Technol. 2014, 123, 11–18. [Google Scholar] [CrossRef]
- Priecel, P.; Kubička, D.; Čapek, L.; Bastl, Z.; Ryšánek, P. The role of Ni species in the deoxygenation of rapeseed oil over NiMo-alumina catalysts. Appl. Catal. A 2011, 397, 127–137. [Google Scholar] [CrossRef]
- Holmgren, J.; Gosling, C.; Marinangeli, R.; Marker, T.; Faraci, G.; Perego, C. New developments in renewable fuels offer more choices: Vegetable oil-based diesel can offer better integration within crude-oil refineries for fuels blending: Refining Developments. Hydrocarbon Process. Int. Ed. 2007, 86, 67–72. [Google Scholar]
- Donnis, B.; Egeberg, R.; Blom, P.; Knudsen, K. Hydroprocessing of Bio-Oils and Oxygenates to Hydrocarbons. Understanding the Reaction Routes. Top. Catal. 2009, 52, 229–240. [Google Scholar] [CrossRef]
- Santillan-Jimenez, E.; Morgan, T.; Lacny, J.; Mohapatra, S.; Crocker, M. Catalytic deoxygenation of triglycerides and fatty acids to hydrocarbons over carbon-supported nickel. Fuel 2013, 103, 1010–1017. [Google Scholar] [CrossRef]
- Peng, B.; Zhao, C.; Kasakov, S.; Foraita, S.; Lercher, J.A. Manipulating catalytic pathways: Deoxygenation of palmitic acid on multifunctional catalysts. Chem. Eur. J. 2013, 19, 4732–4741. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.K.; Huh, B.; Lee, C.H.; Suh, D.J.; Suh, Y.W.; Heo, H.S.; Park, Y.K. The Hydrodeoxygenation of 2-Methoxyphenol over Ni/Kieselguhr Catalysts as a Model Reaction for Bio-oil Upgrading. In Energy Sources Part A: Recovery Utilization Environmental Effects; Taylor & Francis: London, UK, 2013; Volume 35, pp. 271–277. [Google Scholar]
- Hurff, S.J.; Klein, M.T. Reaction pathway analysis of thermal and catalytic lignin fragmentation by use of model compounds. Ind. Eng. Chem. Fundam. 1983, 22, 426–430. [Google Scholar] [CrossRef]
- He, L.; Wu, C.; Cheng, H.; Yuab, Y.; Zhao, F. Highly selective and efficient catalytic conversion of ethyl stearate into liquid hydrocarbons over a Ru/TiO2 catalyst under mild conditions. Catal. Sci. Technol. 2012, 2, 1328–1331. [Google Scholar] [CrossRef]
- Han, J.; Kim, H.J. Thermochemical liquefaction characteristics of microalgae in sub- and supercritical ethanol. Energy Sources 2009, 31, 364–371. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, X.; Zeng, G.; Wang, J.; Li, H.; Zhou, C.; Pei, X.; You, Q.; Chen, L. Pyrolysis Characteristic and Kinetic of Sawdust-Polypropylene Blend. Fuel Process. Technol. 2011, 92, 147–153. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, D.; Wu, Y.; Chen, Y.; Li, J.; Yang, M.; Lu, X. Co-Pyrolysis Behaviors of the Cotton Straw/PP Mixtures and Catalysis Hydrodeoxygenation of Co-Pyrolysis Products over Ni-Mo/Al2O3 Catalyst. Catalysts 2015, 5, 2085-2097. https://doi.org/10.3390/catal5042085
Hua D, Wu Y, Chen Y, Li J, Yang M, Lu X. Co-Pyrolysis Behaviors of the Cotton Straw/PP Mixtures and Catalysis Hydrodeoxygenation of Co-Pyrolysis Products over Ni-Mo/Al2O3 Catalyst. Catalysts. 2015; 5(4):2085-2097. https://doi.org/10.3390/catal5042085
Chicago/Turabian StyleHua, Derun, Yulong Wu, Yu Chen, Jian Li, Mingde Yang, and Xinning Lu. 2015. "Co-Pyrolysis Behaviors of the Cotton Straw/PP Mixtures and Catalysis Hydrodeoxygenation of Co-Pyrolysis Products over Ni-Mo/Al2O3 Catalyst" Catalysts 5, no. 4: 2085-2097. https://doi.org/10.3390/catal5042085





