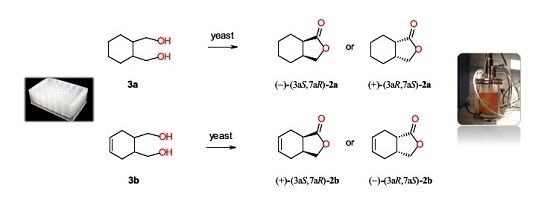
Microbial Stereoselective One-Step Conversion of Diols to Chiral Lactones in Yeast Cultures
Abstract
:1. Introduction
2. Results and Discussion
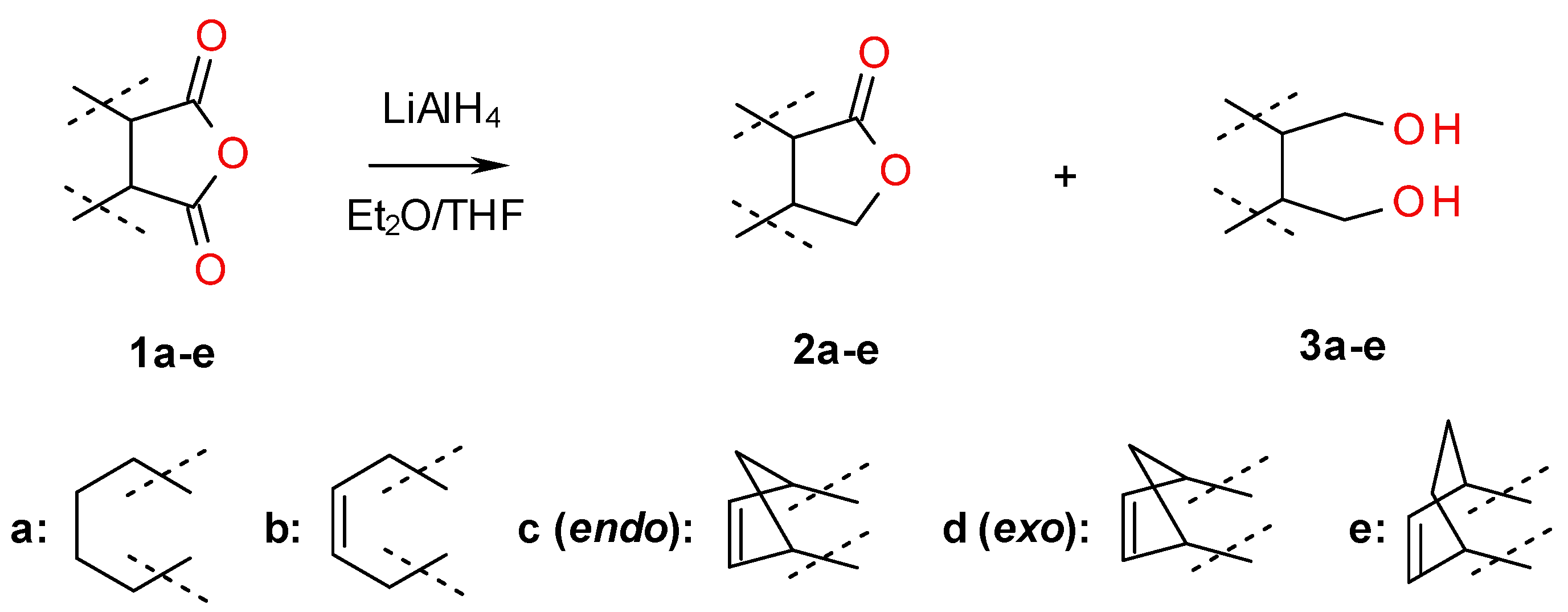
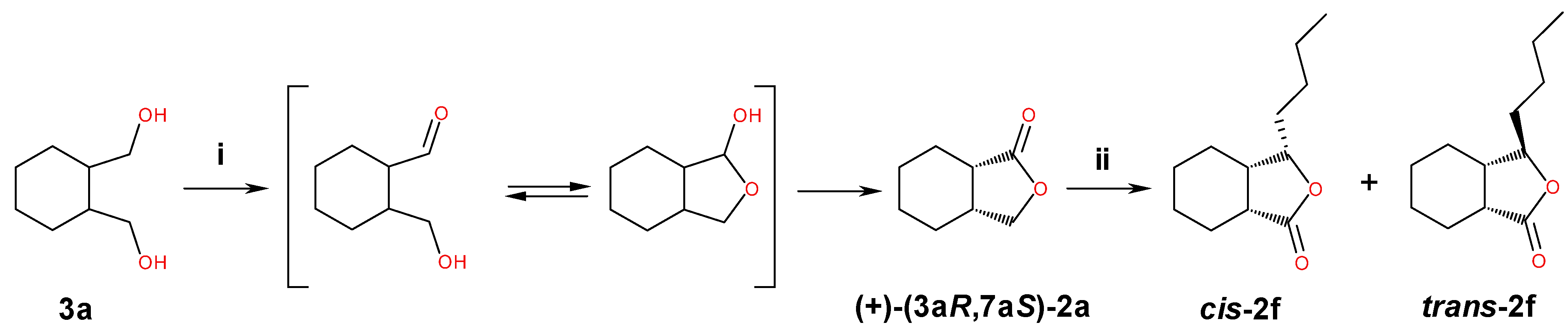
2.1. Synthesis of Diols 3a–f and Lactones 2a–f
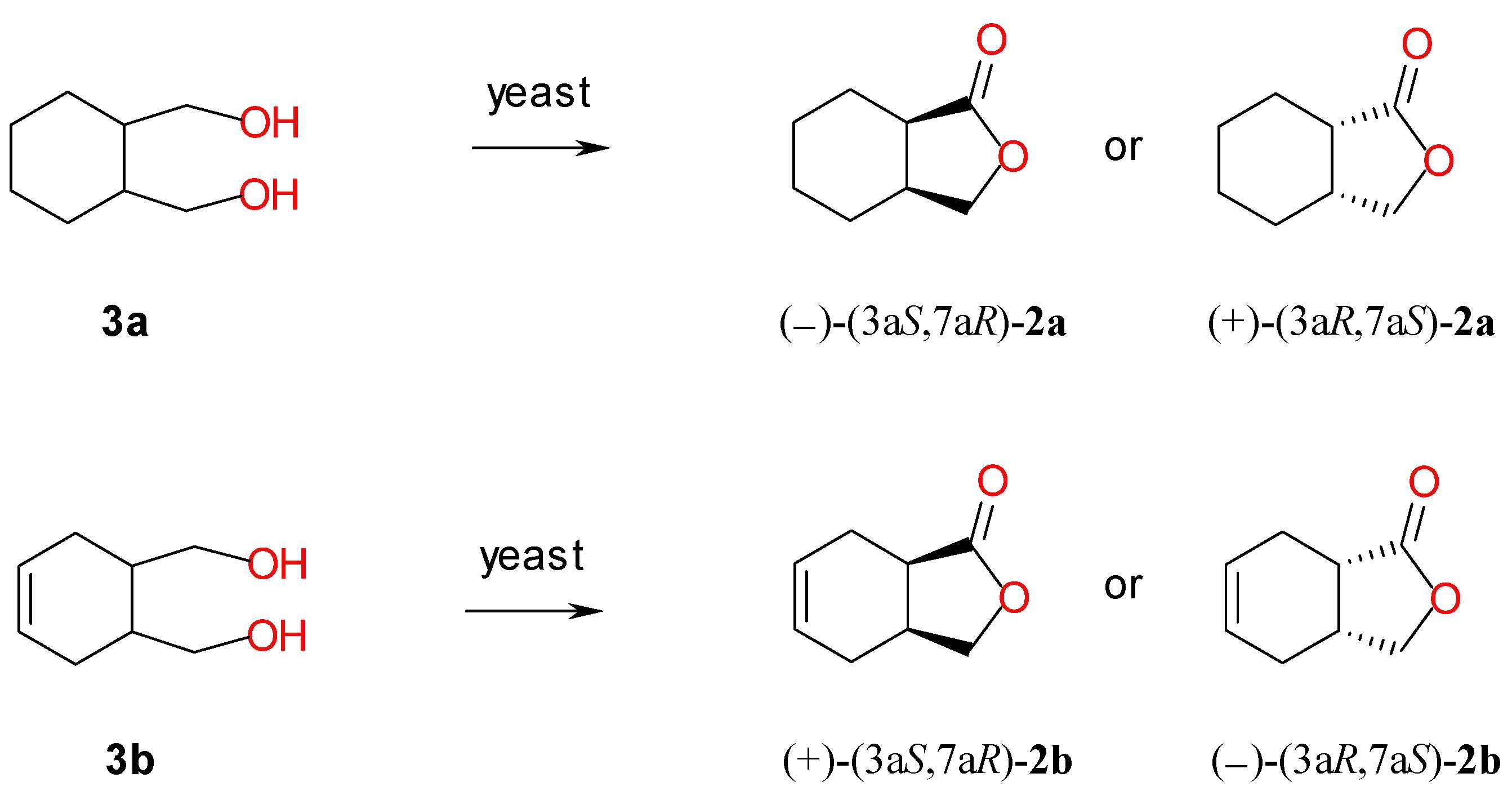
2.2. Screening Scale Biotransformations of Monocyclic Meso Diols 3a–b
| Strain | Time (day) | Conversion of Diol 3a (%) | Lactone 2a | |
|---|---|---|---|---|
| ee (%) | Isomer | |||
| Candida pelliculosa ZP22 | 14 | 92 | 70 | (+)-(3aR,7aS) |
| Candida viswanathi AM120 | 21 | 9 | 0 | racemic |
| Saccharomyces cerevisiae AM464 | 21 | 20 | 95 | (+)-(3aR,7aS) |
| Saccharomyces pastorianus 906 | 21 | >99 | 0 | racemic |
| Yarrowia lipolytica AR71 | 21 | 60 | 68 | (+)-(3aR,7aS) |
| Yarrowia lipolytica AR72 | 21 | 44 | 58 | (+)-(3aR,7aS) |
| Rhodotorula glutinis AM242 | 14 | 20 | 50 | (–)-(3aS,7aR) |
| Rhodotorula marina 77 | 21 | 12 | 10 | (–)-(3aS,7aR) |
| Rhodotorula rubra AM82 | 21 | 28 | 6 | (–)-(3aS,7aR) |
| Rhodotorula rubra AM4 | 21 | 18 | 10 | (–)-(3aS,7aR) |
| Strain | Time (day) | Conversion of Diol 3b (%) | Lactone 2b | |
|---|---|---|---|---|
| ee (%) | Isomer | |||
| Candida pelliculosa ZP22 | 14 | >99 | 68 | (+)-(3aS,7aR) |
| Saccharomyces cerevisiae AM464 | 21 | >99 | 40 | (–)-(3aR,7aS) |
| Yarrowia lipolytica AR71 | 21 | >99 | 50 | (+)-(3aS,7aR) |
| Strain | Time | Lactone 2a | Lactone 2b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH 4.5 | pH 7.2 | pH 8.5 | pH 4.5 | pH 7.2 | pH 8.5 | ||||||||
| day | (%) | ee (%) | (%) | ee (%) | (%) | ee (%) | (%) | ee (%) | (%) | ee (%) | (%) | ee (%) | |
| Candida pelliculosa ZP22 | 14 | >99 | 68 | >99 | 66 | 95 | 64 | >99 | 64 | >99 | 68 | >99 | 70 |
| Saccharomyces cerevisiae AM464 | 21 | 0 | - | 0 | - | 0 | - | >99 | 54 | >99 | 24 | >99 | 50 |
| Yarrowia lipolytica AR71 | 21 | 89 | 58 | 94 | 58 | 94 | 56 | 59 | 50 | 60 | 50 | 52 | 50 |
2.3. Preparative-Scale Biotransformations of Monocyclic Meso Diols 3a–b
2.4. Screening-Scale Biotransformations of Bicyclic Meso Diols 3c–e
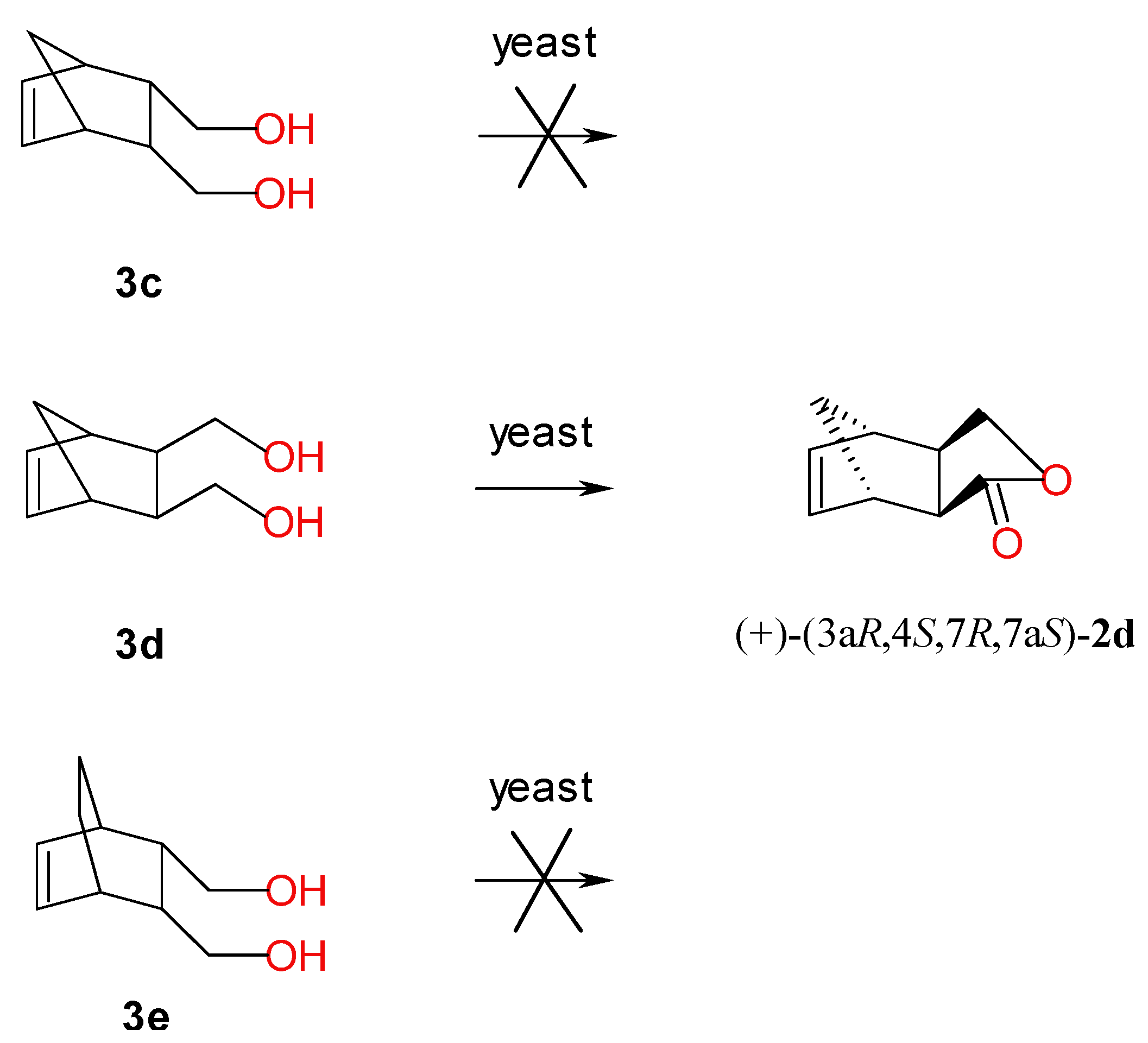
| Strain | Conversion of Diol 3d (%) | Lactone 2d | |
|---|---|---|---|
| ee (%) | Isomer | ||
| Candida viswanathi AM120 | >99 | 64 | (+)-(3aR,4S,7R,7aS) |
| Saccharomyces pastorianus 906 | >99 | 50 | (+)-(3aR,4S,7R,7aS) |
| Yarrowia lipolytica AR71 | >99 | >99 | (+)-(3aR,4S,7R,7aS) |
| Rhodotorula glutinis AM242 | >99 | 54 | (+)-(3aR,4S,7R,7aS) |
| Rhodotorula rubra AM82 | 15 | 80 | (+)-(3aR,4S,7R,7aS) |
| Rhodotorula rubra AM4 | 15 | 76 | (+)-(3aR,4S,7R,7aS) |
2.5. Screening Scale Biotransformations of Diol 3f
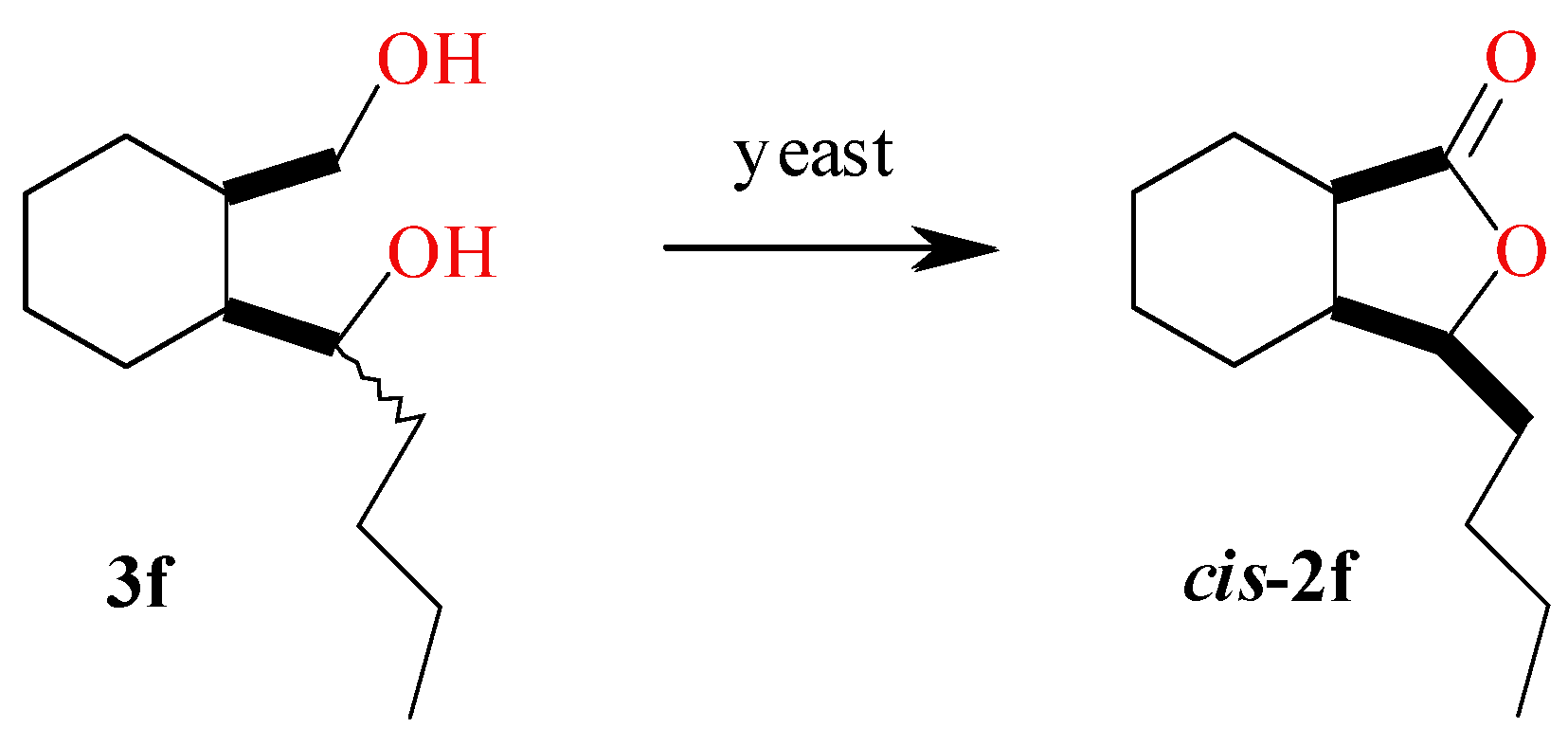
| Strain | Conversion of Diol 3f (%) | Lactone 2f ee (%) |
|---|---|---|
| Candida viswanathi AM120 | 11 | 21 |
| Candida sake AM908 | 18 | >99 |
| Candida parapsilosis AM909 | 22 | 38 |
| Yarrowia lipolytica AR71 | 4 | 62 |
| Rhodotorula marina 77 | 9 | 21 |
| Rhodotorula rubra AM82 | 11 | 98 |
| Rhodotorula rubra AM4 | 17 | >99 |
3. Experimental Section
3.1. Analysis
3.2. Chemicals
3.3. Synthesis of Meso Diols 3a–e and Lactones 2a–e
3.3.1. cis-Hexahydro-1(3H)-isobenzofuranone (±)-(2a)
3.3.2. cis-3a,4,7,7a-Tetrahydro-1(3H)-isobenzofuranone (±)-(2b)
3.3.3. cis-endo-3a,4,7,7a-Tetrahydro-4,7-methanoisobenzofuran-1(3H)-one (±)-(2c)
3.3.4. cis-exo-3a,4,7,7a-Tetrahydro-4,7-methanoisobenzofuran-1(3H)-one (±)-(2d)
3.3.5. cis-endo-3a,4,7,7a-Tetrahydro-4,7-ethanoisobenzofuran-1(3H)-one (±)-(2e)
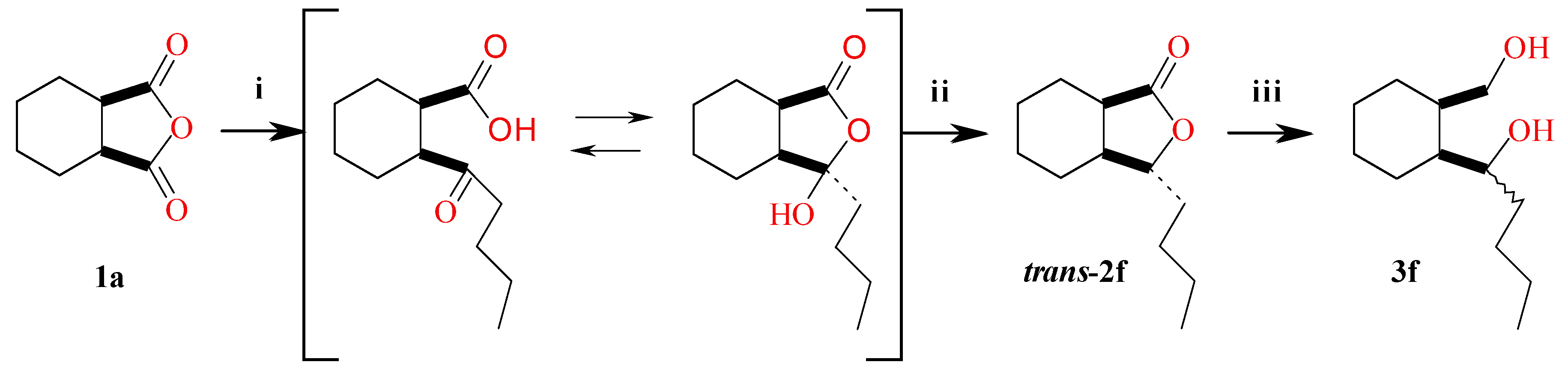
3.4. Synthesis of Diol 3f and Lactone 2f
3.4.1. 1-(2-(Hydroxymethyl)cyclohexyl)pentan-1-ol (±)-(3f)
3.4.2. trans-3-Butylhexahydro-1(3H)-isobenzofuranone (±)-(2f)
3.5. Growth Conditions
- A:
- 40 g glucose, 15 g (NH4)3PO4, 7 g KH2PO4, 0.8 g MgSO4·7H2O, 0.1 g NaCl, 6 × 10−3 g ZnSO4·7H2O, 5 × 10−3 g CuSO4·5H2O, 1 × 10−3 g MnSO4·4H2O;
- C:
- 30 g saccharose, 3 g NaNO3, 1 g KH2PO4, 0.5 g MgSO4·7H2O, 0.5 g KCl, 0.01 g FeSO4;
- E:
- 10 g starch, 4 g yeast extract, 0.1 g K2HPO4, 0.05 g MgSO4·7H2O;
- G:
- 10 g glucose, 0.5 g asparagine, 0.5 g K2HPO4;
- M:
- 40 g glucose, 2 g asparagine, 0.5 g thiamine, 0.5 g KH2PO4, 0.25 g MgSO4·7H2O;
- P:
- 30 g glucose, 10 g peptone;
- S:
- 10 g glucose, 2.5 × 10−3 g genistein, 2.5 g K2HPO4, 2.5 g NaNO3.
3.6. Microorganisms
3.7. Biotransformations of Diols 3a–f
3.7.1. Screening-Scale Biotransformations in Microtiter Plates
3.7.2. Preparative-Scale Biotransformation in a Bioreactor
3.7.3. Preparative Oxidation of Meso Diols 3a–b Catalyzed by Candida pelliculosa ZP22
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Breuer, M.; Ditrich, K.; Habicher, T.; Hauer, B.; Keßeler, M.; Stürmer, R.; Zelinski, T. Industrial methods for the production of optically active intermediates. Angew. Chem. Int. Ed. 2004, 43, 788–824. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Solano, D.; Hoyos, P.; Hernáiz, M.J.; Alcántara, A.R.; Sánchez-Montero, J.M. Industrial biotransformations in the synthesis of building blocks leading to enantiopure drugs. Bioresour. Technol. 2012, 115, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Nestl, B.M.; Nebel, B.A.; Hauer, B. Recent progress in industrial biocatalysis. Curr. Opin. Chem. Biol. 2011, 15, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Brenna, E.; Fuganti, C.; Gatti, F.G.; Serra, S. Biocatalytic methods for the synthesis of enantioenriched odor active compounds. Chem. Rev. 2011, 111, 4036–4072. [Google Scholar] [CrossRef] [PubMed]
- Muschiol, J.; Peters, C.; Oberleitner, N.; Mihovilovic, M.D.; Bornscheuer, U.T.; Rudroff, F. Cascade catalysis—Strategies and challenges en route to preparative synthetic biology. Chem. Commun. 2015, 51, 5798–5811. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gallego, F.; Schmidt-Dannert, C. Multi-enzymatic synthesis. Curr. Opin. Chem. Biol. 2010, 14, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Deasy, R.E.; O’Riordan, N.; Maguire, A.R. Baker’s yeast mediated reduction of 2-acetyl-3-methyl sulfolane. Catalysts 2014, 4, 186–195. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamanaka, R.; Matsuda, T.; Harada, T. Recent developments in asymmetric reduction of ketones with biocatalysis. Tetrahedron Asymmetr. 2003, 14, 2659–2681. [Google Scholar] [CrossRef]
- Brenna, E.; Dei Negri, C.; Fuganti, C.; Serra, S. Baker’s yeast-mediated approach to (−)-cis- and (+)-trans-aerangis lactones. Tetrahedron Asymmetr. 2001, 12, 1871–1879. [Google Scholar] [CrossRef]
- Sohoni, S.; Bapat, P.; Lantz, A. Robust, small-scale cultivation platform for Streptomyces coelicolor. Microb. Cell Fact. 2012, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zang, R.; Zhang, X.; Yang, S.-T. A 24-microwell plate with improved mixing and scalable performance for high throughput cell cultures. Process Biochem. 2012, 47, 612–618. [Google Scholar] [CrossRef]
- Chen, A.; Chitta, R.; Chang, D.; Amanullah, A. Twenty-four well plate miniature bioreactor system as a scale-down model for cell culture process development. Biotechnol. Bioeng. 2009, 102, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Betts, J.; Baganz, F. Miniature bioreactors: Current practices and future opportunities. Microb. Cell Fact. 2006. [Google Scholar] [CrossRef] [PubMed]
- Forchin, M.C.; Crotti, M.; Gatti, F.G.; Parmeggiani, F.; Brenna, E.; Monti, D. A rapid and high-throughput assay for the estimation of conversions of ene-reductase-catalysed reactions. ChemBioChem 2015, 16, 1571–1573. [Google Scholar] [CrossRef] [PubMed]
- Duetz, W. Microtiter plates as mini-bioreactors: Miniaturization of fermentation methods. Trends Microbiol. 2007, 15, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Duetz, W.; Witholt, B. Oxygen transfer by orbital shaking of square vessels and deepwell microtiter plates of various dimensions. Biochem. Eng. J. 2004, 17, 181–185. [Google Scholar] [CrossRef]
- Duetz, W.A.; Rüedi, L.; Hermann, R.; O’Connor, K.; Büchs, J.; Witholt, B. Methods for intense aeration, growth, storage, and replication of bacterial strains in microtiter plates. Appl. Environ. Microb. 2000, 66, 2641–2646. [Google Scholar] [CrossRef]
- Boratyński, F.; Kiełbowicz, G.; Wawrzeńczyk, C. Lactones 34 [1]. Application of alcohol dehydrogenase from horse liver (HLADH) in enantioselective synthesis of δ- and ɛ-lactones. J. Mol. Catal. B 2010, 65, 30–36. [Google Scholar] [CrossRef]
- Boratyński, F.; Pannek, J.; Walczak, P.; Janik-Polanowicz, A.; Huszcza, E.; Szczepańska, E.; Martinez-Rojas, E.; Olejniczak, T. Microbial alcohol dehydrogenase screening for enantiopure lactone synthesis: Down-stream process from microtiter plate to bench bioreactor. Process Biochem. 2014, 49, 1637–1646. [Google Scholar] [CrossRef]
- Boratyński, F.; Smuga, M.; Wawrzeńczyk, C. Lactones 42. Stereoselective enzymatic/microbial synthesis of optically active isomers of whisky lactone. Food Chem. 2013, 141, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Horn, M.; Martinez-Rojas, E.; Görisch, H.; Tressl, R.; Garbe, L.A. Oxidation of 1,4-alkanediols into g-lactones via g-lactols using Rhodococcus erythropolis as biocatalyst. J. Mol. Catal. B 2007, 49, 24–27. [Google Scholar] [CrossRef]
- Romano, A.; Gandolfi, R.; Nitti, P.; Rollini, M.; Molinari, F. Acetic acid bacteria as enantioselective biocatalysts. J. Mol. Catal. B 2002, 17, 235–240. [Google Scholar] [CrossRef]
- Jakovac, I.J.; Goodbrand, H.B.; Lok, K.P.; Jones, J.B. Enzymes in organic synthesis. 24. Preparations of enantiomerically pure chiral lactones via stereospecific horse liver alcohol dehydrogenase catalyzed oxidations of monocyclic meso diols. J. Am. Chem. Soc. 1982, 104, 4659–4665. [Google Scholar] [CrossRef]
- Lok, K.P.; Jakovac, I.J.; Jones, J.B. Enzymes in organic synthesis. 34. Preparations of enantiomerically pure exo- and endo-bridged bicyclic [2.2.1] and [2.2.2] chiral lactones via stereospecific horse liver alcohol dehydrogenase catalyzed oxidations of meso diols. J. Am. Chem. Soc. 1985, 107, 2521–2526. [Google Scholar] [CrossRef]
- Bridges, A.J.; Raman, P.S.; Ng, G.S.Y.; Jones, J.B. Enzymes in organic synthesis. 31. Preparations of enantiomerically pure bicyclic [3.2.1] and [3.3.1] chiral lactones via stereospecific horse liver alcohol dehydrogenase catalyzed oxidations of meso diols. J. Am. Chem. Soc. 1984, 106, 1461–1467. [Google Scholar] [CrossRef]
- Jones, J.B.; Francis, C.J. Enzymes in organic synthesis. 32. Stereospecyfic horse liver alcohol dehydrogenase—Catalyzed oxidations of exo- and endo-oxabicyclic meso diols. Can. J. Chem. 1984, 62, 2578–2582. [Google Scholar] [CrossRef]
- Olejniczak, T.; Boratyński, F.; Białońska, A. Fungistatic activity of bicycle [4.3.0]-g-lactones. J. Agric. Food Chem. 2011, 59, 6071–6081. [Google Scholar] [CrossRef] [PubMed]
- Soni, P.; Banerjee, U.C. Biotransformations for the production of the chiral drug (S)-duloxetine catalyzed by a novel isolate of Candida tropicalis. Appl. Microbiol. Biotechnol. 2005, 67, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, A.; Yamamoto, H.; Kawada, N.; Kobayashi, Y. Industrial production of (R)-1,3-butanediol by new biocatalysts. J. Mol. Catal. B 2001, 11, 513–521. [Google Scholar] [CrossRef]
- Dehli, J.R.; Gotor, V. Dynamic kinetic resolution of 2-oxocycloalkanecarbonitriles: Chemoenzymatic syntheses of optically active cyclic β- and γ-amino alcohols. J. Org. Chem. 2002, 67, 6816–6819. [Google Scholar] [CrossRef] [PubMed]
- Stuermer, R.; Hauer, B.; Hall, M.; Faber, K. Asymmetric bioreduction of activated C=C bonds using enoate reductases from the old yellow enzyme family. Curr. Opin. Chem. Biol. 2007, 11, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Inaba, Y.; Tokitoh, N. Asymmetric reduction of nitroalkenes with baker’s yeast. Tetrahedron Asymmetr. 2001, 12, 309–318. [Google Scholar] [CrossRef]
- Serra, S.; Fuganti, C.; Gatti, F.G. A chemoenzymatic, preparative synthesis of the isomeric forms of p-menth-1-en-9-ol: Application to the synthesis of the isomeric forms of the cooling agent 1-hydroxy-2,9-cineole. Eur. J. Org. Chem. 2008, 1031–1037. [Google Scholar] [CrossRef]
- Fronza, G.; Fuganti, C.; Serra, S. Stereochemical course of baker's yeast mediated reduction of the tri- and tetrasubstituted double bonds of substituted cinnamaldehydes. Eur. J. Org. Chem. 2009, 2009, 6160–6171. [Google Scholar] [CrossRef]
- Sortino, M.A.; Filho, V.C.; Zacchino, S.A. Highly enantioselective reduction of the C–C double bond of N-phenyl-2-methyl- and N-phenyl-2,3-dimethyl-maleimides by fungal strains. Tetrahedron Asymmetr. 2009, 20, 1106–1108. [Google Scholar] [CrossRef]
- Csuk, R.; Glaenzer, B.I. Baker’s yeast mediated transformations in organic chemistry. Chem. Rev. 1991, 91, 49–97. [Google Scholar] [CrossRef]
- Glänzer, B.I.; Faber, K.; Griengl, H. Microbial resolution of o-acetylpantoyl lactone. Enzyme Microb. Technol. 1988, 10, 689–690. [Google Scholar] [CrossRef]
- Patel, R.N.; Hou, C.T.; Laskin, A.I.; Derelanko, P.; Felix, A. Oxidation of secondary alcohols to methyl ketones by yeasts. Appl. Environ. Microb. 1979, 38, 219–223. [Google Scholar]
- Nestl, B.; Voss, C.; Bodlenner, A.; Ellmer-Schaumberger, U.; Kroutil, W.; Faber, K. Biocatalytic racemization of sec-alcohols and α-hydroxyketones using lyophilized microbial cells. Appl. Microb. Biotechnol. 2007, 76, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Momin, R.A.; Nair, M.G. Mosquitocidal, nematicidal, and antifungal compounds from Apium graveolens L. seeds. J. Agric. Food Chem. 2001, 49, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.J.; Chou, S.-C. The structural diversity of phthalides from the Apiaceae. J. Nat. Prod. 2007, 70, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Bartschat, D.; Beck, T.; Mosandl, A. Stereoisomeric flavor compounds. 79. Simultaneous enantioselective analysis of 3-butylphthalide and 3-butylhexahydro-phthalide stereoisomers in celery, celeriac, and fennel. J. Agric. Food Chem. 1997, 45, 4554–4557. [Google Scholar] [CrossRef]
- Walczak, P.; Pannek, J.; Boratyński, F.; Janik-Polanowicz, A.; Olejniczak, T. Synthesis and fungistatic activity of bicyclic lactones and lactams against Botrytis cinerea, Penicillium citrinum and Aspergillus glaucus. J. Agric. Food Chem. 2014, 62, 8571–8578. [Google Scholar] [CrossRef] [PubMed]
- Zaleska, I.; Piegza, M. Asymilacja nietypowych źródeł węgla przez mikroorganizmy o specyficznych uzdolnieniach do życia w wysoko stresogennych środowiskach. Acta Sci. Pol. Biotechnol. 2008, 7, 27–43. [Google Scholar]
- Le Guillou, R.; Fache, F.; Piva, O. Reductive alkylation of anhydrides and lactones: Direct access to monosubstituted lactones. C. R. Chim. 2002, 5, 571–575. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boratyński, F.; Szczepańska, E.; Pannek, J.; Olejniczak, T. Microbial Stereoselective One-Step Conversion of Diols to Chiral Lactones in Yeast Cultures. Catalysts 2015, 5, 2068-2084. https://doi.org/10.3390/catal5042068
Boratyński F, Szczepańska E, Pannek J, Olejniczak T. Microbial Stereoselective One-Step Conversion of Diols to Chiral Lactones in Yeast Cultures. Catalysts. 2015; 5(4):2068-2084. https://doi.org/10.3390/catal5042068
Chicago/Turabian StyleBoratyński, Filip, Ewa Szczepańska, Jakub Pannek, and Teresa Olejniczak. 2015. "Microbial Stereoselective One-Step Conversion of Diols to Chiral Lactones in Yeast Cultures" Catalysts 5, no. 4: 2068-2084. https://doi.org/10.3390/catal5042068