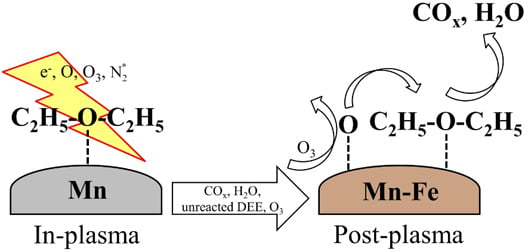
Non-Thermal Plasma Combined with Cordierite-Supported Mn and Fe Based Catalysts for the Decomposition of Diethylether
Abstract
:1. Introduction
2. Results and Discussion
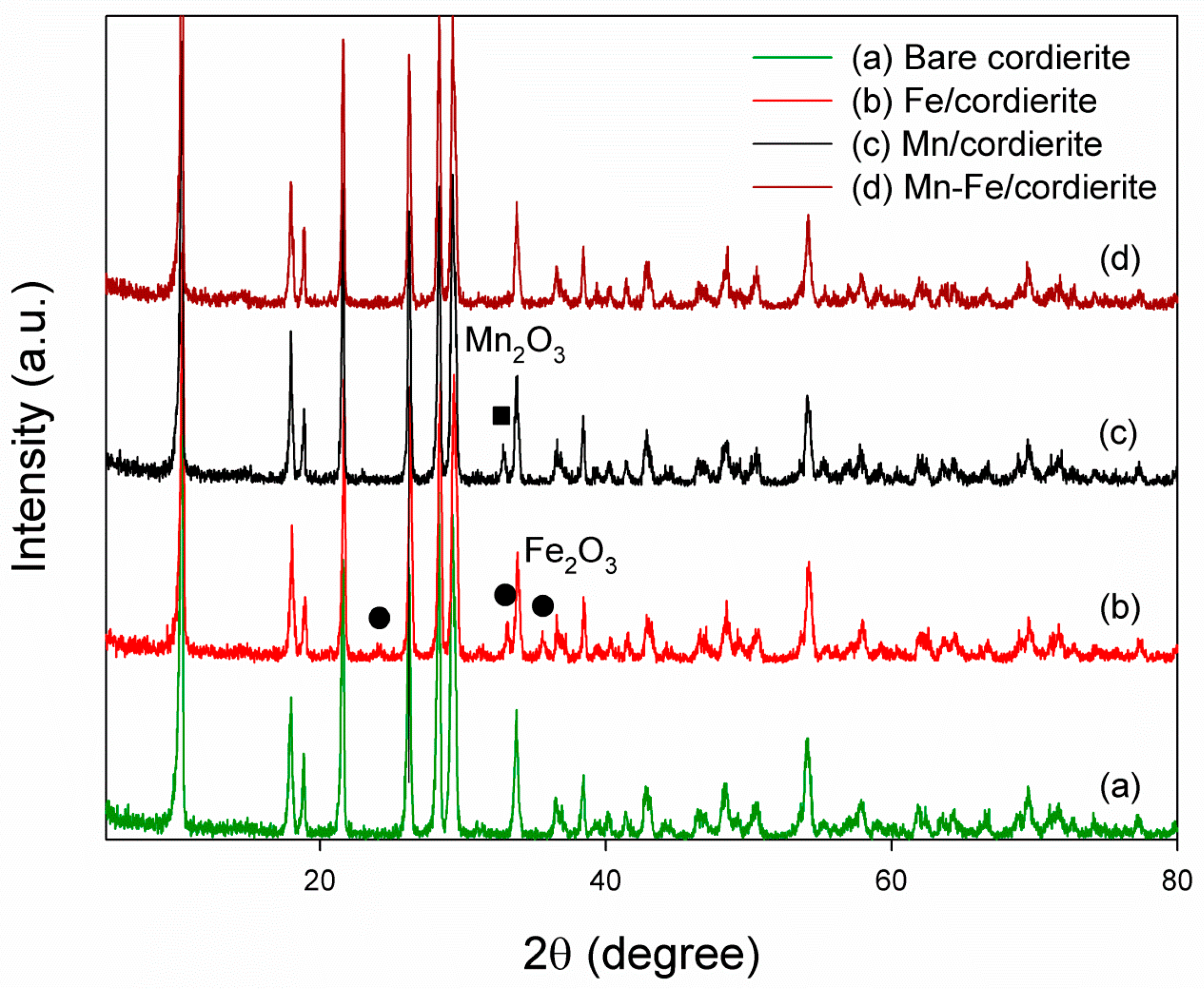
2.1. X-ray Diffraction (XRD) Characterization of Prepared Catalysts
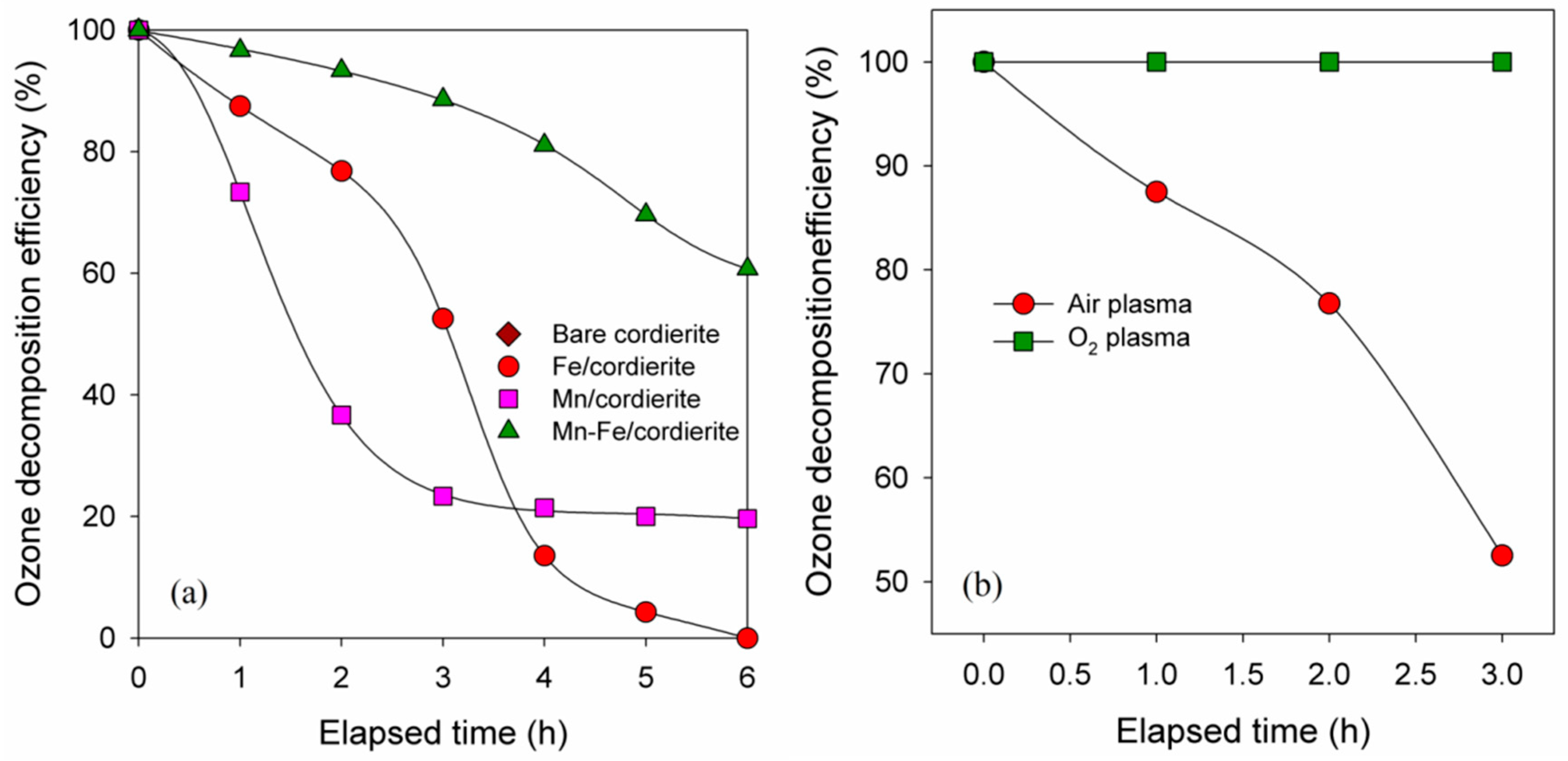
2.2. Catalytic Activities of Prepared Catalysts for Ozone Decomposition
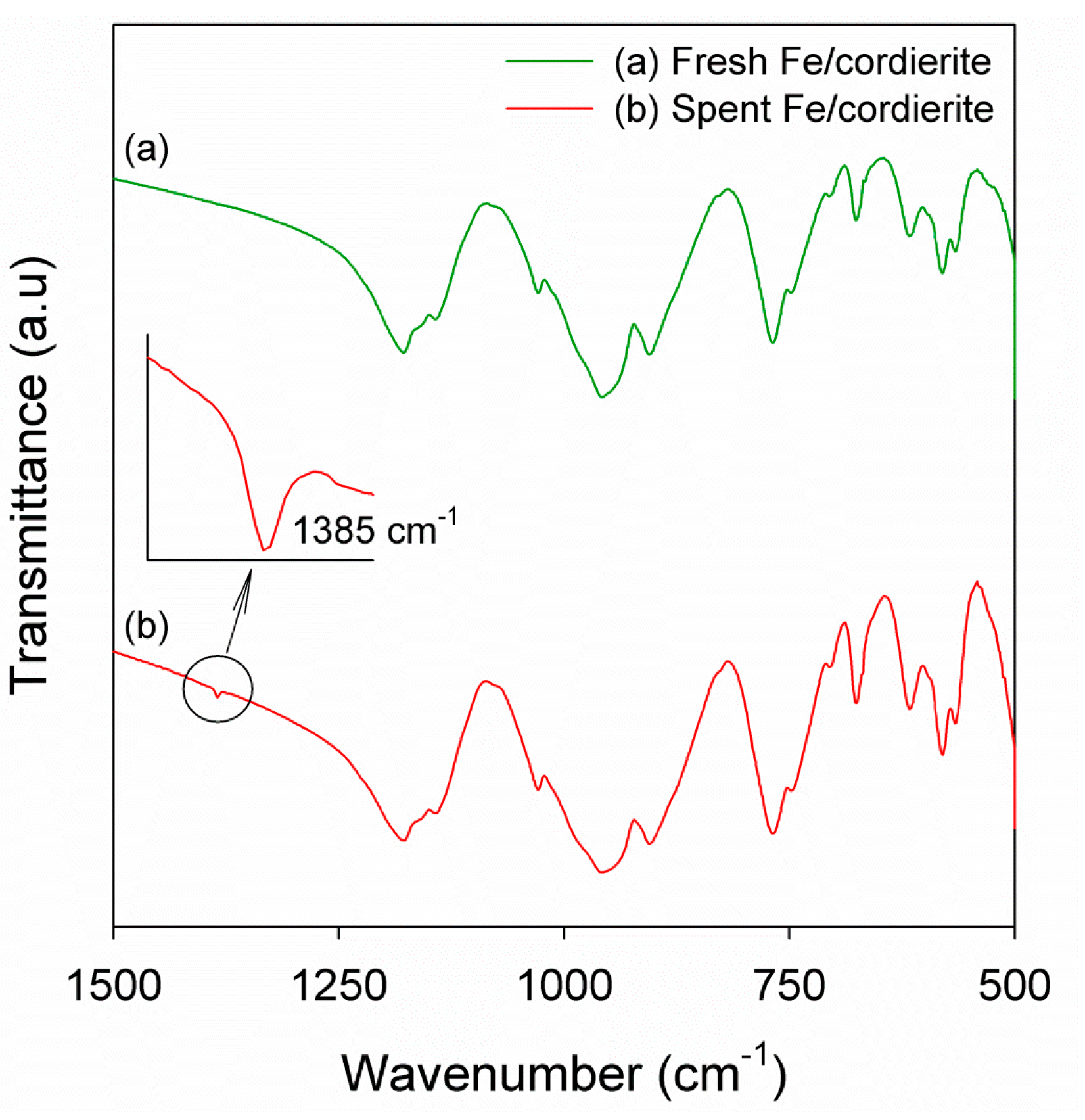
2.3. DEE Decomposition in a One-Stage Reactor
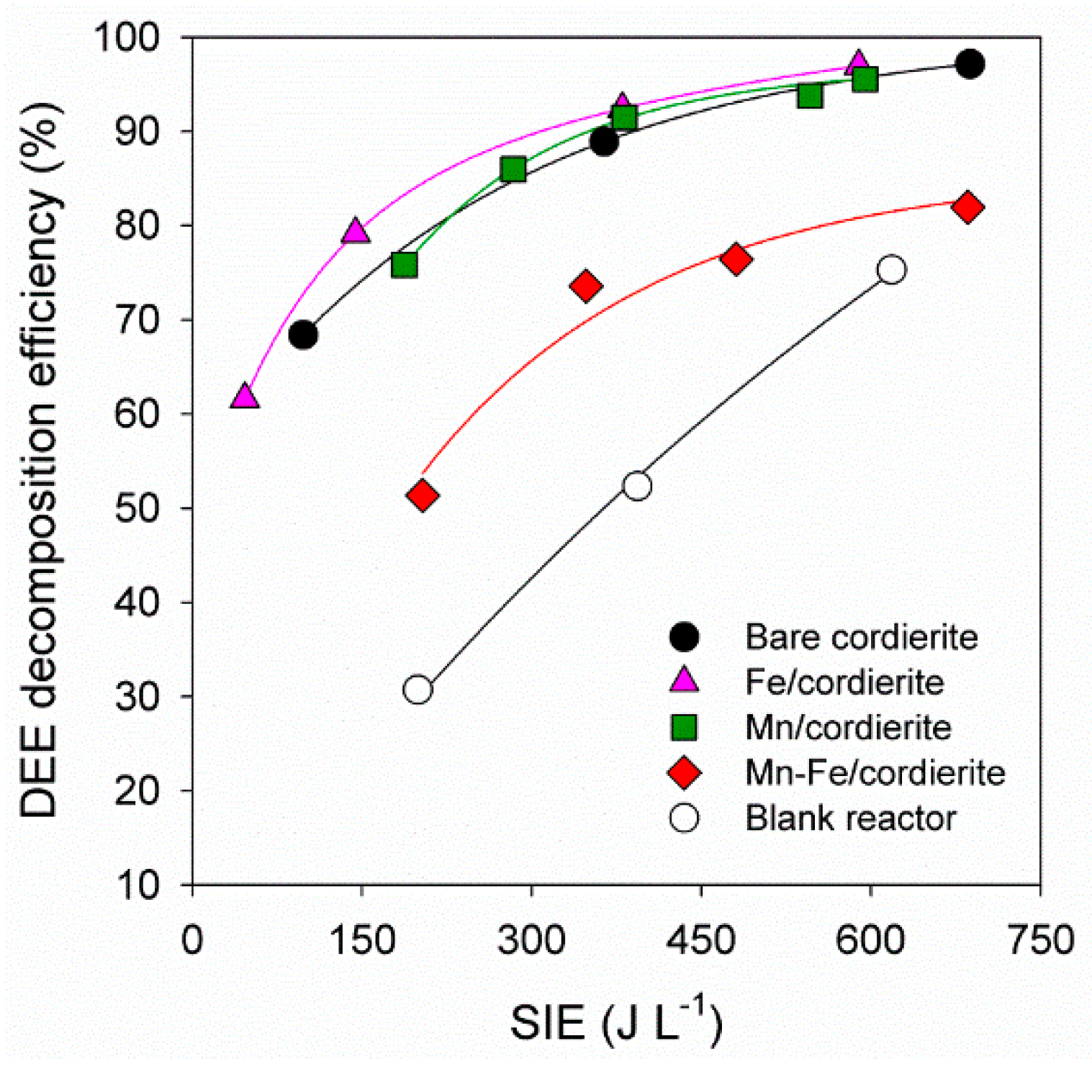


2.4. DEE Decomposition in the Mn+(Mn-Fe) Reactor




3. Experimental Section
3.1. Apparatus and Materials
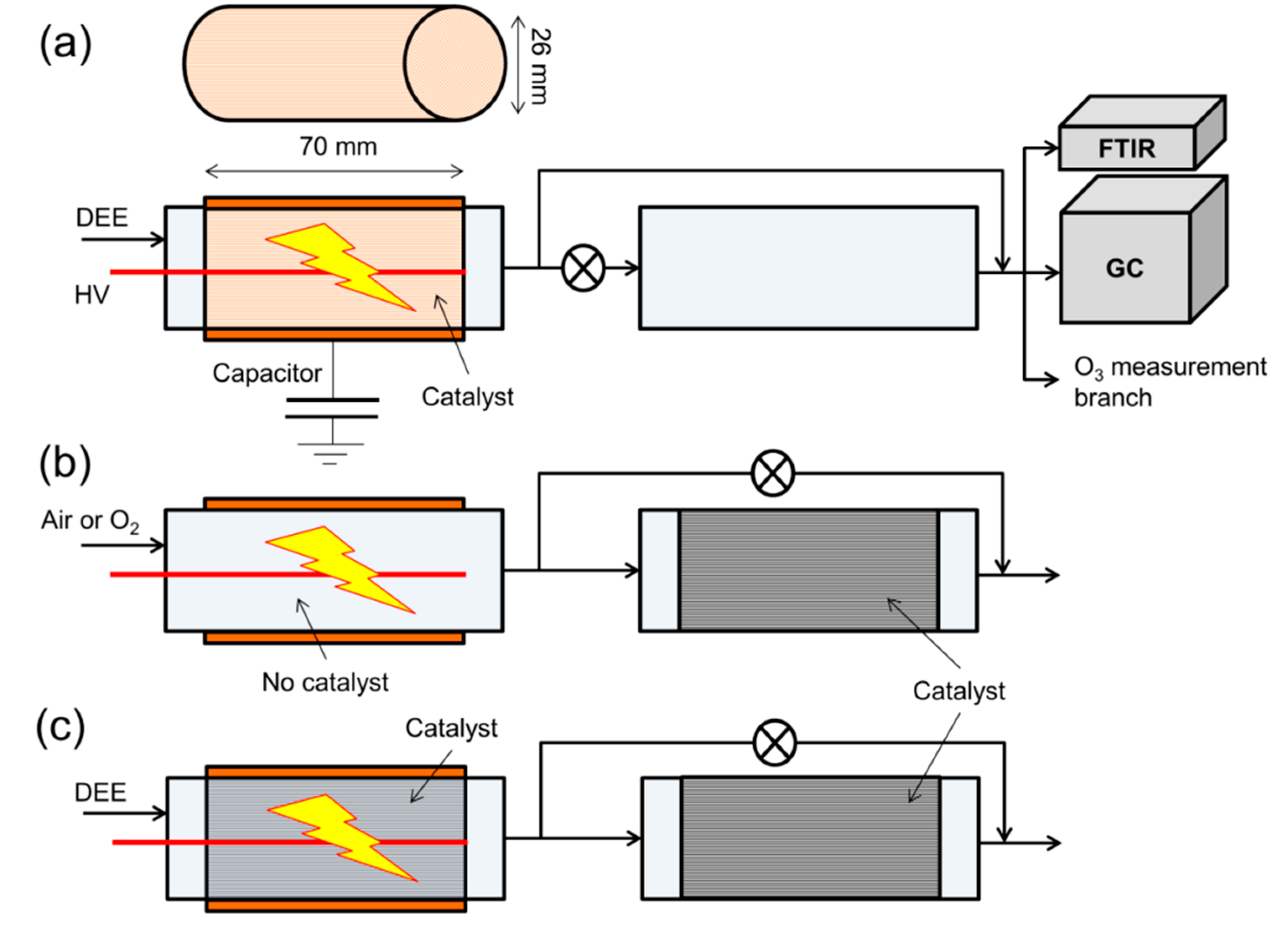
3.2. Measurement Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yamamoto, T. VOC decomposition by nonthermal plasma processing-a new approach. J. Electrostat. 1997, 42, 227–238. [Google Scholar] [CrossRef]
- Roland, U.; Holzer, F.; Kopinke, F.-D. Improved oxidation of air pollutants in a non-thermal plasma. Catal. Today 2002, 73, 315–323. [Google Scholar] [CrossRef]
- Mista, W.; Kacprzyk, R. Decomposition of toluene using non-thermal plasma reactor at room temperature. Catal. Today 2008, 137, 345–349. [Google Scholar] [CrossRef]
- Schmid, S.; Jecklin, M.C.; Zenobi, R. Degradation of volatile organic compounds in a non-thermal plasma air purifier. Chemosphere 2010, 79, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Abd Allah, Z.; Whitehead, J.C.; Martin, P. Remediation of dichloromethane (CH2Cl2) using non-thermal, atmospheric pressure plasma generated in a packed-bed reactor. Environ. Sci. Technol. 2013, 48, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Demidiouk, V.; Chae, J.O. Decomposition of volatile organic compounds in plasma-catalytic system. IEEE Trans. Plasma Sci. 2005, 33, 157–161. [Google Scholar] [CrossRef]
- Zhu, T.; Li, J.; Jin, Y.; Liang, Y.; Ma, G. Decomposition of benzene by non-thermal plasma processing: Photocatalyst and ozone effect. Int. J. Environ. Sci. Tech. 2008, 5, 375–384. [Google Scholar] [CrossRef]
- Chae, J.; Moon, S.; Sun, H.; Kim, K.; Vassiliev, V.A.; Mikholap, E.M. A study of volatile organic compounds decomposition with the use of non-thermal plasma. KSME Int. J. 1999, 13, 647–655. [Google Scholar]
- Kim, H.H.; Ogata, A. Nonthermal plasma activates catalyst: from current understanding and future prospects. Eur. Phys. J. Appl. Phys. 2011, 55, 13806. [Google Scholar] [CrossRef]
- Kim, H.H.; Ogata, A. Interaction of nonthermal plasma with catalyst for the air pollution control. Int. J. Plasma Environ. Sci. Technol. 2012, 6, 43–48. [Google Scholar]
- Whitehead, J.C. Plasma catalysis: A solution for environmental problems. Pure Appl. Chem. 2010, 82, 1329–1336. [Google Scholar] [CrossRef]
- Yamamoto, S.; Yao, S.; Kodama, S.; Mine, C.; Fujioka, Y. Investigation of transition metal oxide catalysts for diesel PM removal under plasma discharge conditions. Open Catal. J. 2008, 1, 11–16. [Google Scholar] [CrossRef]
- Yao, S.; Yamamoto, S.; Kodama, S.; Mine, C.; Fujioka, Y. Characterization of catalyst-supported dielectric barrier discharge reactor. Open Catal. J. 2009, 2, 79–85. [Google Scholar] [CrossRef]
- Zhu, T.; Wan, Y.D.; Li, J.; He, X.W.; Xu, D.Y.; Shu, X.Q.; Liang, W.J.; Jin, Y.Q. Volatile organic compounds decomposition using nonthermal plasma coupled with a combination of catalysts. Int. J. Environ. Sci. Technol. 2011, 8, 621–630. [Google Scholar] [CrossRef]
- Chen, H.L.; Lee, H.M.; Chen, S.H.; Chang, M.B.; Yu, S.J.; Li, S.N. Removal of volatile organic compounds by single-stage and two-stage plasma catalysis systems: A review of the performance enhancement mechanisms, current status, and suitable applications. Environ. Sci. Technol. 2009, 43, 2216–2227. [Google Scholar] [CrossRef] [PubMed]
- Trinh, H.Q.; Mok, Y.S. Plasma-catalytic oxidation of acetone in annular porous monolithic ceramic-supported catalysts. Chem. Eng. J. 2014, 251, 199–206. [Google Scholar] [CrossRef]
- Einaga, H.; Ogata, A. Benzene oxidation with ozone over supported manganese oxide catalysts: effect of catalyst support and reaction conditions. J. Hazard. Mater. 2009, 164, 1236–41. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Feng, F.; Liu, J.; Tang, X.; Zhang, X.; Huang, Y.; Liu, Z.; Yan, K. Toluene decomposition by a two-stage hybrid plasma catalyst system in dry air. IEEE Trans. Plasma Sci. 2014, 42, 3529–3538. [Google Scholar] [CrossRef]
- Jin, M.; Kim, J.H.; Kim, J.M.; Jeon, J.-K.; Jurng, J.; Bae, G.-N.; Park, Y.-K. Benzene oxidation with ozone over MnOx/SBA-15 catalysts. Catal. Today 2013, 204, 108–113. [Google Scholar] [CrossRef]
- Lian, Z.; Ma, J.; He, H. Decomposition of high-level ozone under high humidity over Mn–Fe catalyst: The influence of iron precursors. Catal. Commun. 2015, 59, 156–160. [Google Scholar] [CrossRef]
- Zaloznaya, L.A.; Tkachenko, S.N.; Egorova, G.V.; Tkachenko, I.S.; Sobolev, A.V.; Golosman, E.Z.; Troshina, V.A.; Lunin, V.V. Ozone decomposition and benzene oxidation catalysts based on iron and manganese oxides as industrial wastes from water decontamination by ozone treatment. Catal. Ind. 2009, 1, 224–228. [Google Scholar] [CrossRef]
- Demidiouk, V.; Moon, S.; Chae, J.; Lee, D. Application of a plasma-catalytic system for decomposition of volatile organic compounds. J. Korean Phys. Soc. 2003, 42, 966–970. [Google Scholar]
- Futamura, S.; Einaga, H.; Kabashima, H.; Hwan, L.Y. Synergistic effect of silent discharge plasma and catalysts on benzene decomposition. Catal. Today 2004, 89, 89–95. [Google Scholar] [CrossRef]
- Ayrault, C.; Barrault, J.; Blin-Simiand, N.; Jorand, F.; Pasquiers, S.; Rousseau, A.; Tatibouët, J.M. Oxidation of 2-heptanone in air by a DBD-type plasma generated within a honeycomb monolith supported Pt-based catalyst. Catal. Today 2004, 89, 75–81. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Renken, A.; Kiwi-Minsker, L. Novel catalytic dielectric barrier discharge reactor for gas-phase abatement of isopropanol. Plasma Chem. Plasma Process. 2006, 27, 13–22. [Google Scholar] [CrossRef]
- Jaafar, N.F.; Abdul Jalil, A.; Triwahyono, S.; Muhd Muhid, M.N.; Sapawe, N.; Satar, M.A.H.; Asaari, H. Photodecolorization of methyl orange over α-Fe2O3-supported HY catalysts: The effects of catalyst preparation and dealumination. Chem. Eng. J. 2012, 191, 112–122. [Google Scholar] [CrossRef]
- Boxiong, S.; Yan, Y.A. O.; Hongqing, M.A.; Ting, L.I.U. Ceria modified MnOx/TiO2-pillared clays catalysts for selective catalytic reduction of NO with NH3 at low temperature. Chin. J. Catal. 2011, 32, 1803–1811. [Google Scholar] [CrossRef]
- Kim, H.H.; Kim, J.H.; Ogata, A. Adsorption and oxygen plasma-driven catalysis for total oxidation of VOCs. Int. J. Plasma Environ. Sci. Technol. 2008, 2, 106–112. [Google Scholar]
- Trinh, Q.H.; Gandhi, M.S.; Mok, Y.S. Adsorption and plasma-catalytic oxidation of acetone over zeolite-supported silver catalyst. Jpn. J. Appl. Phys. 2015, 54, 01AG04. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, X.; Zheng, C.; Wang, Z.; Ni, M.; Tu, X. Plasma-catalytic removal of a low concentration of acetone in humid conditions. RSC Adv. 2014, 4, 37796. [Google Scholar] [CrossRef]
- Jo, J.-O.; Lee, S.B.; Jang, D.L.; Mok, Y.S. Plasma–catalytic ceramic membrane reactor for volatile organic compound control. IEEE Trans. Plasma Sci. 2013, 41, 3021–3029. [Google Scholar] [CrossRef]
- Li, W.; Oyama, S.T. Mechanism of ozone decomposition on a manganese oxide catalyst. 2. Steady-state and transient kinetic studies. J. Am. Chem. Soc. 1998, 120, 9047–9052. [Google Scholar]
- Ogata, A.; Saito, K.; Kim, H.-H.; Sugasawa, M.; Aritani, H.; Einaga, H. Performance of an ozone decomposition catalyst in hybrid plasma reactors for volatile organic compound removal. Plasma Chem. Plasma Process. 2009, 30, 33–42. [Google Scholar] [CrossRef]
- Van Durme, J.; Dewulf, J.; Sysmans, W.; Leys, C.; van Langenhove, H. Efficient toluene abatement in indoor air by a plasma catalytic hybrid system. Appl. Catal. B 2007, 74, 161–169. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Morent, R.; De Geyter, N.; Leys, C. Non-thermal plasmas for non-catalytic and catalytic VOC abatement. J. Hazard. Mater. 2011, 195, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Lopaev, D.V.; Malykhin, E.M.; Namiot, V.A. UV absorption of vibrationally excited ozone. J. Phys. B 2008, 41, 085104. [Google Scholar]
- Marinov, D.; Guerra, V.; Guaitella, O.; Booth, J.-P.; Rousseau, A. Ozone kinetics in low-pressure discharges: vibrationally excited ozone and molecule formation on surfaces. Plasma Sources Sci. Technol. 2013, 22, 055018. [Google Scholar] [CrossRef]
- Eliasson, B.; Hirth, M.; Kogelschatz, U. Ozone synthesis from oxygen in dielectric barrier discharges. J. Phys. D 1987, 20, 1421. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ramanathan, K.; Lawless, P.A.; Ensor, D.S.; Newsome, J.R.; Plaks, N.; Ramsey, G.H. Control of volatile organic compounds by an AC energized ferroelectric pellet reactor and a pulsed corona reactor. IEEE Trans. Ind. Appl. 1992, 28, 528–534. [Google Scholar] [CrossRef]
- Yagi, S.; Tanaka, M. Mechanism of ozone generation in air-fed ozonisers. J. Phys. D 1979, 12, 1509–1520. [Google Scholar] [CrossRef]
- Mok, Y.S.; Nam, I.-S. Role of organic chemical additives in pulsed corona discharge process for conversion of NO. J. Chem. Eng. Japan 1998, 31, 391–397. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, Q.H.; Mok, Y.S. Non-Thermal Plasma Combined with Cordierite-Supported Mn and Fe Based Catalysts for the Decomposition of Diethylether. Catalysts 2015, 5, 800-814. https://doi.org/10.3390/catal5020800
Trinh QH, Mok YS. Non-Thermal Plasma Combined with Cordierite-Supported Mn and Fe Based Catalysts for the Decomposition of Diethylether. Catalysts. 2015; 5(2):800-814. https://doi.org/10.3390/catal5020800
Chicago/Turabian StyleTrinh, Quang Hung, and Young Sun Mok. 2015. "Non-Thermal Plasma Combined with Cordierite-Supported Mn and Fe Based Catalysts for the Decomposition of Diethylether" Catalysts 5, no. 2: 800-814. https://doi.org/10.3390/catal5020800