Ruthenium Modification on Mn and Zr-Modified Co/SiO2 Catalysts for Slurry-Phase Fischer-Tropsch Synthesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Noble Metal Addition and Impregnation Order
| Catalyst | Conversion/% | Selectivity/% | C5+ yield/% | STY/g/kg·h | ||||
|---|---|---|---|---|---|---|---|---|
| CO | H2 | CO2 | CH4 | C2–C4 | C5+ | |||
| Co/SiO2 | 30.6 | 36.8 | 2.4 | 12.8 | 9.5 | 75.3 | 23.1 | 281.5 |
| Co+Ru/SiO2 | 31.9 | 38.0 | 1.1 | 9.9 | 3.8 | 85.2 | 27.2 | 310.7 |
| Co+Mn+Zr/SiO2 | 37.4 | 44.7 | 1.9 | 11.1 | 3.4 | 83.5 | 31.2 | 380.4 |
| Pt/Co+Mn+Zr/SiO2 | 34.2 | 42.8 | 2.4 | 17.3 | 6.3 | 74.1 | 25.3 | 308.9 |
| Pt+Co+Mn+Zr/SiO2 | 38.5 | 46.5 | 2.0 | 11.8 | 5.0 | 81.2 | 31.2 | 381.0 |
| Ru/Co+Mn+Zr/SiO2 | 36.9 | 45.3 | 1.7 | 14.3 | 5.0 | 79.1 | 29.2 | 355.6 |
| Ru+Co+Mn+Zr/SiO2 | 44.2 | 53.3 | 1.9 | 11.9 | 3.5 | 82.7 | 36.6 | 445.7 |
| Ru+Co/Mn+Zr/SiO2 | 45.0 | 54.0 | 2.1 | 12.1 | 3.5 | 82.3 | 37.1 | 452.0 |
| Ru+Co+Mn/Zr/SiO2 | 46.2 | 55.2 | 2.1 | 11.0 | 3.0 | 83.9 | 38.8 | 473.0 |

| Catalyst | CO conv./% | H2 consumption/mmol/g-cat | Reduction degree/% (at 673 K) | Reduction degree/% (after hold) | Dispersion/% |
|---|---|---|---|---|---|
| Co/SiO2 | 30.6 | 12.1 | 32 | 94 | 0.66 |
| Co+Ru/SiO2 | 31.9 | 13.1 | 41 | 96 | 0.70 |
| Co+Mn+Zr/SiO2 | 37.4 | 14.7 | 20 | 48 | 1.58 |
| Pt/Co+Mn+Zr/SiO2 | 34.2 | 19.7 | 33 | 68 | 1.49 |
| Pt+Co+Mn+Zr/SiO2 | 38.5 | 22.5 | 34 | 71 | 1.62 |
| Ru/Co+Mn+Zr/SiO2 | 36.9 | 20.4 | 33 | 76 | 1.38 |
| Ru+Co+Mn+Zr/SiO2 | 44.2 | 25.8 | 36 | 79 | 1.67 |
| Ru+Co/Mn+Zr/SiO2 | 45.0 | 24.9 | 37 | 74 | 1.73 |
| Ru+Co+Mn/Zr/SiO2 | 46.2 | 25.9 | 34 | 71 | 1.87 |
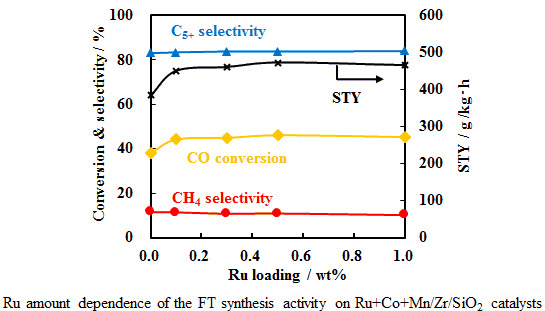
2.2. Effects of Ru Quantity on Ru+Co+Mn/Zr/SiO2
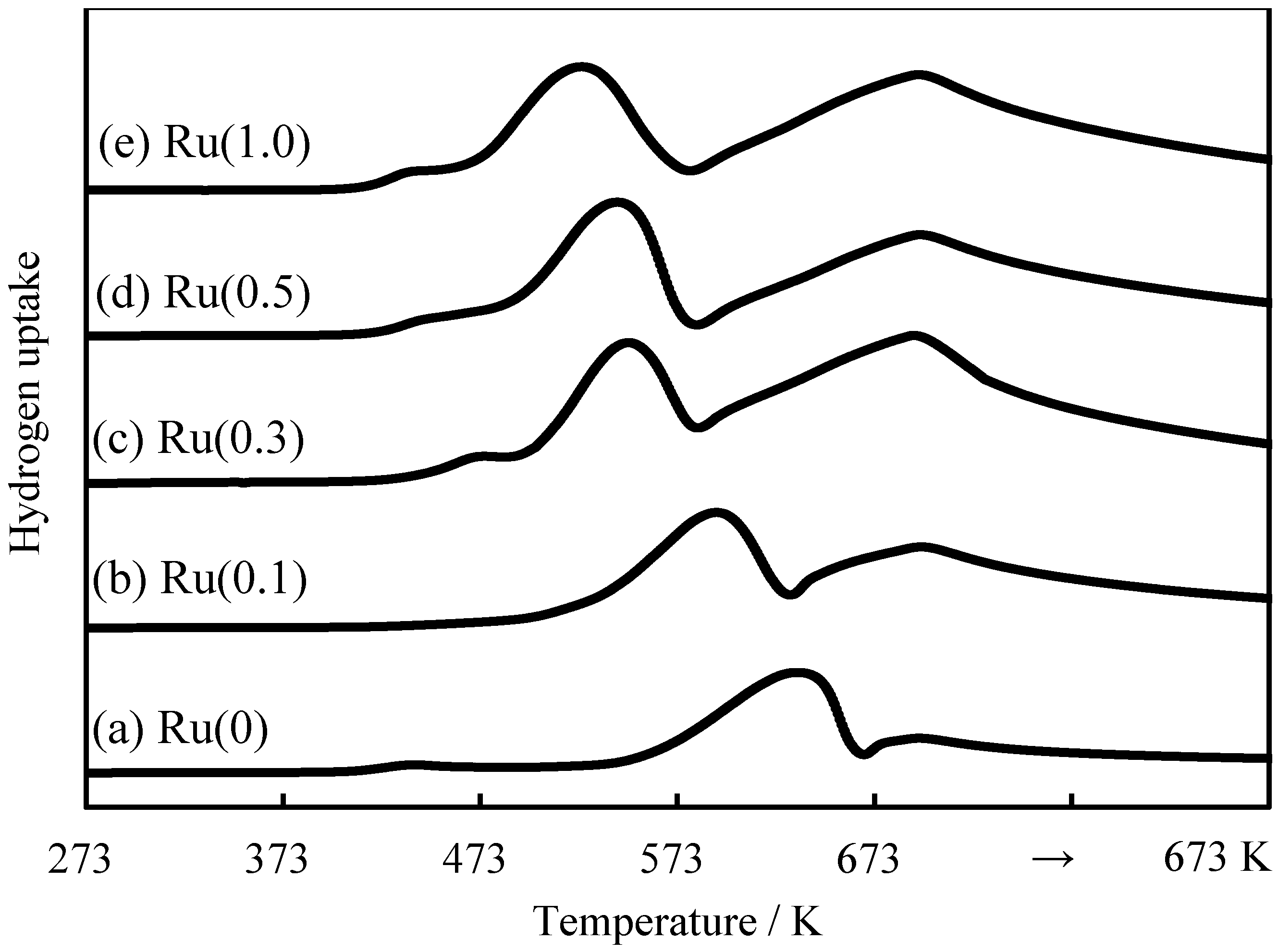
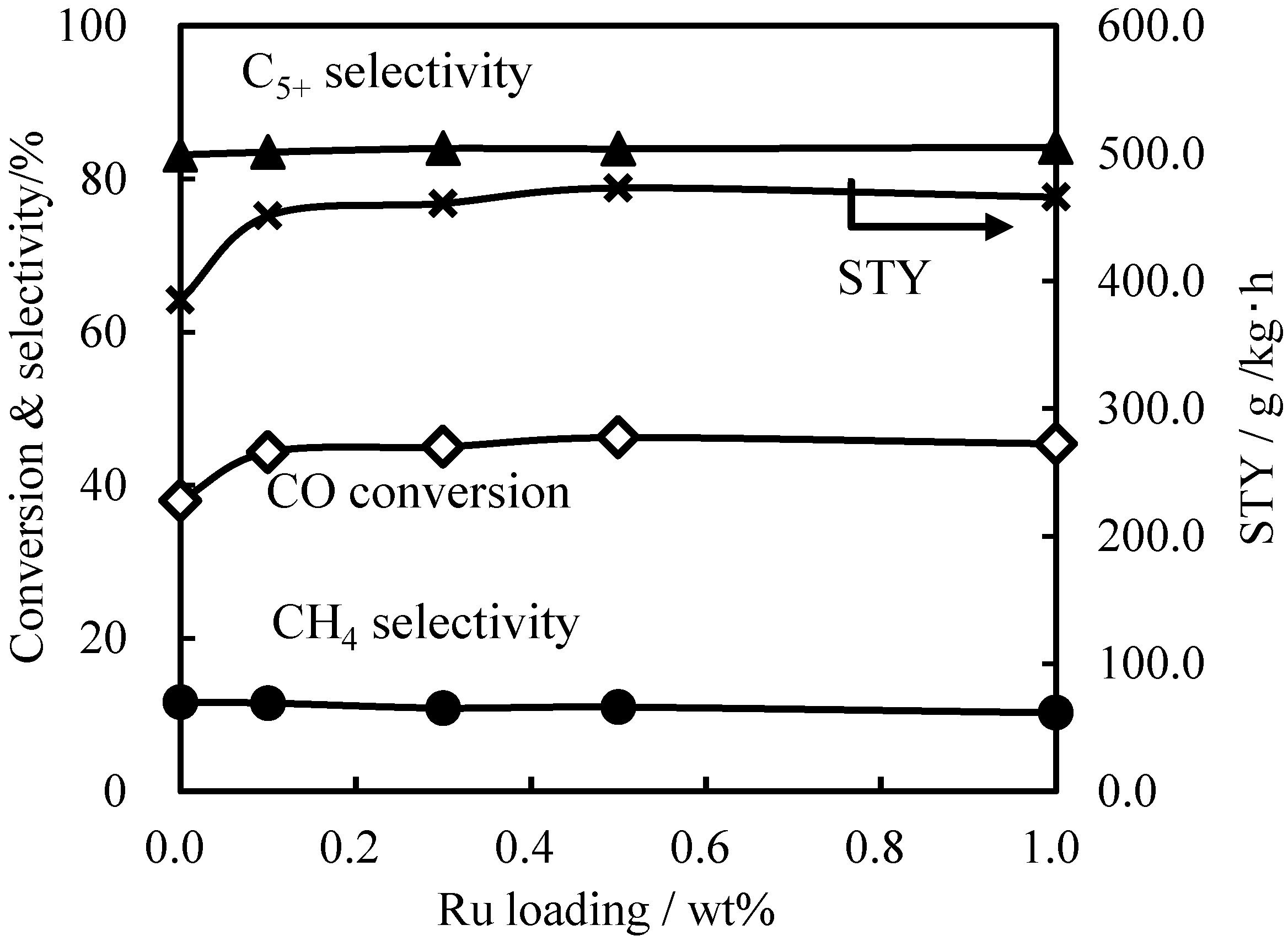
| Ru amount/wt% | CO conv./% | H2 consumption/mmol/g-cat | Reduction degree/% (at 673 K) | Reduction degree/% (after hold) | Dispersion/% |
|---|---|---|---|---|---|
| 0.0 | 38.0 | 14.6 | 20 | 47 | 1.60 |
| 0.1 | 44.3 | 21.1 | 24 | 61 | 1.77 |
| 0.3 | 45.0 | 25.2 | 42 | 71 | 1.84 |
| 0.5 | 46.2 | 25.9 | 34 | 71 | 1.87 |
| 1.0 | 45.4 | 25.7 | 41 | 73 | 1.81 |
2.3. Effect of H2 Reduction Conditions on the Ru+Co+Mn/Zr/SiO2
| Reduction Condition | Conversion/% | Selectivity/% | C5+ yield/% | STY/g/kg·h | ||||
|---|---|---|---|---|---|---|---|---|
| Temperature, Pressure | CO | H2 | CO2 | CH4 | C2–C4 | C5+ | ||
| 673 K, 1.0 MPa | 41.4 | 48.7 | 1.9 | 11.1 | 3.4 | 83.5 | 34.5 | 421.1 |
| 573 K, 0.1 MPa | 46.1 | 54.9 | 2.2 | 11.8 | 3.4 | 82.6 | 38.1 | 464.3 |
| 673 K, 0.1 MPa | 46.2 | 55.2 | 2.1 | 11.0 | 3.0 | 83.9 | 38.8 | 473.0 |
| 773 K, 0.1 MPa | 43.4 | 51.8 | 1.5 | 12.8 | 4.5 | 81.2 | 35.2 | 429.7 |
2.4. FT Synthesis Reaction under High Pressure Conditions
| Catalyst | Pressure/MPa | Conversion/% | Selectivities/% | C5+ yield/% | α | STY/g/kg·h | Dispersion degree b/% | Reduction degree c/% | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | H2 | CO2 | CH4 | C2–C4 | C5+ | |||||||
| Co/SiO2 | 1.0 | 30.6 | 36.8 | 2.4 | 12.8 | 9.5 | 75.3 | 23.1 | 0.86 | 281 | 0.66 | 94 |
| 4.0 | 44.4 | 53.4 | 0.7 | 2.7 | 8.0 | 88.5 | 39.3 | 0.87 | 427 | |||
| Co+Mn+Zr/SiO2 | 1.0 | 37.4 | 44.7 | 1.9 | 11.1 | 3.4 | 83.5 | 31.2 | 0.86 | 380 | 1.57 | 48 |
| 4.0 | 53.6 | 58.7 | 0.5 | 1.6 | 5.9 | 92.0 | 49.3 | 0.90 | 601 | |||
| Ru+Co+Mn/Zr/SiO2 | 1.0 | 46.2 | 55.2 | 2.1 | 11.0 | 3.0 | 83.9 | 38.8 | 0.86 | 473 | 1.87 | 71 |
| 4.0 | 70.5 | 77.8 | 1.4 | 1.3 | 3.4 | 93.9 | 66.2 | 0.90 | 758 | |||
3. Experimental Section
3.1. Catalyst Preparation
3.2. Characterization
3.3. Syngas Preparation
3.4. FT Reaction
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Souza-Aguiar, E.F.; Noronhac, F.B.; Faro, A., Jr. The main catalytic challenges in GTL (gas-to-liquids) processes. Catal. Sci. Technol. 2011, 1, 698–713. [Google Scholar] [CrossRef]
- Luque, R.; de la Osa, A.R.; Campelo, J.M.; Romero, A.A.; Valverde, J.L.; Sanchez, P. Design and development of catalysts for Biomass-to-Liquid-Fischer-Tropsch (BTL-FT) processes for biofuels production. Stud. Surf. Sci. Catal. 2012, 5, 5186–5202. [Google Scholar]
- Schulz, H. Short history and present trends of Fischer-Tropsch synthesis. Appl. Catal. A 1999, 186, 3–12. [Google Scholar] [CrossRef]
- Reuel, R.C.; Bartholomew, C.H. Effects of support and dispersion on the CO hydrogenation activity/selectivity properties of cobalt. J. Catal. 1984, 85, 78–88. [Google Scholar] [CrossRef]
- Schulz, H.; van Steen, E.; Claeys, M. Selectivity and mechanism of Fischer-Tropsch synthesis with iron and cobalt catalysts. Stud. Surf. Sci. Catal. 1994, 81, 455–460. [Google Scholar]
- Iglesia, E.; Soled, S.L.; Fiato, R.A. Fischer-Tropsch synthesis on cobalt and ruthenium. Metal dispersion and support effects on reaction rate and selectivity. J. Catal. 1992, 137, 212–224. [Google Scholar] [CrossRef]
- Iglesia, E. Design, synthesis, and use of cobalt-based Fischer-Tropsch synthesis catalysts. Appl. Catal. A 1997, 161, 59–78. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the Development of Novel Cobalt Fischer-Tropsch Catalysts for Synthesis of Long-Chain Hydrocarbons and Clean Fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef] [PubMed]
- All, S.; Chen, B.; Goodwin, J.G. Zr Promotion of Co/SiO2 for Fischer-Tropsch Synthesis. J. Catal. 1995, 157, 35–41. [Google Scholar] [CrossRef]
- Bae, J.W.; Park, S.J.; Woo, M.H.; Cheon, J.Y.; Ha, K.S.; Jun, K.W.; Lee, D.H.; Jung, H.M. Enhanced Catalytic Performance by Zirconium Phosphate-Modified SiO2-Supported Ru-Co Catalyst for Fischer-Tropsch Synthesis. ChemCatChem 2011, 3, 1342–1347. [Google Scholar] [CrossRef]
- Moradi, G.R.; Basir, M.M.; Taeb, A.; Kiennemann, A. Promotion of Co/SiO2 Fischer-Tropsch catalysts with zirconium. Catal. Commun. 2003, 4, 27–32. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Ren, J.; Li, Y.; Sun, Y. Support effect of Co/Al2O3 catalysts for Fischer-Tropsch synthesis. Fuel 2003, 82, 581–586. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, Y.; Wang, S.; Li, J. Fischer-Tropsch synthesis: The effect of Al2O3 porosity on the performance of Co/Al2O3 catalyst. Catal. Commun. 2005, 6, 512–516. [Google Scholar] [CrossRef]
- Morales, F.; de Smit, E.; de Groot, F.M.F.; Visser, T.; Weckhuysen, B.M. Effects of manganese oxide promoter on the CO and H2 adsorption properties of titania-supported cobalt Fischer-Tropsch catalysts. J. Catal. 2007, 246, 91–99. [Google Scholar] [CrossRef]
- Li, J.; Coville, N.J. The effect of boron on the catalyst reducibility and activity of Co/TiO2 Fischer-Tropsch catalysts. Appl. Catal. A 1999, 181, 201–208. [Google Scholar] [CrossRef]
- Liu, Y.; Hanaoka, T.; Miyazawa, T.; Murata, K.; Okabe, K.; Sakanishi, K. Fischer-Tropsch synthesis in slurry-phase reactors over Mn- and Zr-modified Co/SiO2 catalysts. Fuel Process. Technol. 2009, 90, 901–908. [Google Scholar] [CrossRef]
- Miyazawa, T.; Hanaoka, T.; Shimura, K.; Hirata, S. Mn and Zr modified Co/SiO2 catalysts development in slurry-phase Fischer-Tropsch synthesis. Appl. Catal. A 2013, 467, 47–54. [Google Scholar] [CrossRef]
- Kogelbauer, A.; Goodwin, J.G., Jr.; Oukaci, R. Ruthenium Promotion of Co/Al2O3 Fischer-Tropsch Catalysts. J. Catal. 1996, 160, 125–133. [Google Scholar] [CrossRef]
- Tsubaki, N.; Sun, S.; Fujimoto, K. Different Functions of the Noble Metals Added to Cobalt Catalysts for Fischer-Tropsch Synthesis. J. Catal. 2001, 199, 236–246. [Google Scholar] [CrossRef]
- Jacobs, G.; Das, T.K.; Zhang, Y.; Li, J.; Racoillet, G.; Davis, B.H. Fischer-Tropsch synthesis: Support, loading, and promoter effects on the reducibility of cobalt catalysts. Appl. Catal. A 2002, 233, 263–281. [Google Scholar] [CrossRef]
- Song, S.H.; Lee, S.B.; Bae, J.W.; Sai Prasad, P.S.; Jun, K.W. Influence of Ru segregation on the activity of Ru-Co/γ-Al2O3 during FT synthesis: A comparison with that of Ru-Co/SiO2 catalysts. Catal. Commun. 2008, 9, 2282–2286. [Google Scholar] [CrossRef]
- Ma, W.; Jacobs, G.; Keogh, R.A.; Bukur, D.B.; Davis, B.H. Fischer-Tropsch synthesis: Effect of Pd, Pt, Re, and Ru noble metal promoters on the activity and selectivity of a 25%Co/Al2O3 catalyst. Appl. Catal. A 2012, 437–438, 1–9. [Google Scholar] [CrossRef]
- Pirola, C.; Scavini, M.; Galli, F.; Vitali, S.; Comazzi, A.; Manenti, F.; Ghigna, P. Fischer-Tropsch synthesis: EXAFS study of Ru and Pt bimetallic Co based catalysts. Fuel 2014, 132, 62–70. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Griboval-Constant, A.; Bechara, R.; Zholobenko, V.L. Pore Size Effects in Fischer Tropsch Synthesis over Cobalt-Supported Mesoporous Silicas. J. Catal. 2002, 206, 230–241. [Google Scholar] [CrossRef]
- Zhang, Y.; Nagamori, S.; Hinchiranan, S.; Vitidsant, T.; Tsubaki, N. Promotional Effects of Al2O3 Addition to Co/SiO2 Catalysts for Fischer-Tropsch Synthesis. Energy Fuels 2006, 20, 417–421. [Google Scholar] [CrossRef]
- Koizumi, N.; Suzuki, S.; Niiyama, S.; Shindo, T.; Yamada, M. Preparation of Co/SiO2 Using Several Glycols for Enhanced Fischer-Tropsch Synthesis Activity and Dispersion of Co0 Nanoparticles with Unique Co0 Particle Size Effect. Catal. Lett. 2011, 141, 931–938. [Google Scholar] [CrossRef]
- Hanaoka, T.; Miyazawa, T.; Nurunnabi, M.; Hirata, S.; Sakanishi, K. Liquid Fuel Production from Woody Biomass via Oxygen-enriched Air/CO2 Gasification on a Bench Scale. J. Jpn. Inst. Energy 2011, 90, 1072–1080. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazawa, T.; Hanaoka, T.; Shimura, K.; Hirata, S. Ruthenium Modification on Mn and Zr-Modified Co/SiO2 Catalysts for Slurry-Phase Fischer-Tropsch Synthesis. Catalysts 2015, 5, 26-37. https://doi.org/10.3390/catal5010026
Miyazawa T, Hanaoka T, Shimura K, Hirata S. Ruthenium Modification on Mn and Zr-Modified Co/SiO2 Catalysts for Slurry-Phase Fischer-Tropsch Synthesis. Catalysts. 2015; 5(1):26-37. https://doi.org/10.3390/catal5010026
Chicago/Turabian StyleMiyazawa, Tomohisa, Toshiaki Hanaoka, Katsuya Shimura, and Satoshi Hirata. 2015. "Ruthenium Modification on Mn and Zr-Modified Co/SiO2 Catalysts for Slurry-Phase Fischer-Tropsch Synthesis" Catalysts 5, no. 1: 26-37. https://doi.org/10.3390/catal5010026
APA StyleMiyazawa, T., Hanaoka, T., Shimura, K., & Hirata, S. (2015). Ruthenium Modification on Mn and Zr-Modified Co/SiO2 Catalysts for Slurry-Phase Fischer-Tropsch Synthesis. Catalysts, 5(1), 26-37. https://doi.org/10.3390/catal5010026