Guanidine Hydrochloride/ZnI2 as Heterogeneous Catalyst for Conversion of CO2 and Epoxides to Cyclic Carbonates under Mild Conditions
Abstract
:1. Introduction
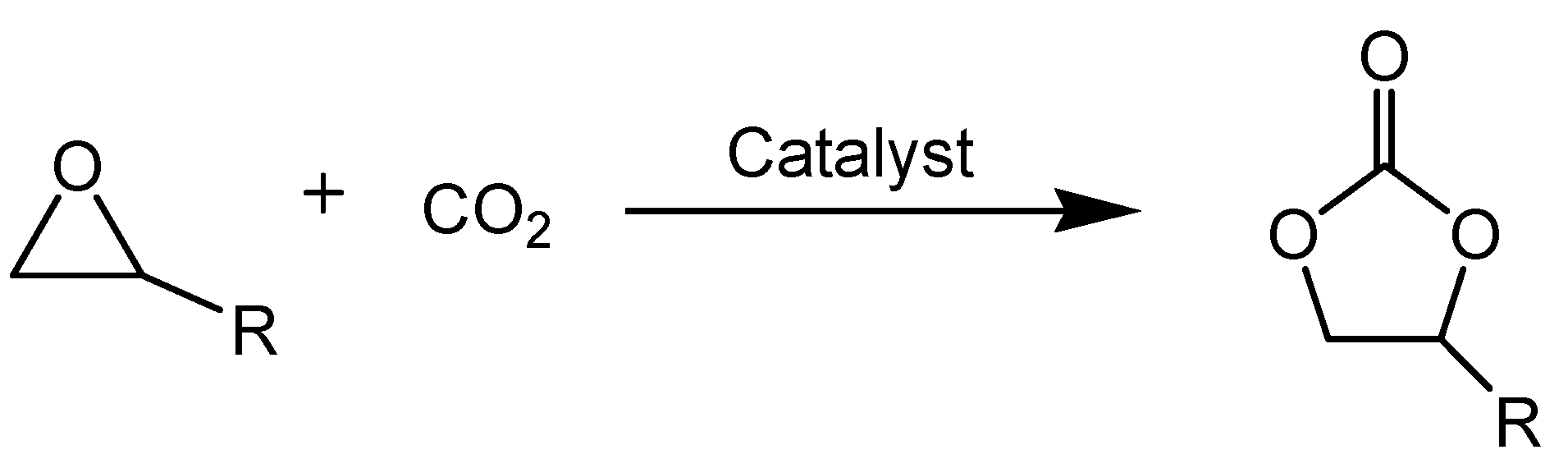
2. Results and Discussion
2.1. Screening of Catalysts and Their Reusability
| Entry | Catalyst system | Catalytic results b | ||
|---|---|---|---|---|
| Yield (%) | Selectivity (%) | |||
| 1 | GndCl | — | trace | — |
| 2 | — | ZnI2 | trace | — |
| 3 c | GndCl | — | 27 | ≥98 |
| 4 | GndCl | ZnI2 | 94 | ≥99 |
| 5 | GndCl | ZnBr2 | 43 | ≥99 |
| 6 | GndCl | ZnCl2 | 12 | ≥99 |
| 7 | GndCl | ZnO | trace | ≥99 |
| 8 | GndCl | Zn(NO3)2 | trace | — |
| 9 | GndCl | CdCl2 | trace | — |
| 10 | GndCl | MnCl2 | trace | — |
| 11 | GndCl | CoCl2 | trace | — |
| 12 | GndCl | FeCl3 | 5 | ≥99 |
| 13 d | GndCl | ZnI2 | 37 | ≥99 |
| 14 e | GndCl | ZnI2 | 95 | ≥99 |
| 15 f | GndCl | ZnI2 | 80 | ≥99 |
| 16 g | GndCl | ZnI2 | 90 | ≥98 |
2.2. Effect of Reaction Parameters
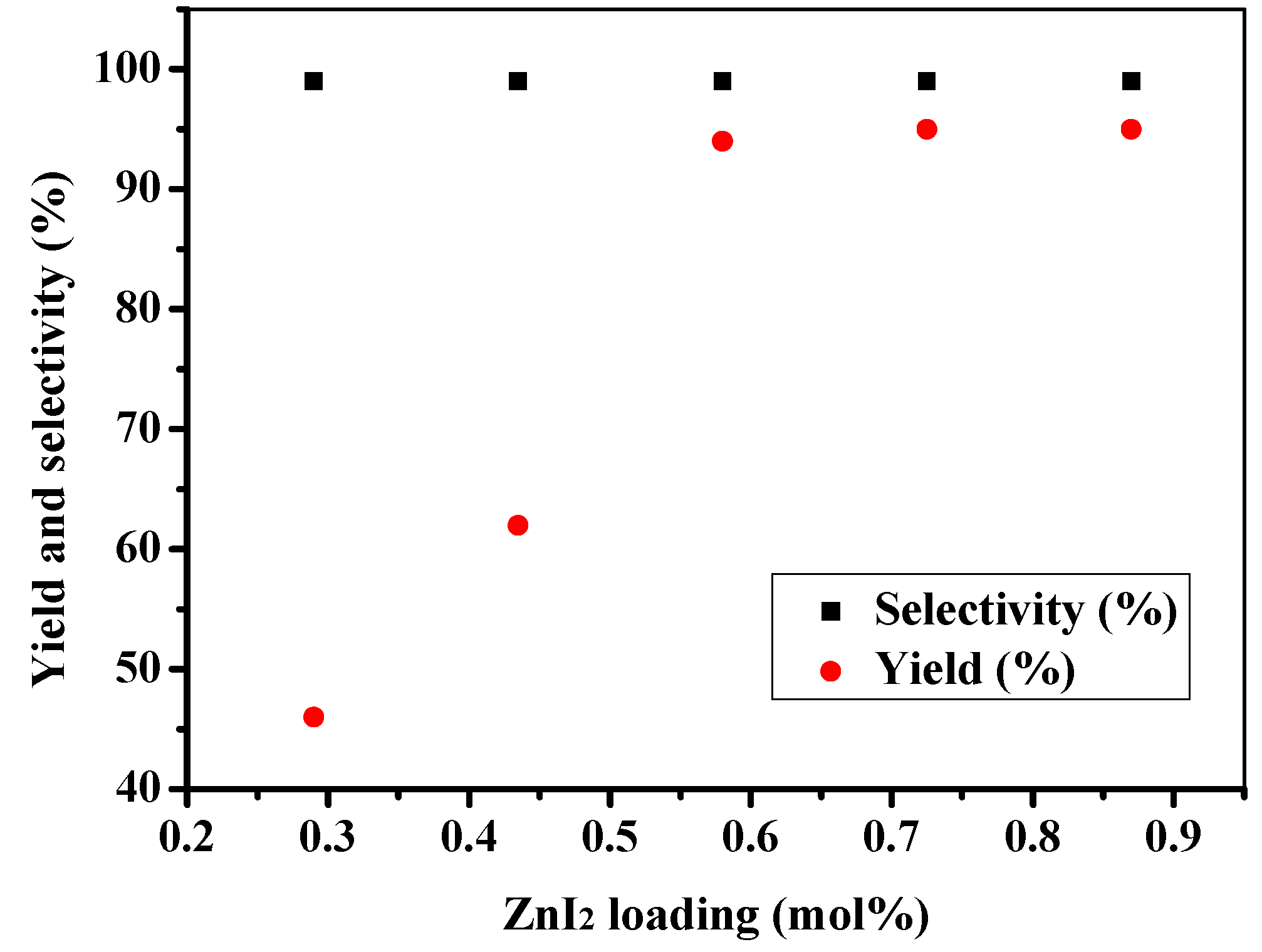
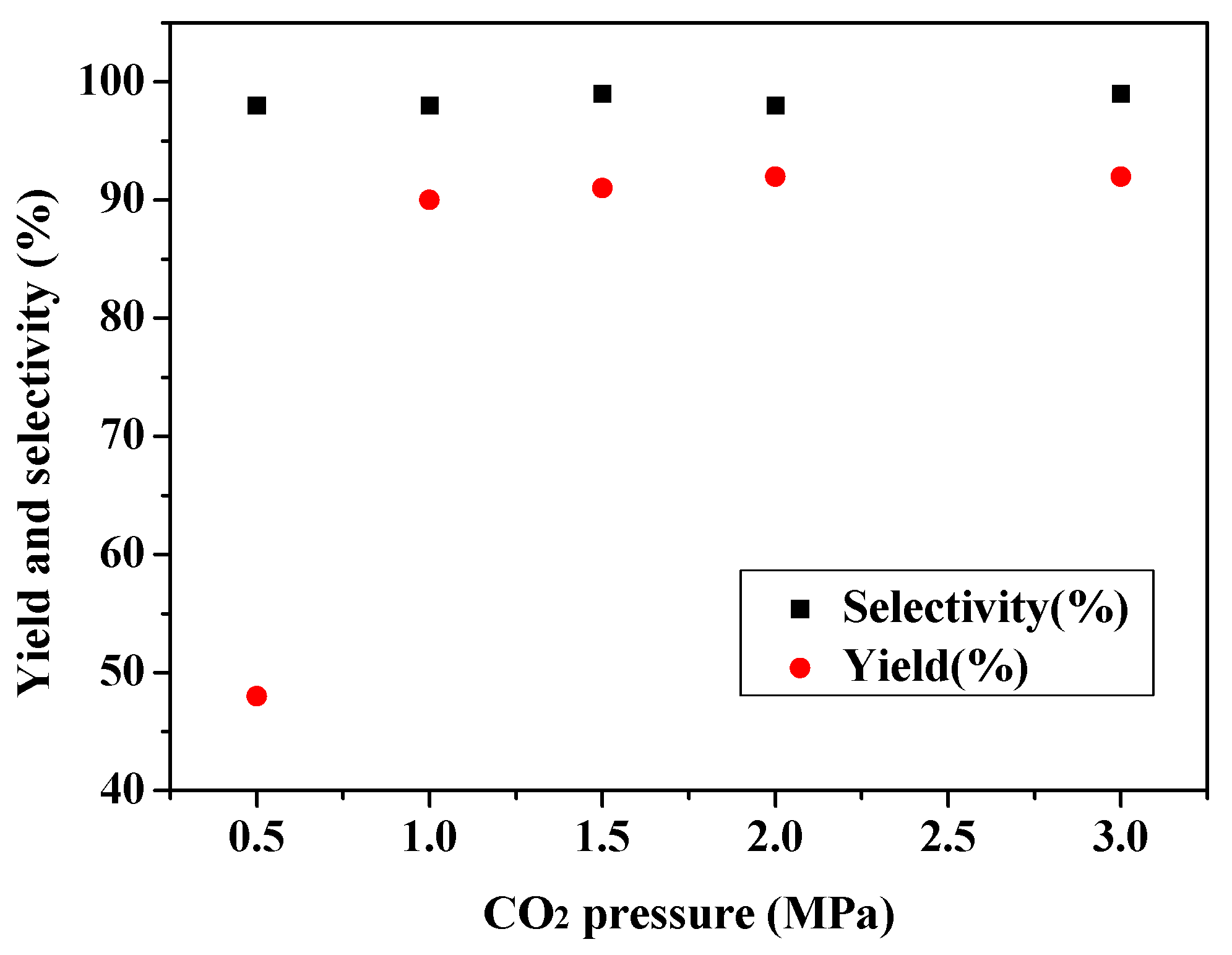


2.3. Catalytic Activity towards Other Epoxides
| Entry | Epoxide | Product | Time (h) | Reaction results b | |
|---|---|---|---|---|---|
| Yield (%) | Selectivity (%) | ||||
| 1 c | 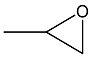 |  | 1.5 | 94 | 99 |
| 2 | 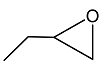 | 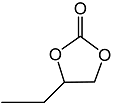 | 1.5 | 92 | 99 |
| 3 | 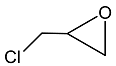 | 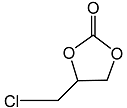 | 1.5 | 92 | 99 |
| 4 | 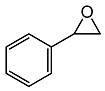 |  | 3 | 80 | 98 |
| 5 d | 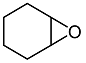 | 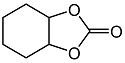 | 11 | 71 | 98 |
2.4. Proposed Reaction Mechanism
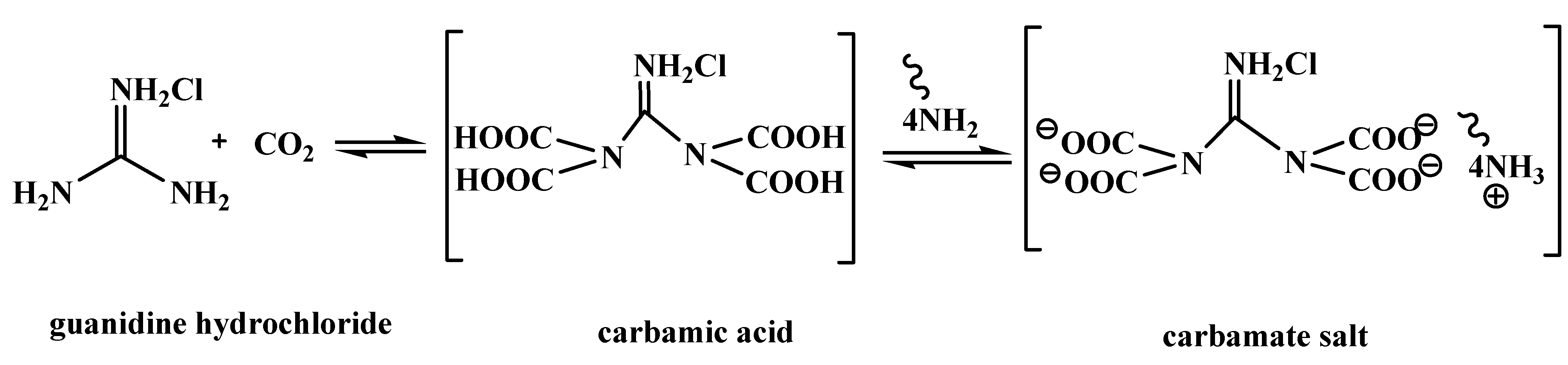
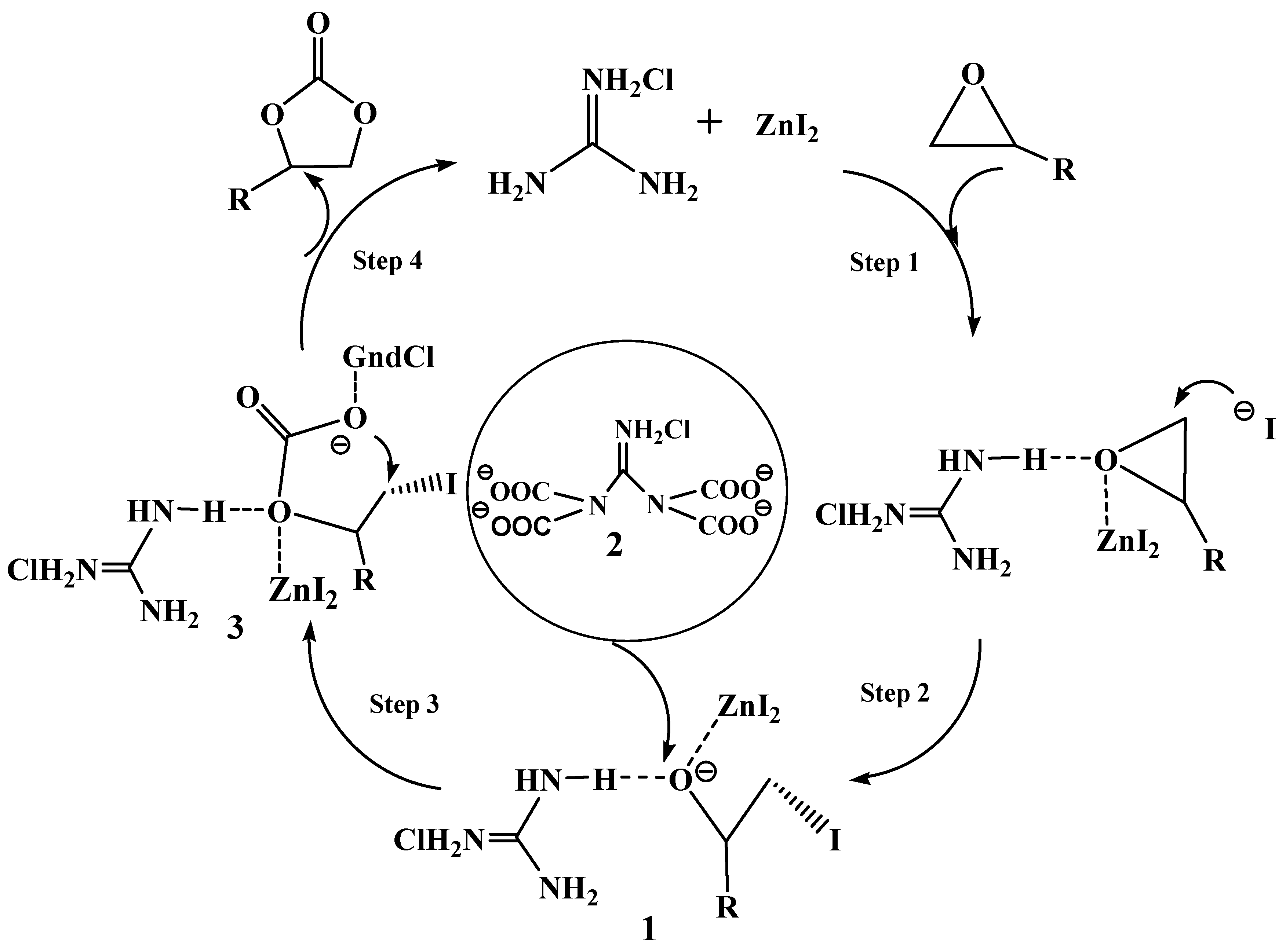
3. Experimental Section
3.1. Materials and Instruments
3.2. Typical Procedure for the CO2 Cycloaddition Reaction to Propylene Oxide
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aresta, M.; Dibenedetto, A. Utilisation of CO2 as a chemical feedstock: Opportunities and challenges. Dalton Trans. 2007, 2975–2992. [Google Scholar]
- Lee, T.; van de Meene, S. Comparative studies of urban climate co-benefits in Asian cities: An analysis of relationships between CO2 emissions and environmental indicators. J. Clean. Prod. 2013, 58, 15–24. [Google Scholar]
- Decortes, A.; Castilla, A.M.; Kleij, A.W. Salen-complex-mediated formation of cyclic carbonates by cycloaddition of CO2 to epoxides. Angew. Chem. Int. Ed. 2010, 49, 9822–9837. [Google Scholar]
- Sakakura, T.; Choi, J.C.; Yasuda, H. Transformation of carbon dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar]
- Yamaguchi, K.; Ebitani, K.; Yoshida, T.; Yoshida, H.; Kaneda, K. Mg-Al mixed oxides as highly active acid-base catalysts for cycloaddition of carbon dioxide to epoxides. J. Am. Chem. Soc. 1999, 121, 4526–4527. [Google Scholar]
- Dai, W.L.; Yin, S.F.; Guo, R.; Luo, S.L.; Du, X.; Au, C.T. Synthesis of propylene carbonate from carbon dioxide and propylene oxide using Zn-Mg-Al composite oxide as high-efficiency catalyst. Catal. Lett. 2010, 136, 35–44. [Google Scholar]
- Sako, T.; Fukai, T.; Sahashi, R. Cycloaddition of oxirane group with carbon dioxide in the supercritical homogeneous state. Ind. Eng. Chem. Res. 2002, 41, 5353–5358. [Google Scholar]
- Ahmadi, F.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Baltork, I.M.; Khosropour, A.R. Electron-deficient tin (IV) tetraphenylporphyrin perchlorate: A highly efficient catalyst for chemical fixation of carbon dioxide. Polyhedron 2012, 32, 68–72. [Google Scholar]
- Sun, J.; Zhang, S.J.; Cheng, W.G.; Ren, J.Y. Hydroxyl-functionalized ionic liquid: A novel efficient catalyst for chemical fixation of CO2 to cyclic carbonate. Tetrahedron Lett. 2008, 49, 3588–3591. [Google Scholar]
- Tsutsumi, Y.; Yamakawa, K.; Yoshida, M.; Ema, T.; Sakai, T. Bifunctional organocatalyst for activation of carbon dioxide and epoxide to produce cyclic carbonate: Betaine as a new catalytic motif. Org. Lett. 2010, 12, 5728–5731. [Google Scholar]
- Takahashi, T.; Watahiki, T.; Kitazume, S.; Yasuda, H.; Sakakura, T. Synergistic hybrid catalyst for cyclic carbonate synthesis: Remarkable acceleration caused by immobilization of homogeneous catalyst on silica. Chem. Commun. 2006, 1664–1666. [Google Scholar]
- Zhou, H.; Wang, Y.M.; Zhang, W.Z.; Qu, J.P.; Lu, X.B. N-Heterocyclic carbene functionalized MCM-41 as an efficient catalyst for chemical fixation of carbon dioxide. Green Chem. 2011, 13, 644–650. [Google Scholar]
- Cheng, W.G.; Chen, X.; Sun, J.; Wang, J.Q.; Zhang, S.J. SBA-15 supported triazolium-based ionic liquids as highly efficient and recyclable catalysts for fixation of CO2 with epoxides. Catal. Today 2013, 200, 117–124. [Google Scholar]
- Wang, Y.M.; Wu, Z.Y.; Wei, Y.L.; Zhu, J.H. In situ coating metal oxide on SBA-15 in one-pot synthesis. Micropor. Mesopor. Mater. 2005, 84, 127–136. [Google Scholar]
- Dai, W.L.; Jin, B.; Luo, S.L.; Luo, X.B.; Tu, X.M.; Au, C.T. Novel functionalized guanidinium ionic liquids: Efficient acid-base bifunctional catalysts for CO2 fixation with epoxides. J. Mol. Catal. A 2013, 378, 326–332. [Google Scholar]
- Rojas, M.F.; Bernard, F.L.; Aquino, A.; Borges, J.; Vecchia, F.D.; Menezes, S.; Ligabue, R.; Einloft, S. Poly(ionic liquid)s as efficient catalyst in transformation of CO2 to cyclic carbonate. J. Mol. Catal. A 2014, 392, 83–88. [Google Scholar]
- Sun, J.M.; Fujita, S.I.; Zhao, F.Y.; Arai, M. A highly efficient catalyst system of ZnBr2/n-Bu4NI for the synthesis of styrene carbonate from styrene oxide and supercritical carbon dioxide. Appl. Catal. A 2005, 287, 221–226. [Google Scholar]
- Liu, M.S.; Liu, B.; Shi, L.; Wang, F.X.; Liang, L.; Sun, J.M. Melamine-ZnI2 as heterogeneous catalysts for efficient chemical fixation of carbon dioxide to cyclic carbonate. RSC Adv. 2015, 5, 960–966. [Google Scholar]
- Kim, M.I.; Choi, S.J.; Kim, D.W.; Park, D.W. Catalytic performance of zinc containing ionic liquids immobilized on silica for the synthesis of cyclic carbonates. J. Ind. Eng. Chem. 2014, 20, 3102–3107. [Google Scholar]
- Lee, J.K.; Kim, Y.J.; Choi, Y.S.; Lee, H.; Lee, J.S.; Hong, J.; Jeong, E.K.; Kim, H.S.; Cheong, M. Zn-containing ionic liquids bearing dialkylphosphate ligands for the coupling reactions of epoxides and CO2. Appl. Catal. B 2012, 111–112, 621–627. [Google Scholar]
- Wang, J.Q.; Sun, J.; Cheng, W.G.; Dong, K.; Zhang, X.P.; Zhang, S.J. Experimental and theoretical studies on hydrogen bond-promoted fixation of carbon dioxide and epoxides in cyclic carbonates. Phys. Chem. Chem. Phys. 2012, 14, 11021–11026. [Google Scholar]
- Xiao, L.F.; Lv, D.W.; Wu, W. Brønsted acidic ionic liquids mediated metallic salts catalytic system for the chemical fixation of carbon dioxide to form cyclic carbonates. Catal. Lett. 2011, 141, 1838–1844. [Google Scholar]
- Motokura, K.; Itagaki, S.; Iwasawa, Y.; Miyaji, A.; Baba, T. Zinc-accelerated cycloaddition of carbon dioxide to styrene oxide catalyzed by pyrrolidinopyridinium iodides. Top Catal. 2014, 57, 953–959. [Google Scholar]
- Ema, T.; Miyazaki, Y.; Shimonishi, J.; Maeda, C.; Hasegawa, J.Y. Bifunctional porphyrin catalysts for the synthesis of cyclic carbonates from epoxides and CO2: Structural optimization and mechanistic study. J. Am. Chem. Soc. 2014, 136, 15270–15279. [Google Scholar]
- Adam, F.; Batagarawa, M.S. Tetramethylguanidine-silica nanoparticles as an efficient and reusable catalyst for the synthesis of cyclic propylene carbonate from carbon dioxide and propylene oxide. Appl. Catal. A 2013, 454, 164–171. [Google Scholar]
- Miao, C.X.; Wang, J.Q.; Wu, Y.; Du, Y.; He, L.N. Bifunctional metal-salen complexes as efficient catalysts for the fixation of CO2 with epoxides under solvent-free conditions. ChemSusChem. 2008, 1, 236–241. [Google Scholar]
- Yang, Z.Z.; Jiang, D.E.; Zhu, X.; Tian, C.C.; Brown, S.; Thanh, C.L.; He, L.N.; Dai, S. Coordination effect-regulated CO2 capture with an alkali metal onium salts/crown ether system. Green Chem. 2014, 16, 253–258. [Google Scholar]
- Yue, C.T.; Su, D.; Zhang, X.; Wu, W.; Xiao, L.F. Amino-functional imidazolium ionic liquids for CO2 activation and conversion to form cyclic carbonate. Catal. Lett. 2014, 144, 1313–1321. [Google Scholar]
- Zhong, S.F.; Liang, L.; Liu, B.; Sun, J.M. ZnBr2/DMF as simplest highly active Acid-Base homogeneous catalysts for cycloaddition of carbon dioxide to propylene oxide. J. CO2 Util. 2014, 6, 75–79. [Google Scholar]
- Prasetyanto, E.A.; Ansari, M.B.; Min, B.H.; Park, S.E. Melamine tri-silsesquioxane bridged periodic mesoporous organosilica as an efficient metal-free catalyst for CO2 activation. Catal. Today 2010, 158, 252–257. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Liu, M.; Liang, L.; Sun, J. Guanidine Hydrochloride/ZnI2 as Heterogeneous Catalyst for Conversion of CO2 and Epoxides to Cyclic Carbonates under Mild Conditions. Catalysts 2015, 5, 119-130. https://doi.org/10.3390/catal5010119
Liu B, Liu M, Liang L, Sun J. Guanidine Hydrochloride/ZnI2 as Heterogeneous Catalyst for Conversion of CO2 and Epoxides to Cyclic Carbonates under Mild Conditions. Catalysts. 2015; 5(1):119-130. https://doi.org/10.3390/catal5010119
Chicago/Turabian StyleLiu, Bo, Mengshuai Liu, Lin Liang, and Jianmin Sun. 2015. "Guanidine Hydrochloride/ZnI2 as Heterogeneous Catalyst for Conversion of CO2 and Epoxides to Cyclic Carbonates under Mild Conditions" Catalysts 5, no. 1: 119-130. https://doi.org/10.3390/catal5010119





