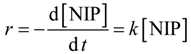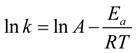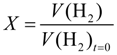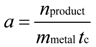2.1. Cyclic Voltammetry
Cyclic voltammetry (CV) was used to study the electrochemical activity of the mono- and bi-metallic (Pt, PtBi and PtPb) nanoparticles stabilized onto ethoxylated polyethylene imine (PEIPEO). A special focus is on the poisoning of the catalysts by carbon monoxide, and for this, the electrochemical oxidation of formic acid can be studied (see
Scheme 1). There are two paths in which formic acid (HCOOH) can be oxidized on the surface of a Pt electrode, namely dehydrogenation and dehydration [
6,
16]. As shown in
Scheme 1, these processes involve either the formation of a reactive intermediate that yields CO
2 as the final product (dehydrogenation) or the formation of CO via the dehydration of formic acid. The adsorbed CO is then oxidized to CO
2. Thus, the electrode can only be poisoned by CO if the formic acid is decomposed via the dehydration path. Taking this into account, valuable information about the oxidation of formic acid on different electrodes can be obtained by a cyclic voltammogram.
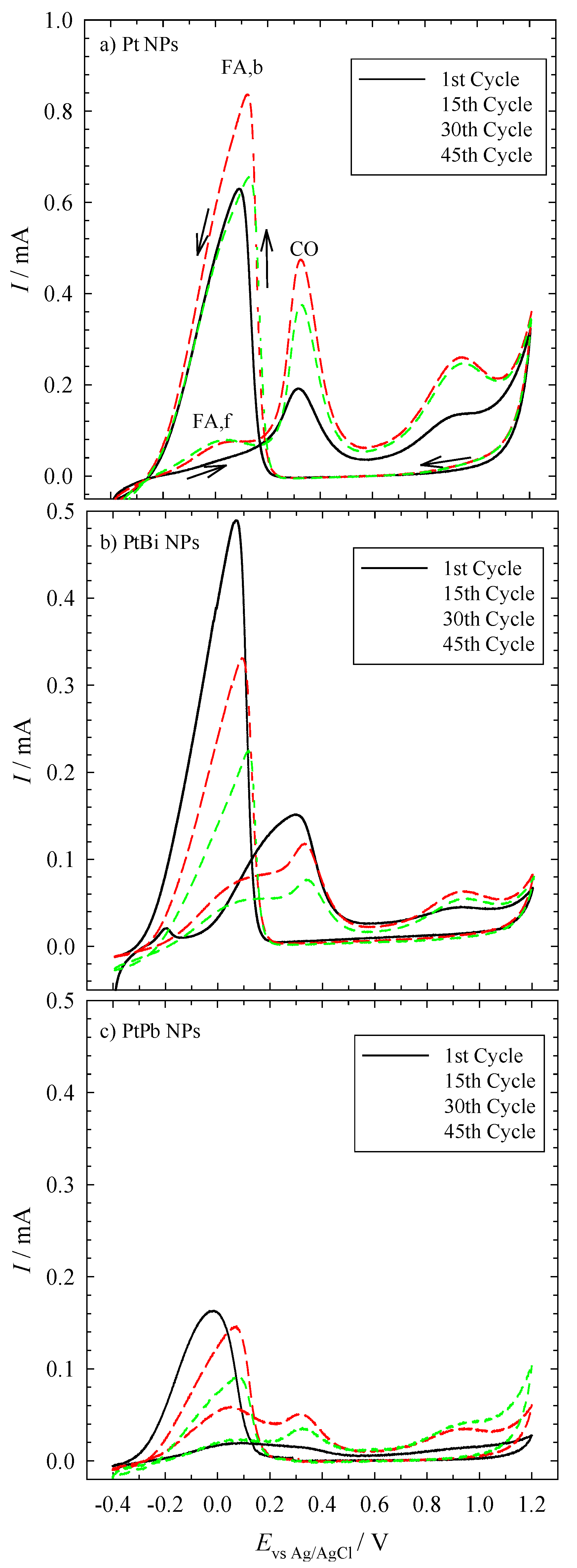
In
Figure 1, one can see the cyclic voltammograms of the electrochemical oxidation of formic acid on the three different nanoparticles over the course of 45 cycles. The three NP-coated glassy carbon (GC) electrodes show the typical peaks obtained for platinum.
All cyclic voltammograms can be explained in terms of the dual path mechanism, as shown in
Scheme 1 [
16]. At low potentials, HCOOH is oxidized via both the direct and the indirect pathways. This can be seen in
Figure 1, where the first peak occurs at +0.1 V (
IFA,f). However, the indirect pathway leads to the formation of CO. Note that at low electrode potentials, CO acts as a surface poisoning species, while at high electrode potentials, it is an active intermediate [
17]. The poisoning effect can be seen in the CV, with the reaction rate of the direct path declining as the number of platinum surface sites available for the direct path diminishes [
16]. At higher potentials, the CO starts to be oxidized (
ICO), giving rise to a clean platinum surface, which results in the oxidation of more formic acid. Simultaneously, the current increases until platinum is oxidized (
IPt). PtO, however, is inactive for the oxidation of formic acid. In the backward sweep, the current increases rapidly due to the reduction of the platinum oxide. In addition, the current is much higher than in the forward sweep, due to the absence of the poisonous CO (
IFA,b). At too low of a potential, however, CO is formed again, and hence, the current decreases significantly [
18]. Looking at the currents of the NP-coated GC electrodes, one sees that the currents measured with the Pt-coated electrode are higher than those measured with the electrodes coated with the bimetallic NPs. However, the available surface area of the nanoparticles on the electrodes very likely was not the same for the three samples, because different amounts of nanoparticles may be present on the glassy carbon disc. Unfortunately, the experimental set-up does not allow quantifying the surface area, and thus, a quantitative analysis of the curves cannot be done at this point.
Scheme 1.
Suggested dual-path mechanism of formic acid oxidation on a Pt electrode [
6,
16]. When formic acid is oxidized on the Pt electrode via the dehydrogenation process, no CO is produced as a side product, and hence, there cannot be any poisoning of the electrode. However, CO is produced via the dehydration of formic acid.
Scheme 1.
Suggested dual-path mechanism of formic acid oxidation on a Pt electrode [
6,
16]. When formic acid is oxidized on the Pt electrode via the dehydrogenation process, no CO is produced as a side product, and hence, there cannot be any poisoning of the electrode. However, CO is produced via the dehydration of formic acid.
Please note that the onset potential (Evs Ag/AgCl = −0.1 V) of the three different electrodes for the oxidation of formic acid is almost the same. This shows that the intermetallic nanoparticles are electrochemically as active as the monometallic platinum nanoparticles.
To gain further information and to study the influence of the poisonous CO on the surface of the electrode, one can compare the ratio of the currents of the oxidation of HCOOH (
IFA,b) and of CO (
ICO) in the forward sweep and the ratio of the currents of the oxidation of HCOOH in the forward (
IFA,f) and in the backward sweep (
IFA,b) for the three different electrodes [
19]. This allows a direct comparison of the different electrodes, even without knowing the available surface area of the nanoparticles. The first ratio compares the currents of the oxidation of formic acid to CO
2 with the currents of the oxidation of CO to CO
2, which occurs at higher potentials. This ratio illustrates how much CO is produced via the indirect pathway. The produced CO can block the active sites for the oxidation of the HCOOH of platinum. The second ratio compares the current of the oxidation of formic acid in the forward sweep with the current of the oxidation of formic acid in the backward sweep. While in the forward sweep, the electrode’s surface can be blocked by CO, in the backward sweep, the electrode’s surface is completely reduced, and therefore, all of the active sites can be used to oxidize formic acid [
18]. The higher the ratios, the less affected the electrodes are by CO. The results clearly show a superior behavior for the bimetallic NPs compared to the monometallic Pt NPs (see
Table 1 and
Table 2).
Figure 1.
Cyclic voltammograms of a glassy carbon (GC) electrode coated with mono- and bi-metallic NPs. The electrolyte contained 0.5 M formic acid/0.1 M sulfuric acid. The sweep rate is 10 mV∙s−1. The GC electrode was coated with (a) Pt NP, (b) PtBi NP and (c) PtPb NP, and the potentials are measured against an Ag/AgCl electrode. Please note that the y-scale of the upper figure is different from the two lower ones.
Figure 1.
Cyclic voltammograms of a glassy carbon (GC) electrode coated with mono- and bi-metallic NPs. The electrolyte contained 0.5 M formic acid/0.1 M sulfuric acid. The sweep rate is 10 mV∙s−1. The GC electrode was coated with (a) Pt NP, (b) PtBi NP and (c) PtPb NP, and the potentials are measured against an Ag/AgCl electrode. Please note that the y-scale of the upper figure is different from the two lower ones.
Table 1.
The ratio of the current resulting from the oxidation of HCOOH to CO2 (IFA,f) and from the oxidation of CO to CO2 (ICO) in the forward scan of the cyclic voltammogram of the three different electrodes coated with the mono- and bi-metallic nanoparticles.
Table 1.
The ratio of the current resulting from the oxidation of HCOOH to CO2 (IFA,f) and from the oxidation of CO to CO2 (ICO) in the forward scan of the cyclic voltammogram of the three different electrodes coated with the mono- and bi-metallic nanoparticles.
| Cycle | IFA,f/ICO |
|---|
| Pt NPs | PtBi NPs | PtPb NPs |
|---|
| 1stCycle | 0.18 ± 0.01 | 0.50 ± 0.10 | 2.50 ± 0.02 |
| 5thCycle | 0.14 ± 0.01 | 0.75 ± 0.20 | 3.00 ± 0.50 |
| 10thCycle | 0.14 ± 0.02 | 0.75 ± 0.35 | 1.90 ± 0.20 |
| 15thCycle | 0.15 ± 0.03 | 0.67 ± 0.20 | 1.15 ± 0.01 |
| 30thCycle | 0.17 ± 0.04 | 0.51 ± 0.04 | 0.64 ± 0.03 |
| 45thCycle | 0.18 ± 0.04 | 0.55 ± 0.20 | 0.61 ± 0.05 |
Table 2.
The ratio of the current resulting from the oxidation of HCOOH to CO2 (IFA,f) in the forward sweep and from the oxidation of HCOOH to CO2 (IFA,b) in the backward sweep of the cyclic voltammogram of the three different electrodes coated with the mono- and bi-metallic nanoparticles.
Table 2.
The ratio of the current resulting from the oxidation of HCOOH to CO2 (IFA,f) in the forward sweep and from the oxidation of HCOOH to CO2 (IFA,b) in the backward sweep of the cyclic voltammogram of the three different electrodes coated with the mono- and bi-metallic nanoparticles.
| Cycle | IFA,f/IFA,b |
|---|
| Pt NPs | PtBi NPs | PtPb NPs |
|---|
| 1st Cycle | 0.09 ± 0.02 | 0.30 ± 0.05 | 0.65 ± 0.15 |
| 5th Cycle | 0.08 ± 0.02 | 0.42 ± 0.10 | 0.75 ± 0.20 |
| 10th Cycle | 0.08 ± 0.02 | 0.28 ± 0.07 | 0.84 ± 0.35 |
| 15th Cycle | 0.08 ± 0.01 | 0.25 ± 0.05 | 0.70 ± 0.50 |
| 30th Cycle | 0.09 ± 0.01 | 0.18 ± 0.02 | 0.40 ± 0.30 |
| 45th Cycle | 0.10 ± 0.02 | 0.20 ± 0.01 | 0.36 ± 0.20 |
Over a run of 45 cycles, however, the selectivity towards the HCOOH oxidation decreases for the intermetallic nanoparticles, which can be explained by the dissolution of the less noble metal from the electrode [
20].
Although it is well known in the literature that intermetallic PtBi and PtPb electrodes are less affected by CO poisoning compared to monometallic Pt electrodes [
5,
6,
9,
21,
22,
23], it is interesting to find out whether the mixture of phases present in our nanoparticles [
13,
24] has an effect on the activity and selectivity of the electrochemical reaction. For this purpose, we compare the ratios discussed above with the ratios calculated from the literature.
Our results are completely in line with data found by Casado-Rivera
et al. for their monometallic platinum and bimetallic PtBi electrodes. If one calculates the first ratio
IFA,f/
ICO based on the data published in [
6], one obtains 0.21 for Pt and 0.7 for PtBi. Comparing these figures with those measured in the present work, which are 0.18 ± 0.01 for the GC/Pt NPs and 0.5 ± 0.1 for the glassy carbon electrode coated with the bimetallic PtBi NPs, one clearly sees that the results are in perfect agreement for the first cycle. In other words, the mixture of phases found in the PtBi nanoparticles does not influence the electrochemical activity compared to a pure PtBi phase. The only advantage of the pure intermetallic PtBi phase is the enhanced performance regarding the onset potential, while our data does not show any change in the onset potential in comparison to the monometallic platinum nanoparticles. Furthermore, the CVs measured by Volpe
et al. for a pure PtBi phase led to the same value for the second ratio (
IFA,f/
IFA,b ~ 0.34 [
8]) as our measurements for the first cycle (
IFA,f/
IFA,b = 0.3 ± 0.05).
The results found by Casado-Rivera
et al. [
5] and Zhang
et al. [
25] for a bimetallic PtPb electrode, however, show a greater CO tolerance compared to our data, with an
IFA,f/
IFA,b ratio of around 1.5, which is about twice the value we found for the GC electrode coated with the bimetallic PtPb nanoparticles. From the data measured by Volpe
et al., one can calculate an even greater value of
IFA,f/
IFA,b ~ 2.4. However, this CV was measured at 2000 rpm to reduce the occlusion of the surface by bubble formation [
8]. The general trend observed,
i.e., that the pure PtPb electrodes show a greater resistance towards CO poisoning than our bimetallic nanoparticles, could be due to the mixture of phases found in our nanoparticles. Furthermore, our bimetallic PtPb NPs do not show any enhanced electrochemical activity, as it is the case for pure PtPb phases [
5,
8].
2.2. Reduction of 4-Nitrophenol
To learn more about the catalytic activity of the three different nanoparticles, stabilized onto ethoxylated polyethylene imine, the reduction of 4-nitrophenol (NIP) to 4-aminophenol (see
Scheme 2) was studied via UV-Vis spectroscopy.
Scheme 2.
Reaction mechanism for the reduction of 4-nitrophenol to 4-aminophenol. 4-nitrophenol is first hydrogenated to 4-nitrosophenol, which is further hydrogenated to 4-hydroxyaminophenol. In the last step, 4-hydroxyaminophenol is hydrogenated to the final product, 4-aminophenol [
26].
Scheme 2.
Reaction mechanism for the reduction of 4-nitrophenol to 4-aminophenol. 4-nitrophenol is first hydrogenated to 4-nitrosophenol, which is further hydrogenated to 4-hydroxyaminophenol. In the last step, 4-hydroxyaminophenol is hydrogenated to the final product, 4-aminophenol [
26].
The reaction can be described as follows: the catalyst reacts with borohydride ions to form the metal hydride, and at the same time, 4-nitrophenol adsorbs onto the metal surface. Here, 4-nitrophenol is reduced to 4-aminophenol via two intermediates, namely 4-nitrosophenol and 4-hydroxyaminophenol, which desorbs afterwards. As reported in [
27], the reaction follows the Langmuir–Hinshelwood mechanism. However, if a large enough amount of NaBH
4 is used (at least >100-times more than 4-nitrophenol), the kinetics simplify to a pseudo first-order reaction [
28,
29,
30,
31,
32]. Thus, to test the catalytic activity of the synthesized nanoparticles, we used an excess of NaBH
4 and treated the reaction as a pseudo first-order reaction.
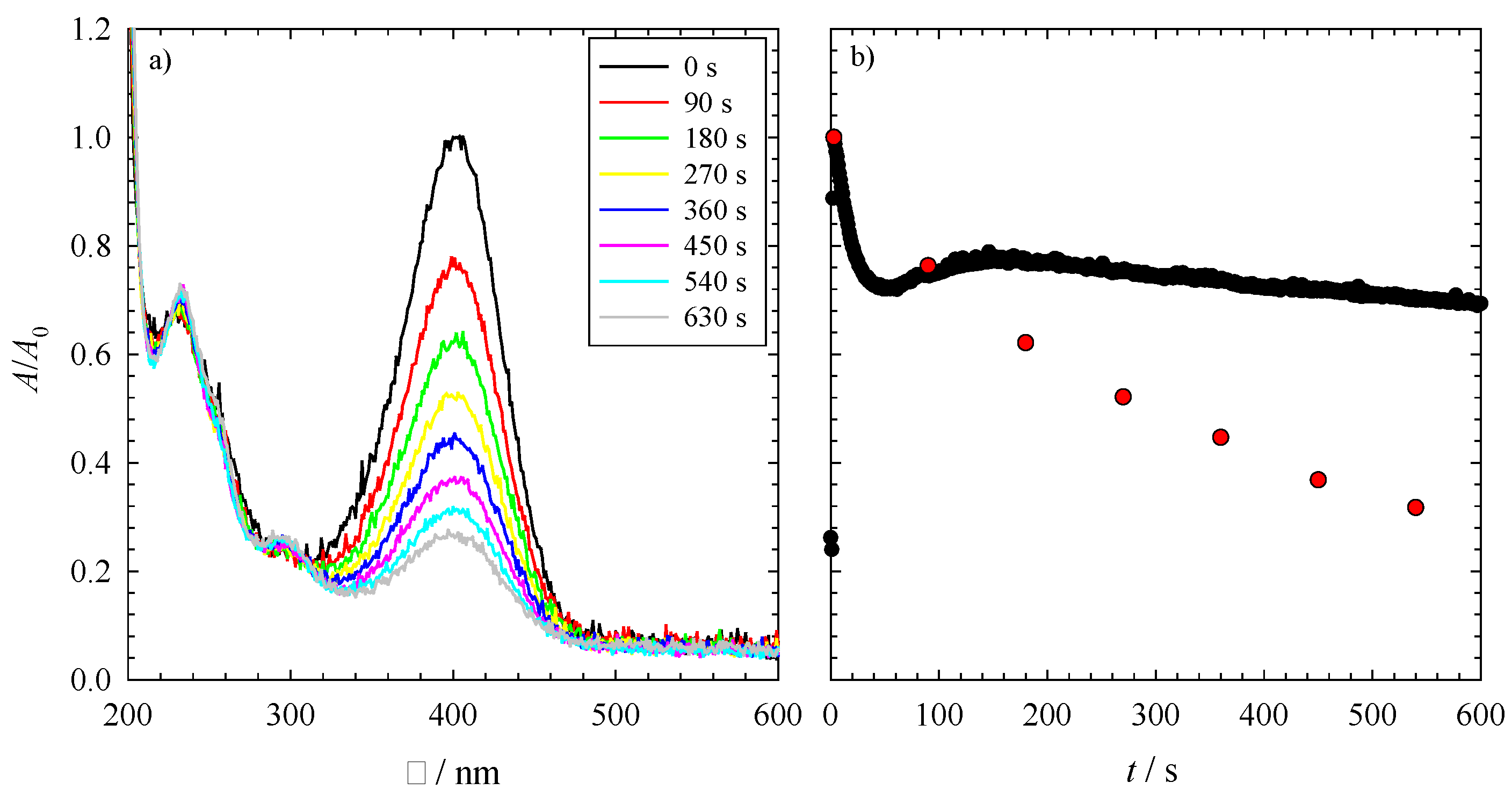
As an example, the UV-Vis spectra of the reduction of 4-nitrophenol with NaBH
4 catalyzed by PtPb nanoparticles are displayed in
Figure 2a as a function of time. At λ = 400 nm, one can see the decrease of the absorption of the nitrophenolate ion, while the increasing peak at around λ = 300 nm indicates the increasing concentration of 4-aminophenol. Note that rising hydrogen bubbles in the measuring cell disturb the visibility of the three isosbestic points at around 280, 320 and 470 nm. Looking at
Figure 2a, one observes that even after 630 s, the reaction is not complete,
i.e., not all of the 4-nitrophenol is converted to 4-aminophenol. The time evolution of this reduction can be seen in detail via the red spheres in
Figure 2b. However, to monitor the reaction on an adequate time scale, the UV-Vis absorbance spectra were measured directly at λ = 400 nm,
i.e., at the position of the nitrophenolate ion peak as a function of time. The resulting time evolution over a range of 600 s of this absorption band is seen in
Figure 2b (black spheres). Please note, the deviation between the two sets of data is in the experimental error. However, one can immediately see via the time-resolved data that the reduction of 4-nitrophenol occurs via different steps, which will be discussed in the following. At the very first couple of seconds, one can see a rise in the absorbance at 400 nm. This increase indicates a delay of the dissociation of 4-nitrophenol to the nitrophenolate ion after the addition of NaBH
4, which is caused by the mixing of the solutions. The start of the reaction is defined as the time where 4-nitrophenol is completely dissociated,
i.e., the highest point of the absorbance spectra. Although some nitrophenolate ions are reduced within these first seconds, this effect will be neglected in the following discussion. After 2 s, the solution is completely mixed, the 4-nitrophenol is deprotonated and the reaction starts. This can be seen by the steep decrease of absorbance within the first couple of seconds. This decrease is followed by an increase of absorbance up until around 150 s after the start of the reaction, before a second, but less steep decrease indicates a slower reduction compared to the initial process. An explanation for these different reaction rates can be given by looking at the reaction mechanism of 4-nitrophenol to 4-aminophenol (see
Scheme 2). Firstly, 4-nitrophenol is hydrogenated to 4-nitrosophenol, followed by a hydrogenation to 4-hydroxyaminophenol. This is then hydrogenated to the final product, 4-aminophenol [
26]. A possible explanation for the different reaction rates found for the reduction of 4-nitrophenol is thus the stepwise reduction via 4-nitrosophenol (steep decrease) and 4-hydroxyaminophenol (slow increase) before the latter is reduced to 4-aminophenol (slow decrease). We will use the stepwise reduction displayed in
Scheme 2 to describe the experimental results.
Figure 2.
(a) The UV-Vis absorbance spectrum of the reduction of 4-nitrophenol to 4-aminophenol via PtPb nanoparticles as the catalyst; (b) The UV-Vis absorbance spectrum for the reduction of 4-nitrophenol over 600 s (black spheres), monitored at 400 nm. The red spheres show the absorbance of the measurement from the spectrum displayed in (a). Both reductions were performed at 23 °C with 1 mg of catalyst (i.e., 0.3 mg PtPb NPs and 0.7 mg polymer), an initial NaBH4 concentration of cNABH4 = 6.196 mM and an initial 4-nitrophenol concentration of cNip = 0.0317 mM.
Figure 2.
(a) The UV-Vis absorbance spectrum of the reduction of 4-nitrophenol to 4-aminophenol via PtPb nanoparticles as the catalyst; (b) The UV-Vis absorbance spectrum for the reduction of 4-nitrophenol over 600 s (black spheres), monitored at 400 nm. The red spheres show the absorbance of the measurement from the spectrum displayed in (a). Both reductions were performed at 23 °C with 1 mg of catalyst (i.e., 0.3 mg PtPb NPs and 0.7 mg polymer), an initial NaBH4 concentration of cNABH4 = 6.196 mM and an initial 4-nitrophenol concentration of cNip = 0.0317 mM.
Note that amines may poison the catalyst, which is why only the first part of the reaction, i.e., the fast reduction of 4-nitrophenol to 4-nitrosophenol, will be evaluated. This allows not only the exclusion of catalyst poisoning, but also the consideration of the NaBH4 concentration as constant, which, in turn, justifies treating the first step as a reaction of pseudo first order.
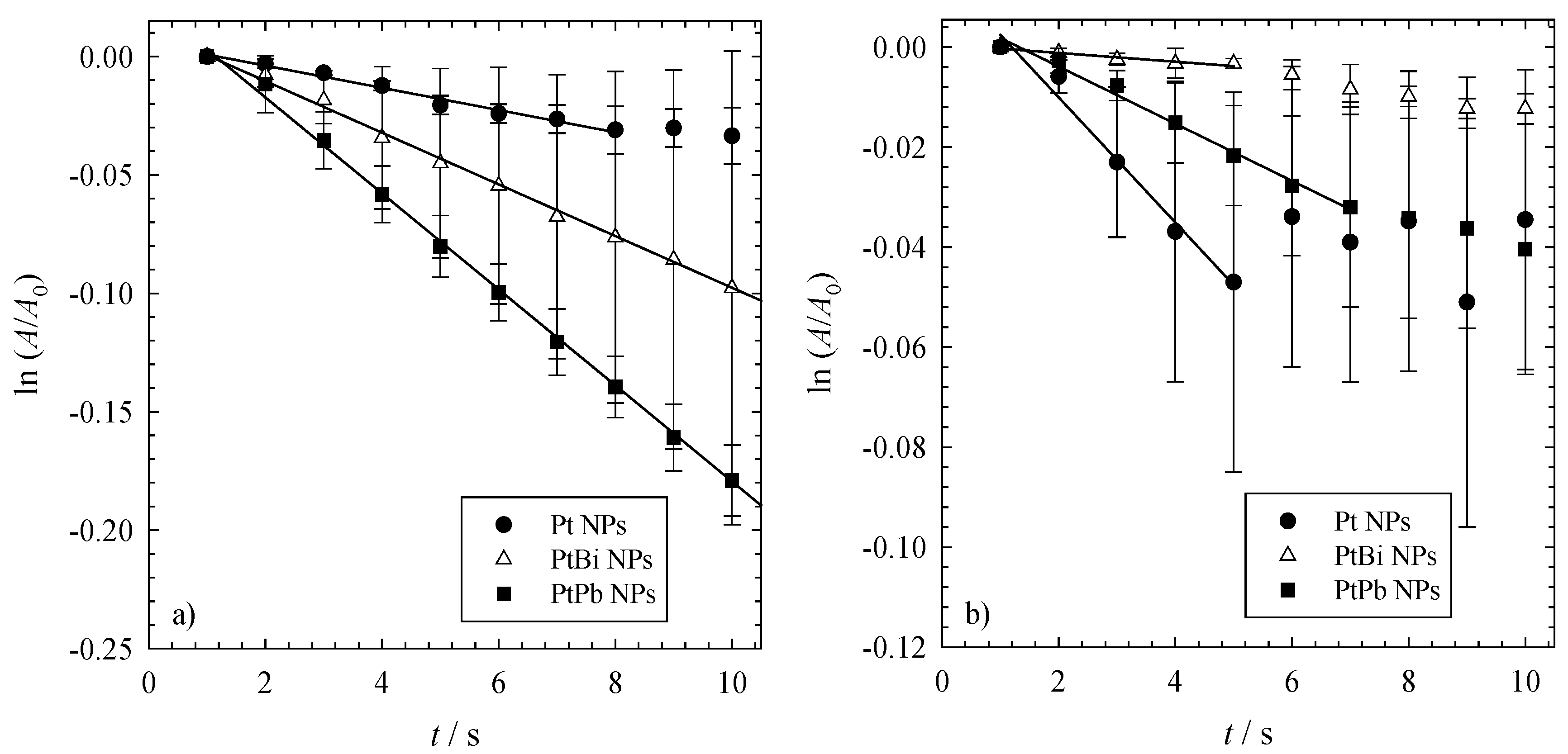
To obtain more information about the kinetics of the reduction of 4-nitrophenol via NaBH
4 using the three different nanoparticles as the catalyst, one can plot ln(
A/
A0) as a function of time for the first 10 s after the start of the reaction (see
Figure 3).
Figure 3.
The time-dependent change of the concentration of 4-nitrophenol ln(A/A0) versus time t. All reductions were performed with 1mg of catalyst (i.e., 0.3 mg NPs and 0.7 mg polymer), an initial NaBH4 concentration of cNABH4 = 6.196 mM and an initial 4-nitrophenol concentration of cNip = 0.0317 mM at (a) 23 °C and (b) 36 °C.
Figure 3.
The time-dependent change of the concentration of 4-nitrophenol ln(A/A0) versus time t. All reductions were performed with 1mg of catalyst (i.e., 0.3 mg NPs and 0.7 mg polymer), an initial NaBH4 concentration of cNABH4 = 6.196 mM and an initial 4-nitrophenol concentration of cNip = 0.0317 mM at (a) 23 °C and (b) 36 °C.
The data shown in
Figure 3 allow calculating the rate constants of the first reaction step,
i.e., of the hydrogenation of 4-nitrophenol to 4-nitrosophenol using first order kinetics (see Equation (1) in
Section 3.4). It is evident that the rate constants of the mono- and bi-metallic nanoparticles change with the temperature at which the reaction is performed. While at ambient temperature (see
Figure 3a), one finds that the bimetallic nanoparticles are more active for the reduction of 4-NIP, the monometallic platinum nanoparticles are more active at higher temperatures (see
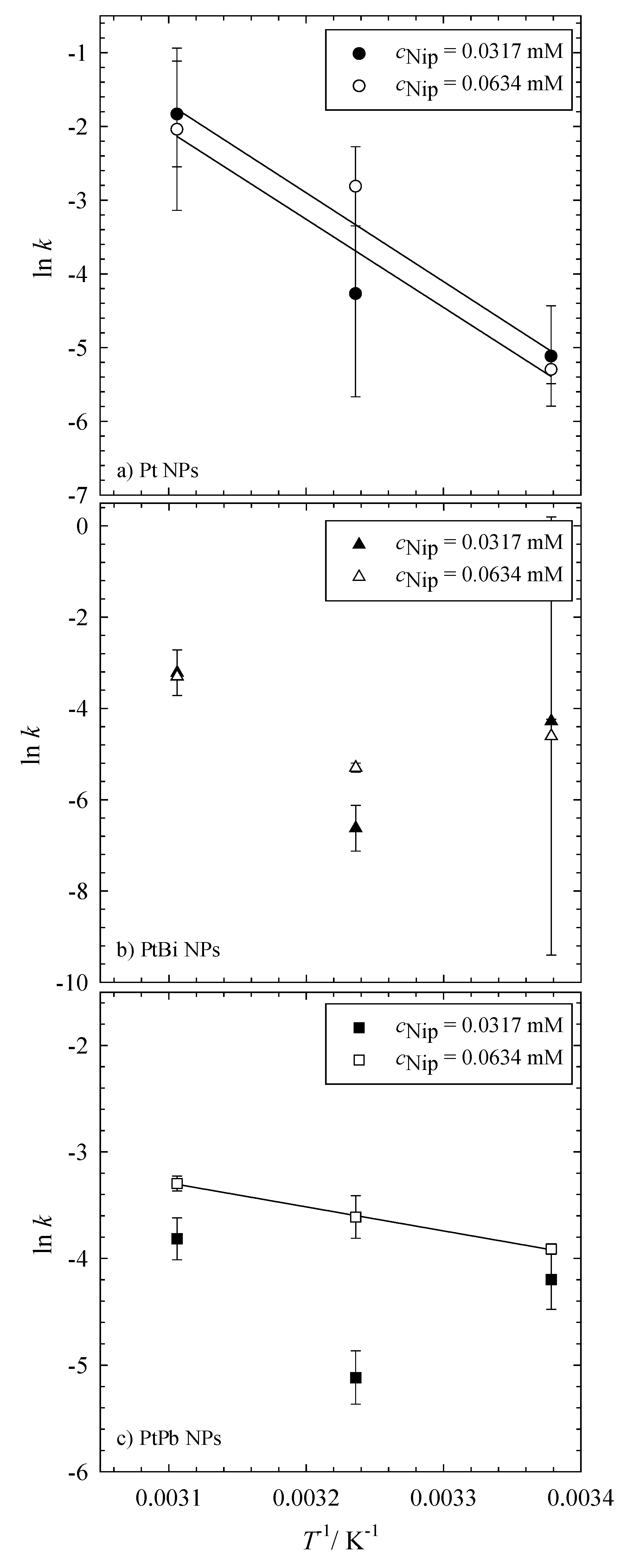
Figure 3b). This can be further seen by the kinetic rate constants shown in
Table 3. For the reaction catalyzed with the monometallic platinum nanoparticles, the kinetic rate constants
k' increase with increasing temperature and can be regarded as almost independent of the initial concentration of 4-nitrophenol (see
Table 3). A linear increase of the rate constant with temperature, as displayed in
Figure 4a, underlines the pseudo first-order reaction kinetics of the reduction of 4-nitrophenol to 4-nitrosophenol via NaBH
4 catalyzed by the platinum nanoparticles. Unfortunately, the rate constants calculated for the reaction catalyzed by the bimetallic PtBi and PtPb nanoparticles do not follow this trend (see
Figure 4b,c). It is obvious that the calculated rate constants are not independent of the initial concentration of 4-nitrophenol and that their values are not related to each other.
Table 3.
The first-order rate constants for the reduction of 4-nitrophenol to 4-nitrosophenol using NaBH4 as the reducing agent and three different nanoparticles as the catalyst. All reductions were performed with 1 mg of catalysts (i.e., 0.3 mg Pt, PtPb, PtBi NPs and 0.7 mg polymer) and a constant concentration of the reducing agent (6.196 mM NaBH4). The reduction was measured at 23 °C, 36 °C and 49 °C.
Table 3.
The first-order rate constants for the reduction of 4-nitrophenol to 4-nitrosophenol using NaBH4 as the reducing agent and three different nanoparticles as the catalyst. All reductions were performed with 1 mg of catalysts (i.e., 0.3 mg Pt, PtPb, PtBi NPs and 0.7 mg polymer) and a constant concentration of the reducing agent (6.196 mM NaBH4). The reduction was measured at 23 °C, 36 °C and 49 °C.
| Catalysts | Initial reactant concentration (cNip)/mM | Rate constants (k'/s−1) at Different temperatures |
|---|
| T = 23 °C | T = 36 °C | T = 49 °C |
|---|
| Pt/PEIPEO | 0.0317 | 0.006 ± 0.004 | 0.014 ± 0.012 | 0.16 ± 0.11 |
| 0.0634 | 0.005 ± 0.001 | 0.05 ± 0.02 | 0.13 ± 0.09 |
| PtBi/PEIPEO | 0.0317 | 0.014 ± 0.001 | 0.013 ± 0.009 | 0.04 ± 0.015 |
| 0.0634 | 0.01 ± 0.01 | 0.005 ± 0.0005 | 0.037 ± 0.003 |
| PtPb/PEIPEO | 0.0317 | 0.015 ± 0.004 | 0.006 ± 0.002 | 0.022 ± 0.004 |
| 0.0634 | 0.020 ± 0.001 | 0.027 ± 0.005 | 0.037 ± 0.003 |
The trend seen in
Figure 4 for the bimetallic nanoparticles supports the idea that the kinetics of the reduction of 4-nitrophenol cannot be simply described by a pseudo first-order reaction, but more complex mechanisms need to be considered,
i.e., the influence of the support material (PEIPEO). A similar trend was seen by Mei
et al. and Lu
et al. for the reduction of 4-nitrophenol catalyzed by palladium nanoparticles and silver nanoparticles, respectively [
31,
33]. Their nanoparticles were stabilized on a poly(
N-isopropylacrylamide) (PNIPA) network, the structure of which was strongly influenced by the temperature of the reaction. The shrinking of the network, due to an expulsion of water, is followed by a slowing down of the diffusion of the reactants within the polymer network. This process, in turn, lowers the rate of the reaction catalyzed by the nanoparticles. At higher temperatures, the expected increase in the kinetic rate constants is overcompensated by the diffusional barrier. If the temperature is increased further, the density of the network stays constant and the increase of
k with
T dominates, so that the reaction rate was found to rise again [
31]. Their findings could indeed explain the trend seen in our measurements, especially for the reaction catalyzed by the bimetallic nanoparticles.
It is evident that only the reduction of NIP catalyzed by the monometallic platinum nanoparticles shows an Arrhenius-like behavior, and the data can hence be used to calculate an apparent activation energy using the Arrhenius equation (Equation (2) in
Section 3.4). In comparison to the results found in the literature, the apparent activation energy
EA for the reaction catalyzed via the Pt NPs is rather high with
EA = 99 ± 23 kJ∙mol
−1 compared to the values between 30 and 45 kJ∙mol
−1, which are typical for platinum and platinum-based nanoparticles catalyzed reactions [
27,
28,
31]. This high apparent activation energy could be explained by desorption of the NaBH
4 from the catalyst surface at high temperatures. At low temperatures, NaBH
4 adsorbs at the surface of the catalyst, leaving less empty active sites for the reduction of 4-nitrophenol. With increasing temperature, NaBH
4 desorbs and leaves a higher number of active sites, which, in turn, increases the rate constant significantly.
Since the kinetic analysis of the reduction of 4-nitrophenol is very complex and since our results for the bimetallic nanoparticles do not follow a pseudo first-order kinetic, another model reaction was used to obtain a better picture of the catalytic activity of the nanoparticles.
Figure 4.
Arrhenius plot of the reduction of 4-nitrophenol to 4-nitrosophenol using NaBH4 as a reducing agent catalyzed via (a) Pt, (b) PtBi and (c) PtPb nanoparticles. All reductions were performed with 1 mg of catalysts (i.e., 0.3 mg Pt, PtPb, PtBi NPs and 0.7 mg of polymer) and a constant concentration of the reducing agent (6.196 mM NaBH4). The reduction was measured at 23 °C, 36 °C and 49 °C. The filled symbols denote the measurements with an initial concentration of cNip = 0.0317 mM, while the empty symbols denote the measurements with cNip = 0.0634 mM.
Figure 4.
Arrhenius plot of the reduction of 4-nitrophenol to 4-nitrosophenol using NaBH4 as a reducing agent catalyzed via (a) Pt, (b) PtBi and (c) PtPb nanoparticles. All reductions were performed with 1 mg of catalysts (i.e., 0.3 mg Pt, PtPb, PtBi NPs and 0.7 mg of polymer) and a constant concentration of the reducing agent (6.196 mM NaBH4). The reduction was measured at 23 °C, 36 °C and 49 °C. The filled symbols denote the measurements with an initial concentration of cNip = 0.0317 mM, while the empty symbols denote the measurements with cNip = 0.0634 mM.
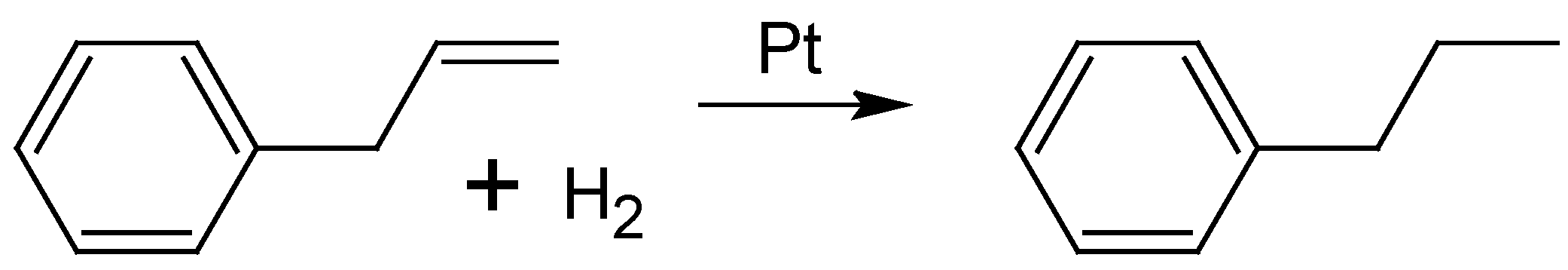
2.3. Hydrogenation of Allylbenzene
To obtain quantitative information about the catalytic activity of the bimetallic nanoparticles stabilized onto ethoxylated polyethylene imine, the hydrogenation of allylbenzene (AB) to propylbenzene (see
Scheme 3) was studied.
Scheme 3.
Hydrogenation of allylbenzene to propylbenzene using platinum catalysts.
Scheme 3.
Hydrogenation of allylbenzene to propylbenzene using platinum catalysts.
The conversion of allylbenzene can be monitored directly by studying the consumption of hydrogen (see Equation (3) in
Section 3.4). To obtain the exact conversion of AB, a sample was taken after the reaction and was then analyzed via gas chromatography. In
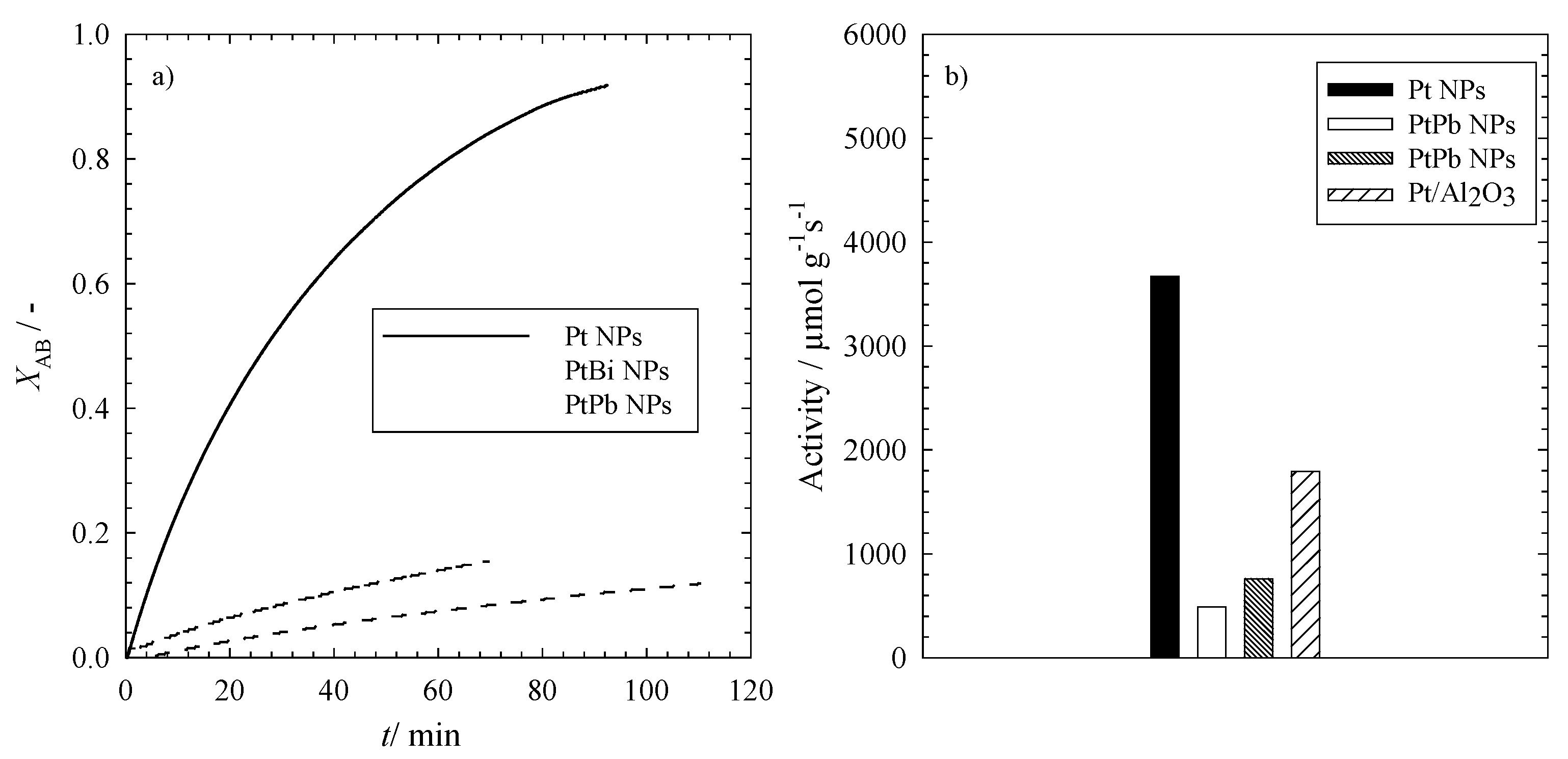
Figure 5a, the conversion of allylbenzene to propylbenzene
XAB, calculated from Equation (3), is plotted
versus time for the three different nanoparticles. It can be clearly seen that the three different types of nanoparticles convert AB on different time scales. The full conversion via the monometallic Pt NPs takes around 120 min, which is also the best catalyst for this reaction. With the bimetallic nanoparticles containing lead (PtPb NPs), the conversion is significantly slower compared to the former, but it is still faster than the conversion with the bimetallic platinum-bismuth nanoparticles (PtBi NPs). This is in line with the trend observed for the reduction of 4-nitrophenol.
To gain further insight into the kinetics of the reaction, one can calculate the initial reaction rates
r0 for the hydrogenation of allylbenzene via Equation (4) in
Section 3.4. The first ten percent of the conversion were taken to calculate the initial rate of the hydrogenation.
Figure 5.
(a) The conversion of allylbenzene (XAB) to propylbenzene in the presence of monometallic platinum nanoparticles (Pt NPs) and the intermetallic platinum-bismuth (PtBi NPs) and platinum-lead (PtPb NPs) nanoparticles; (b) The activity of the different catalysts of the hydrogenation of allylbenzene to propylbenzene using Pt, PtBi and PtPb nanoparticles and a commercially available Pt catalyst. Please note that the activity refers to the amount of metal in the catalysts (see Equation (5)). All reactions were performed at T = 20 °C, pH2 = 1.1 MPa, n = 1200 rpm and V = 100 mL.
Figure 5.
(a) The conversion of allylbenzene (XAB) to propylbenzene in the presence of monometallic platinum nanoparticles (Pt NPs) and the intermetallic platinum-bismuth (PtBi NPs) and platinum-lead (PtPb NPs) nanoparticles; (b) The activity of the different catalysts of the hydrogenation of allylbenzene to propylbenzene using Pt, PtBi and PtPb nanoparticles and a commercially available Pt catalyst. Please note that the activity refers to the amount of metal in the catalysts (see Equation (5)). All reactions were performed at T = 20 °C, pH2 = 1.1 MPa, n = 1200 rpm and V = 100 mL.
It is evident from
Figure 5a that the platinum nanoparticles catalyze the reaction much faster with an initial reaction rate of
r0 = 2.3 ± 0.9 mM∙min
−1, which is 10 times higher than for the bimetallic PtPb nanoparticles (
r0 = 0.20 ± 0.05 mM∙min
−1), followed by the lowest initial reaction rate
r0 = 0.10 ± 0.05 mM∙min
−1 for the PtBi nanoparticles.
To compare the performance of our mono- and bi-metallic nanoparticles with that of a commercially available catalyst, it is necessary to calculate the activity of the catalysts
a via Equation (5) in
Section 3.4. In
Figure 5b, the activities of the mono- and bi-metallic nanoparticles (Pt, PtBi and PtPb NPs) and of a commercially available Pt/Al
2O
3 catalyst are displayed. The activity of the synthesized monometallic platinum nanoparticles supported by ethoxylated polyethylene imine is significantly higher than that of the commercially available platinum nanoparticles supported by Al
2O
3, but it should be mentioned that a direct comparison of the results for the two supported catalysts is not possible. Although the same amount of Pt was used in the experiments and although the particles had comparable sizes, the activity of the two supported catalysts may differ, due to the following reasons: the impact of the support material, the accessible platinum surface area, pore or film diffusion effects, reactant distribution in the polymer-supported catalyst, oxidized Pt nanoparticles on the surface,
etc. Since the focus of our research was the synthesis of nanoparticles via the microemulsion route, we did not carry out a systematic study, which allows comparing the different catalysts quantitatively. What we wanted to show is the fact that this route leads to active mono- and bi-metallic catalysts, clear proof for which is seen in
Figure 5. However, the activity of the bimetallic nanoparticles is much lower compared to both the “home-made” and the commercially available monometallic Pt catalyst. Note that for all experiments with the mono- and bi-metallic catalysts, the total amount of metal was kept constant. Consequently, the amount of Pt in the bimetallic catalyst was much lower compared to the monometallic counterparts. It is well known that Pt is a good hydrogenation catalyst; therefore, the lower amount of Pt in the bimetallic catalysts might be the major reason for the lower activity in comparison to the monometallic ones. Consistent with the results found for the reduction of 4-nitrophenol, the bimetallic platinum-lead nanoparticles show slightly higher activities compared to the platinum-bismuth nanoparticles.