Dehydration of Methanol to Dimethyl Ether—Current State and Perspectives
Abstract
:1. Introduction
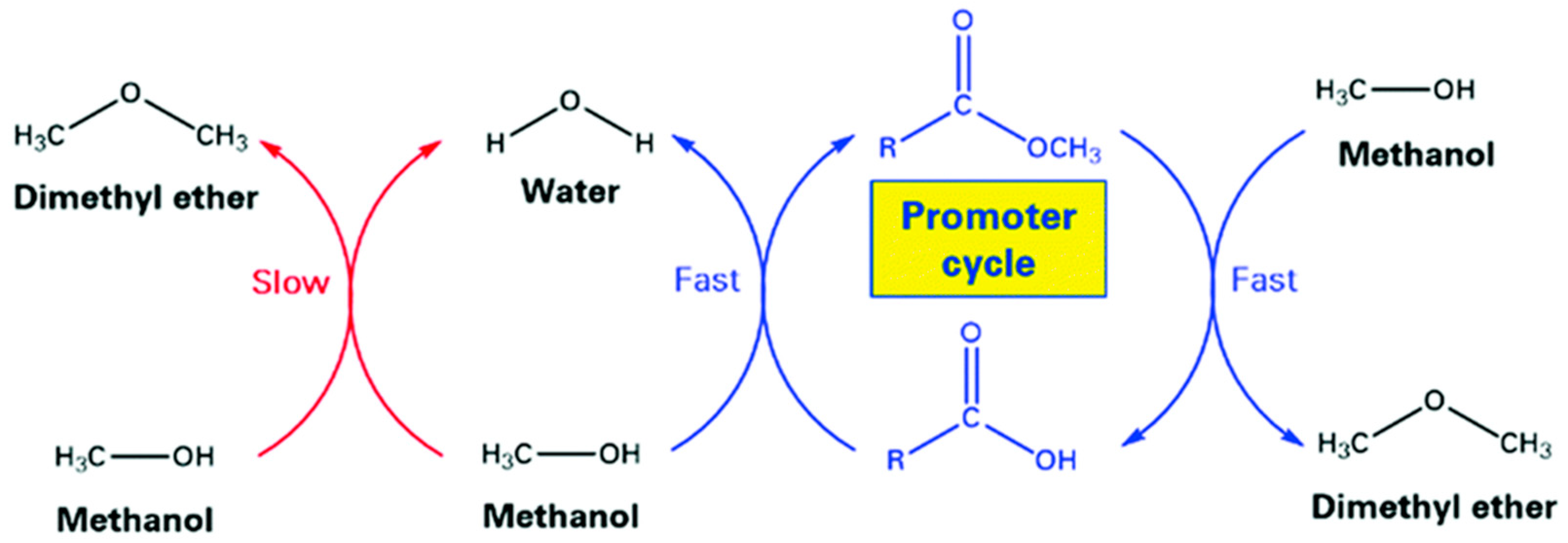
2. Methanol to DME (MTD Process)
2.1. Al2O3-Based Catalysts
2.2. Zeolite-Based Catalysts
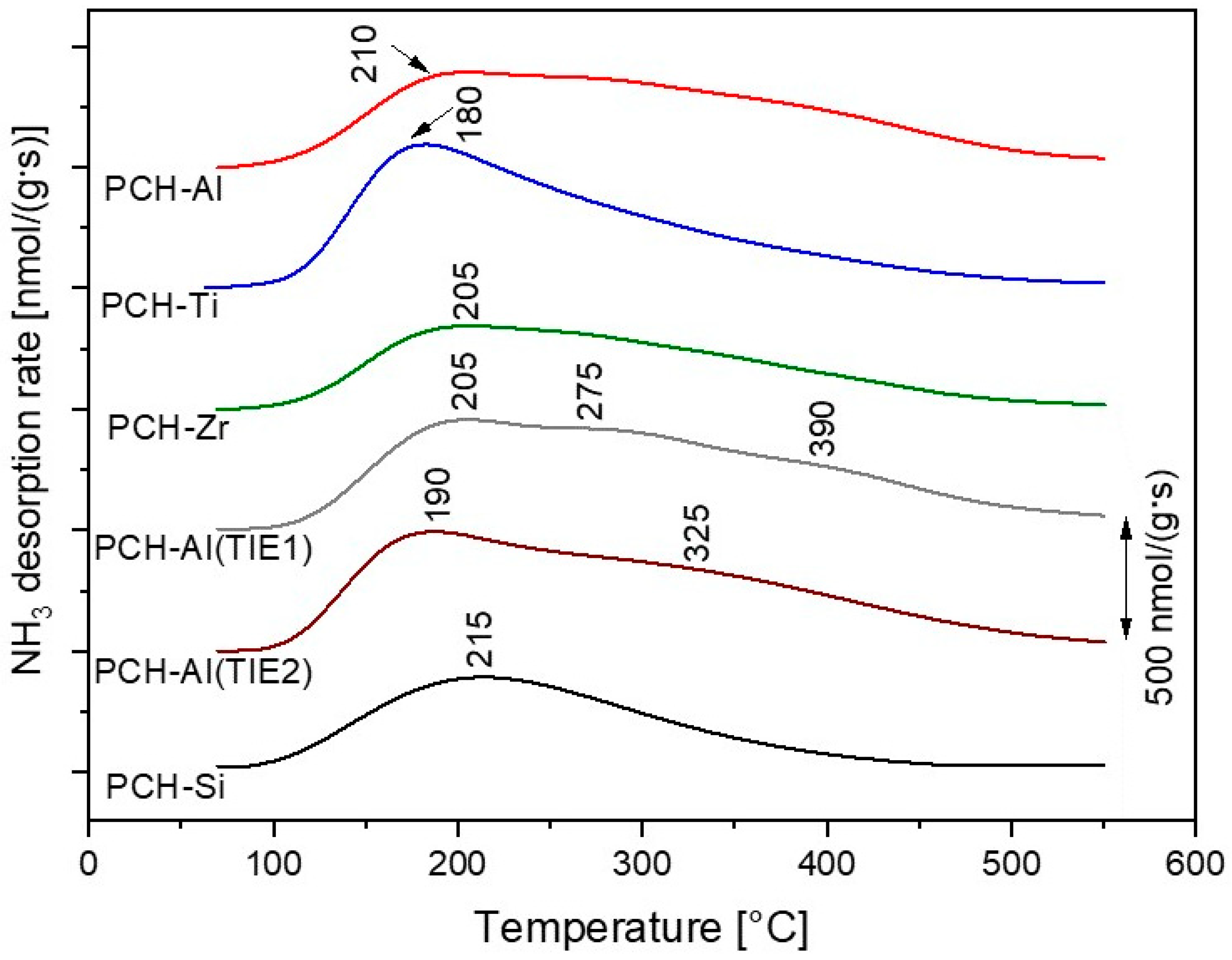
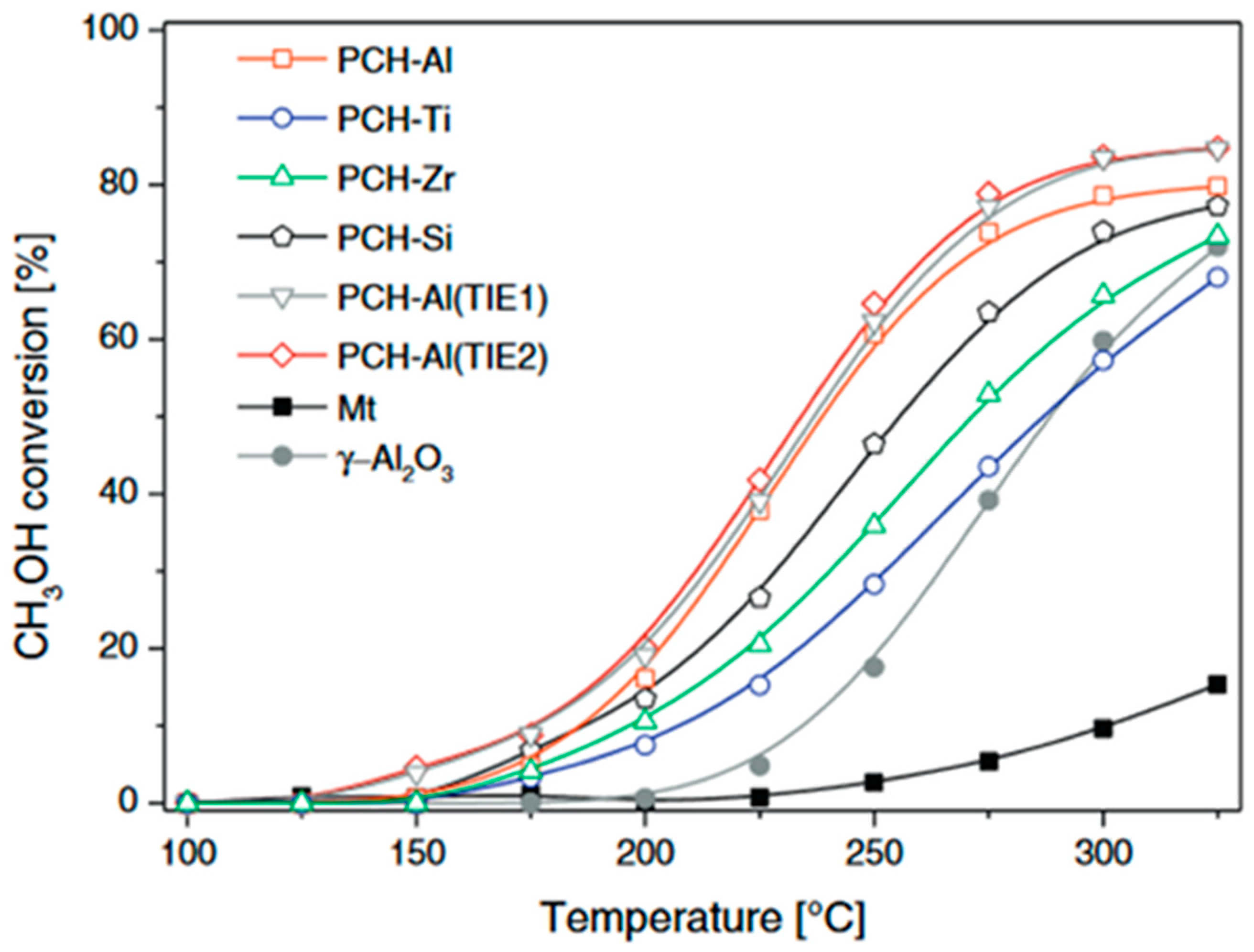
2.3. Clay Mineral-Based Catalysts
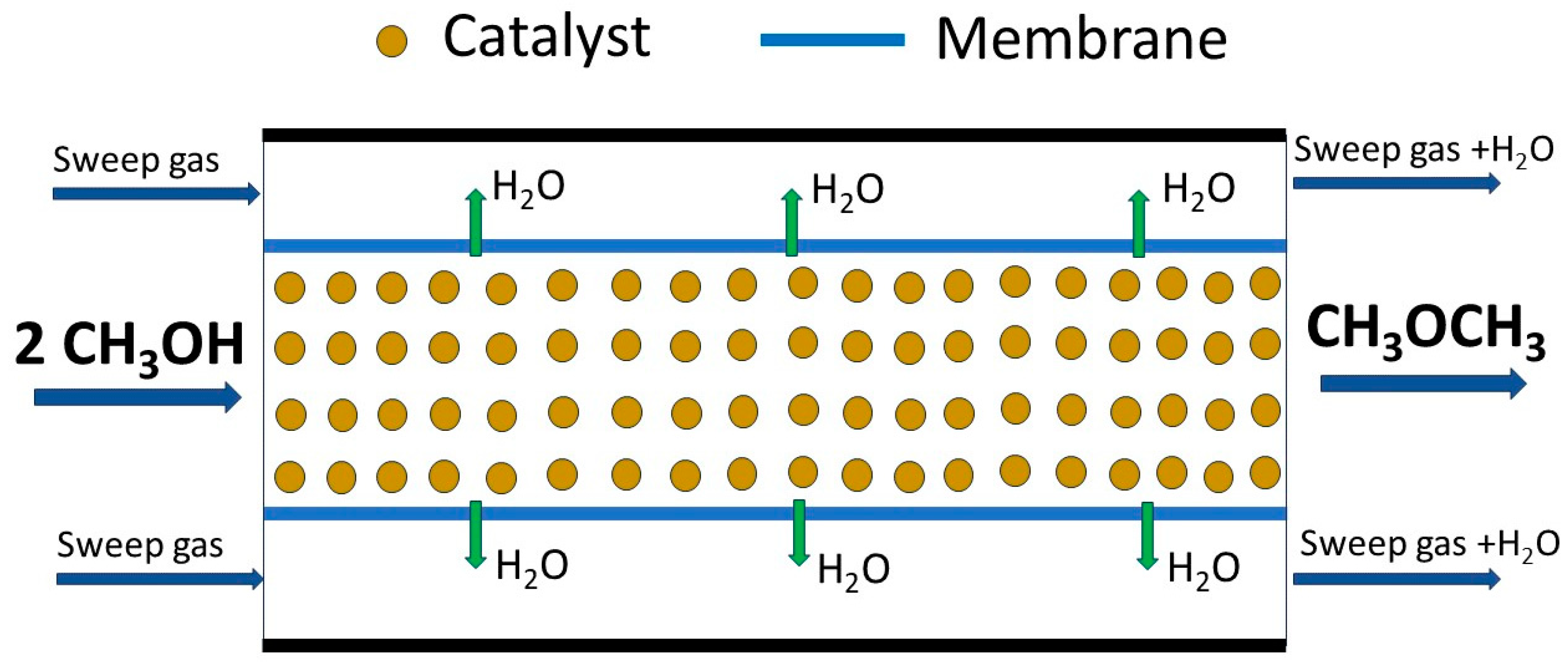
2.4. Membrane-Based Catalytic Systems
3. Summary and Perspectives
Funding
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| BAS | Brønsted acid sites |
| DME | dimethyl ether |
| DEE | diethyl ether |
| FT-IR | Fourier transform infrared spectroscopy |
| F-T | Fischer–Tropsch process |
| HMB | hexamethyl benzene |
| LAS | Lewis acid sites |
| LPG | liquefied petroleum gas |
| MFI | mordenite framework inverted (zeolites) |
| Mt | montmorillonite |
| MTD | methanol to dimethyl ether conversion |
| NH3-TPD | temperature-programmed desorption of ammonia |
| PCH | porous clay heterostructure |
| PILCs | pillared interlayered clays |
| TIE | template ion-exchange method, one cycle (TIE1) and two cycles (TIE2) |
| ULEV | ultra-low-emission vehicle |
| WHSV | weight hourly space velocity |
References
- Dumas, J.B.; Péligot, E.M. Mémoire sur l’esprit-de-bois et les divers composés éthéres qui en proviennent. Ann. Chim. Phys. 1835, 58, 5–74. [Google Scholar]
- Chai, M.; Chen, Z.; Nourozieh, H.; Yang, M.; Chai, B. Introduce dimethyl ether (DME) as a solvent for steam-assisted gravity drainage (SAGD) co-injection: An effective and environmental application. Fuel 2023, 341, 127639. [Google Scholar] [CrossRef]
- Naik, S.P.; Ryu, T.; Bui, V.; Miller, J.D.; Drinnan, N.B.; Zmierczak, W. Synthesis of DME from CO2/H2 gas mixture. Chem. Eng. J. 2011, 167, 362–368. [Google Scholar] [CrossRef]
- Herring, H. Energy Efficiency—A Critical Review. Energy 2006, 31, 10–20. [Google Scholar] [CrossRef]
- Sun, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Catalysis chemistry of dimethyl ether synthesis. ACS Catal. 2014, 4, 3346–3356. [Google Scholar] [CrossRef]
- Erik Rotheim—Famous Inventor. Available online: http://www.edubilla.com/inventor/erik-rotheim/ (accessed on 6 May 2024).
- Chen, W.H.; Lin, B.J.; Lee, H.M.; Huang, M.H. One-step synthesis of dimethyl ether from the gas mixture containing CO2 with high space velocity. Appl. Energy 2012, 98, 92–101. [Google Scholar]
- Dimethyl Ether Market Size—Global Industry, Share, Analysis, Trends and Forecast 2023–2032. Available online: https://www.acumenresearchandconsulting.com/dimethyl-ether-market (accessed on 6 May 2024).
- Mondal, U.; Yadav, G.D. Perspective of dimethyl ether as fuel: Part I. Catalysis. J. CO2 Util. 2019, 32, 299–320. [Google Scholar] [CrossRef]
- Brunetti, A.; Migliori, M.; Cozza, D.; Catizzone, E.; Giordano, G.; Barbieri, G. Methanol Conversion to Dimethyl Ether in Catalytic Zeolite Membrane Reactors. ACS Sustain. Chem. Eng. 2020, 8, 10471–10479. [Google Scholar] [CrossRef]
- Putrasari, Y.; Lim, O. Dimethyl Ether as the Next Generation Fuel to Control Nitrogen Oxides and Particulate Matter Emissions from Internal Combustion Engines: A Review. ACS Omega 2022, 7, 32–37. [Google Scholar] [CrossRef]
- Arcoumanis, C.; Bae, C.; Crookes, R.; Kinoshita, E. The potential of di-methyl ether (DME) as an alternative fuel for compression-ignition engines: A review. Fuel 2008, 87, 1014–1030. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Borup, R.L.; Greene, H.L. Dimethyl ether (DME) as an alternative fuel. J. Power Sources 2006, 156, 497–511. [Google Scholar] [CrossRef]
- Dimethyl Ether Market Size, Share, Industry, Forecast and Outlook (2023–2030). Available online: https://www.datamintelligence.com/research-report/dimethyl-ether-market (accessed on 6 May 2024).
- Zhou, C.; Wang, N.; Qian, Y.; Liu, X.; Caro, J.; Huang, A. Efficient Synthesis of Dimethyl Ether from Methanol in a Bifunctional Zeolite Membrane Reactor. Angew. Chem. Int. Ed. 2016, 55, 12678–12682. [Google Scholar] [CrossRef]
- Fedosov, D.; Smirnov, A.; Shkirskiy, V.; Voskoboynikov, T.; Ivanova, I. Methanol dehydration in NaA zeolite membrane reactor. J. Membr. Sci. 2015, 486, 189–194. [Google Scholar] [CrossRef]
- Farsi, M.; Jahanmiri, A. Enhancement of DME Production in an Optimized Membrane Isothermal Fixed-Bed Reactor. Int. J. Chem. React. Eng. 2011, 9, A74. [Google Scholar] [CrossRef]
- Volkov, V.; Novitskii, E.; Dibrov, G.; Samokhin, P.; Kipnis, M.; Yaroslavtsev, A. Catalytic conversion of methanol to dimethyl ether on polymer/ceramic composite membranes. Catal. Today 2012, 193, 31–36. [Google Scholar] [CrossRef]
- Iliuta, I.; Larachi, F.; Fongarland, P. Dimethyl Ether Synthesis with in situ H2O Removal in Fixed-Bed Membrane Reactor: Model and Simulations. Ind. Eng. Chem. Res. 2010, 49, 6870–6877. [Google Scholar] [CrossRef]
- Baracchini, G.; Klumpp, M.; Arnold, P.; Dittmeyer, R. Direct synthesis of dimethyl ether: A simulation study on the influence of the catalyst configuration. Chem. Eng. J. 2020, 396, 125155. [Google Scholar] [CrossRef]
- Moghaddam, A.L.; Ghavipour, M.; Kopyscinski, J.; Hazlett, M.J. Methanol dehydration to dimethyl ether over KFI zeolites. Effect of template concentration and crystallization time on catalyst properties and activity. Appl. Catal. A Gen. 2024, 158, 119594. [Google Scholar] [CrossRef]
- Luque-Álvarez, L.A.; González-Arias, J.; Romero-Sarria, F.; Reina, T.R.; Bobadilla, L.F.; Odriozola, J.A. Mechanistic insights into methanol carbonylation to methyl acetate over an efficient organic template-free Cu-exchanged mordenite. Catal. Sci. Technol. 2024, 15, 128–136. [Google Scholar] [CrossRef]
- Leonzio, G. State of art and perspectives about the production of methanol, dimethyl ether and syngas by carbon dioxide hydrogenation. J. CO2 Util. 2018, 27, 326–354. [Google Scholar] [CrossRef]
- Li, H.; Ren, S.; Zhang, S.; Padinjarekutt, S.; Sengupta, B.; Liang, X.; Lic, S.; Yu, M. The high-yield direct synthesis of dimethyl ether from CO2 and H2 in a dry reaction environment. J. Mater. Chem. A 2021, 9, 2678–2682. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazabal, G.O.; Perez-Ramırez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar]
- Waugh, K.C. Methanol Synthesis. Catal. Lett. 2012, 142, 1153–1166. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar]
- Peinado, C.; Liuzzi, D.; Retuerto, M.; Boon, J.; Peña, M.A.; Rojas, S. Study of catalyst bed composition for the direct synthesis of dimethyl ether from CO2-rich syngas. Chem. Eng. J. Adv. 2020, 4, 100039. [Google Scholar] [CrossRef]
- Bakhtyari, A.; Parhoudeh, M.; Rahimpour, M.R. Optimal conditions in converting methanol to dimethyl ether, methyl formate, and hydrogen utilizing a double membrane heat exchanger reactor. J. Nat. Gas Sci. Eng. 2016, 28, 31–45. [Google Scholar] [CrossRef]
- Akarmazyan, S.S.; Panagiotopoulou, P.; Kambolis, A.; Papadopoulou, C.; Kondarides, D.I. Methanol dehydration to dimethylether over Al2O3 catalysts. Appl. Catal. B Environ. 2014, 145, 136–148. [Google Scholar] [CrossRef]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-liquid via synthesis of methanol, DME or Fischer–Tropsch-fuels: A review. Energy Environ. Sci. 2020, 13, 3207–3252. [Google Scholar] [CrossRef]
- Kansha, Y.; Ishizuka, M.; Song, C.; Tsutsumi, A. An innovative dimethyl ether (DME) production using self-heat recuperation. Chem. Eng. Trans. 2014, 39, 109–114. [Google Scholar]
- Banivaheb, S.; Pitter, S.; Delgado, K.H.; Rubin, M.; Sauer, J.; Dittmeyer, R. Recent Progress in Direct DME Synthesis and Potential of Bifunctional Catalysts. Chemie Ingenieur Technik 2022, 94, 240–255. [Google Scholar] [CrossRef]
- Gao, X.-Y.; Jiao, W.-H.; Zuo, Z.-J.; Gao, Z.-H.; Huang, W. DME synthesis from methanol over hydrated γ-Al2O3(110) surface in slurry bed using continuum and atomistic models. J. Theor. Comp. Chem. 2017, 16, 1750029. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Ying, W.; Fang, D. Dehydration of methanol to dimethyl ether over γ-Al2O3 catalyst: Intrinsic kinetics and effectiveness factor. Can. J. Chem. Eng. 2013, 91, 1538–1546. [Google Scholar] [CrossRef]
- Ardy, A.; Rizkiana, J.; Gunawan, M.L.; Susanto, H. Characterization and Catalytic Activity of γ-Al2O3-ITB on Methanol Dehydration. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012054. [Google Scholar] [CrossRef]
- Sung, D.M.; Kim, Y.H.; Park, E.D.; Yie, J.E. Correlation between acidity and catalytic activity for the methanol dehydration over various aluminum oxides. Res. Chem. Intermed. 2010, 36, 653–660. [Google Scholar] [CrossRef]
- Keshavarz, A.R.; Rezaei, M.; Yaripour, F. Preparation of nanocrystalline γ-Al2O3 catalyst using different procedures for methanol dehydration to dimethyl ether. J. Nat. Gas Chem. 2011, 20, 334–338. [Google Scholar] [CrossRef]
- Lertjiamratn, K.; Praserthdam, P.; Arai, M.; Panpranot, J. Modification of acid properties and catalytic properties of AlPO4 by hydrothermal pretreatment for methanol dehydration to dimethyl ether. Appl. Catal. A Gen. 2010, 378, 119–123. [Google Scholar] [CrossRef]
- Seo, C.W.; Jung, K.D.; Lee, K.Y.; Yoo, K.S. Influence of Structure Type of Al2O3 on Dehydration of Methanol for Dimethyl Ether Synthesis. Ind. Eng. Chem. Res. 2008, 47, 6573–6578. [Google Scholar] [CrossRef]
- Xu, M.; Lunsford, J.H.; Goodman, D.W.; Bhattacharyya, A. Synthesis of dimethyl ether (DME) from methanol over solid-acid catalysts. Appl. Catal. A Gen. 1997, 149, 289–301. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; Rooney, D.W.; Halawy, S.A.; Mohamed, M.A.; Abdelkader, A. Effect of precursor on the performance of alumina for the dehydration of methanol to dimethyl ether. Appl. Catal. B Environ. 2012, 127, 307–315. [Google Scholar] [CrossRef]
- Bateni, H.; Able, C. Development of Heterogeneous Catalysts for Dehydration of Methanol to Dimethyl Ether: A Review. Catal. Ind. 2019, 11, 7–33. [Google Scholar] [CrossRef]
- Rahmanpour, O.; Shariati, A.; Nikou, M.R.K. New Method for Synthesis Nano Size γ-Al2O3 Catalyst for Dehydration of Methanol to Dimethyl Ether. Int. J. Chem. Eng. Appl. 2012, 3, 125–128. [Google Scholar] [CrossRef]
- Keshavarz, A.R.; Rezaei, M.; Yaripour, F. Nanocrystalline gamma-alumina: A highly active catalyst for dimethyl ether synthesis. Powder Technol. 2010, 199, 176–179. [Google Scholar] [CrossRef]
- Hosseini, Z.; Taghizadeh, M.; Yaripour, F. Synthesis of nanocrystalline γ-Al2O3 by sol-gel and precipitation methods for methanol dehydration to dimethyl ether. J. Nat. Gas Chem. 2011, 20, 128–134. [Google Scholar] [CrossRef]
- Mollavali, M.; Yaripour, F.; Atashi, H.; Sahebdelfar, S. Intrinsic Kinetics Study of Dimethyl Ether Synthesis from Methanol on γ-Al2O3 Catalysts. Ind. Eng. Chem. Res. 2008, 47, 3265–3273. [Google Scholar] [CrossRef]
- Sahebdelfar, S.; Bijani, P.M.; Yaripour, F. Deactivation kinetics of γ-Al2O3 catalyst in methanol dehydration to dimethyl ether. Fuel 2022, 310, 122443. [Google Scholar] [CrossRef]
- Raoof, F.; Taghizadeh, M.; Eliassi, A.; Yaripour, F. Effects of temperature and feed composition on catalytic dehydration of methanol to dimethyl ether over γ-alumina. Fuel 2008, 87, 2967–2971. [Google Scholar] [CrossRef]
- Boon, J.; Kampen, J.; Hoogendoorn, R.; Tanase, S.; Berkel, F.P.F.; Sint Annaland, M. Reversible deactivation of γ-alumina by steam in the gas-phase dehydration of methanol to dimethyl ether. Catal. Commun. 2019, 119, 22–27. [Google Scholar] [CrossRef]
- Yaripour, F.; Shariatinia, Z.; Sahebdelfar, S.; Irandoukht, A. The effects of synthesis operation conditions on the properties of modified γ-alumina nanocatalysts in methanol dehydration to dimethyl ether using factorial experimental design. Fuel 2015, 139, 40–50. [Google Scholar] [CrossRef]
- Nogueira, D.J.; Arl, M.; Köerich, J.S.; Simioni, C.; Ouriques, L.C.; Vicentini, D.S.; Matias, W.G. Comparison of cytotoxicity of α-Al2O3 and η-Al2O3 nanoparticles toward neuronal and bronchial cells. Toxicol. In Vitro 2019, 61, 104596. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K. Kinetic Investigation of η-Al2O3 Catalyst for Dimethyl Ether Production. Catal. Lett. 2018, 148, 1236–1245. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; Abdelkader, A.; Hassan, N.M.; Laffir, F.; McLaren, M.; Rooney, D. Silver-Modified η-Al2O3 Catalyst for DME Production. J. Phys. Chem. C 2017, 121, 25018–25032. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; Rooney, D.W.; Thompson, J.; Halawy, S.A.; Mohamed, M.A. Surface hydrophobicity and acidity effect on alumina catalyst in catalytic methanol dehydration reaction. J. Chem. Technol. Biotechnol. 2017, 92, 2952–2962. [Google Scholar] [CrossRef]
- Jo, H.; Jung, H.; Park, J.; Jung, K.-D. Surface Modification of η–Al2O3 by SiO2 Impregnation to Enhance Methanol Dehydration Activity. Bull. Korean Chem. Soc. 2017, 38, 307–312. [Google Scholar] [CrossRef]
- Khaleel, A. Titanium-doped alumina for catalytic dehydration of methanol to dimethyl ether at relatively low temperatures. Fuel 2011, 90, 2422–2427. [Google Scholar] [CrossRef]
- Liu, D.; Yao, C.; Zhang, J.; Fang, D.; Chen, D. Catalytic dehydration of methanol to dimethyl ether over modified γ-Al2O3 catalyst. Fuel 2011, 90, 1738–1742. [Google Scholar] [CrossRef]
- Khaleel, A.; Ahmed, M.; Sowaid, S.B. Ti-doped γ-Al2O3 versus ZSM5 zeolites for methanol to dimethyl ether conversion: In-situ DRIFTS investigation of surface interactions and reaction mechanism. Colloid. Surf. A Physicochem. Eng. Asp. 2019, 571, 174–181. [Google Scholar] [CrossRef]
- Vishwanathan, V.; Jun, K.W.; Kim, J.W.; Roh, H.S. Vapour phase dehydration of crude methanol to dimethyl ether over Na-modified H-ZSM-5 catalysts. Appl. Catal. A Gen. 2004, 276, 251–255. [Google Scholar] [CrossRef]
- Hassanpour, S.; Taghizadeh, M.; Yaripour, F. Preparation, Characterization, and Activity Evaluation of H-ZSM-5 Catalysts in Vapor-Phase Methanol Dehydration to Dimethyl Ether. Ind. Eng. Chem. Res. 2010, 49, 4063–4069. [Google Scholar] [CrossRef]
- Rownaghi, A.A.; Rezaei, F.; Stante, M.; Hedlund, J. Selective dehydration of methanol to dimethyl ether on ZSM-5 nanocrystals. Appl. Catal. B Environ. 2012, 119–120, 56–61. [Google Scholar] [CrossRef]
- Hassanpour, S.; Yaripour, F.; Taghizadeh, M. Performance of modified H-ZSM-5 zeolite for dehydration of methanol to dimethyl ether. Fuel Process. Technol. 2010, 91, 1212–1221. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.T.; Zhang, C.; Kwak, G.; Jun, K.-W. Effect of Reaction Conditions on the Catalytic Dehydration of Methanol to Dimethyl Ether Over a K-modified HZSM-5 Catalyst. Catal. Lett. 2017, 147, 792–801. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Arundhathi, R.; Kasture, M.W.; Samanta, C.; Vankayala, R.; Thota, C. Temperature-dependent synthesis of dimethyl ether (DME) from methanol over beta zeolite: A novel approach to a sustainable fuel. R. Soc. Open Sci. 2023, 10, 230524. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Macina, D.; Mirocha-Kubień, N.; Piwowarska, Z.; Chmielarz, L. Hierarchically structured ZSM-5 obtained by desilication as new catalyst for DME synthesis from methanol. Appl. Catal. B Environ. 2015, 174–175, 336–343. [Google Scholar]
- Dalena, F.; Giglio, E.; Giorgianni, G.; Cozza, D.; Marino, A.; Aloise, A. DME Production via Methanol Dehydration with H form and Desilicated ZSM-5 Type Zeolitic Catalysts: Study on the Correlation Between Acid Sites and Conversion. Chem. Eng. Trans. 2021, 84, 211–216. [Google Scholar]
- Aloise, A.; Marino, A.; Dalena, F.; Giorgianni, G.; Migliori, M.; Frusteri, L.; Cannilla, C.; Bonura, G.; Frusteri, F.; Giordano, G. Desilicated ZSM-5 zeolite: Catalytic performances assessment in methanol to DME dehydration. Micropor. Mesopor. Mater. 2020, 302, 110198. [Google Scholar] [CrossRef]
- Jin, D.; Zhu, B.; Hou, Z.; Fei, J.; Lou, H.; Zheng, X. Dimethyl ether synthesis via methanol and syngas over rare earth metals modified zeolite Y and dual Cu–Mn–Zn catalysts. Fuel 2007, 86, 2707–2713. [Google Scholar] [CrossRef]
- Fei, J.; Hou, Z.; Zhu, B.; Lou, H.; Zheng, X. Synthesis of dimethyl ether (DME) on modified HY zeolite and modified HY zeolite-supported Cu–Mn–Zn catalysts. Appl. Catal. A Gen. 2006, 304, 49–54. [Google Scholar] [CrossRef]
- Moradi, G.R.; Yaripour, F.; Vale-Sheyda, P. Catalytic dehydration of methanol to dimethyl ether over mordenite catalysts. Fuel Process. Technol. 2010, 91, 461–468. [Google Scholar] [CrossRef]
- Catizzone, E.; Aloise, A.; Migliori, M.; Giordano, G. From 1-D to 3-D zeolite structures: Performance assessment in catalysis of vapour-phase methanol dehydration to DME. Micropor. Mesopor. Mater. 2017, 243, 102–111. [Google Scholar] [CrossRef]
- Migliori, M.; Catizzone, E.; Aloise, A.; Bonura, G.; Gómez-Hortigüela, L.; Frusteri, L.; Cannilla, C.; Frusteri, F.; Giordano, G. New insights about coke deposition in methanol-to-DME reaction over MOR-, MFI- and FER-type zeolites. J. Ind. Eng. Chem. 2018, 68, 196–208. [Google Scholar] [CrossRef]
- Zheng, J.; Ma, J.; Wang, Y.; Bai, Y.; Zhang, X.; Li, R. Synthesis and Catalytic Property of a Zeolite Composite for Preparation of Dimethyl Ether from Methanol Dehydration. Catal. Lett. 2009, 130, 672–678. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, H.; Zheng, Y.; Wang, J.; Li, H.; Zhang, J. Catalytic dehydration of methanol to dimethyl ether over micro–mesoporous ZSM-5/MCM-41 composite molecular sieves. Appl. Catal. A Gen. 2012, 413–414, 36–42. [Google Scholar] [CrossRef]
- Ulfah, M.; Octavia, S.; Suherman, H.; Laniwati, M.; Makerthiharta, I.G.B.N.; Subagjo. Synthesis and characterization of modified γ-Alumina-NaA and γ-Alumina-NaX zeolite composites as methanol dehydration catalysts in synthesis Dimethyl Ether (DME). IOP Conf. Ser. Mater. Sci. Eng. 2020, 823, 012010. [Google Scholar] [CrossRef]
- Catizzone, E.; Aloise, A.; Giglio, E.; Ferrarelli, G.; Bianco, M.; Migliori, M.; Giordano, G. MFI vs. FER zeolite during methanol dehydration to dimethyl ether: The crystal size plays a key role. Catal. Commun. 2021, 149, 106214. [Google Scholar] [CrossRef]
- Rutkowska, M.; Macina, D.; Piwowarska, Z.; Gajewska, M.; Díaz, U.; Chmielarz, L. Hierarchically structured ZSM-5 obtained by optimized mesotemplate-free method as active catalyst for methanol to DME conversion. Catal. Sci. Technol. 2016, 6, 4849–4862. [Google Scholar] [CrossRef]
- Abbasian, S.; Taghizadeh, M. Preparation of H-ZSM-5 Nano-Zeolite Using Mixed Template Method and its Activity Evaluation for ethanol to DME Reaction. Int. J. Nanosci. Nanotechnol. 2014, 10, 171–180. [Google Scholar]
- Aboul-Fotouh, S.M.K.; Aboul-Gheit, N.A.K.; Naghmash, M.A. Dimethylether production on zeolite catalysts activated by Cl-, F- and/or ultrasonication. J. Fuel Chem. Technol. 2016, 44, 428–436. [Google Scholar] [CrossRef]
- Dennis-Smither, B.J.; Yang, Z.; Buda, C.; Liu, X.; Sainty, N.; Tan, X.; Sunley, G.J. Getting zeolite catalysts to play your tune: Methyl carboxylate esters as switchable promoters for methanol dehydration to DME. Chem. Commun. 2019, 55, 13804–13807. [Google Scholar] [CrossRef]
- Masih, D.; Rohani, D.; Kondo, J.N.; Tatsumi, T. Low-temperature methanol dehydration to dimethyl ether over various small-pore zeolites. Appl. Catal. B Environ. 2017, 217, 247–255. [Google Scholar] [CrossRef]
- Catizzone, E.; Aloise, A.; Migliori, M.; Giordano, G. The effect of FER zeolite acid sites in methanol-to-dimethyl-ether catalytic dehydration. J. Energy Chem. 2016, 26, 406–415. [Google Scholar] [CrossRef]
- Marosz, M.; Samojeden, B.; Kowalczyk, A.; Rutkowska, M.; Motak, M.; Díaz, U.; Palomares, A.E.; Chmielarz, L. MCM-22, MCM-36, and ITQ-2 Zeolites with Different Si/Al Molar Ratios as Effective Catalysts of Methanol and Ethanol Dehydration. Materials 2020, 13, 2399. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanpour, A.; Rimer, J.D.; Grabow, L.C. Computational Assessment of the Dominant Factors Governing the Mechanism of Methanol Dehydration over H-ZSM-5 with Heterogeneous Aluminum Distribution. ACS Catal. 2016, 6, 2287–2298. [Google Scholar] [CrossRef]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef]
- Święs, A.; Kowalczyk, A.; Gil, B.; Chmielarz, L. Dehydration of methanol and ethanol over ferrierite originated layered zeolites—The role of acidity and porous structure. RSC Adv. 2022, 12, 9395–9403. [Google Scholar] [CrossRef]
- Chmielarz, L.; Dziembaj, R. Modified Layered Silicas as Catalysts for Conversion of Nitrogen Pollutants in Flue Gases—A Review. Catalysts 2021, 11, 644. [Google Scholar] [CrossRef]
- Silva, A.C.; Ciuffi, K.J.; Reis, M.J.; Calefi, P.S. Emerson Henrique de Faria, Influence of physical/chemical treatments to delamination of nanohybrid kaolinite-dipicolinate. Appl. Clay Sci. 2016, 126, 251–258. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kowalczyk, A.; Wojciechowska, M.; Boroń, P.; Dudek, M.; Michalik, M. Montmorillonite Intercalated with SiO2, SiO2-Al2O3 or SiO2-TiO2 Pillars by Surfactant-Directed Method as Catalytic Supports for DeNOx Process. Chem. Pap. 2014, 68, 1219–1227. [Google Scholar] [CrossRef]
- Chmielarz, L.; Piwowarska, Z.; Kuśtrowski, P.; Węgrzyn, A.; Gil, B.; Kowalczyk, A.; Dudek, B.; Dziembaj, R.; Michalik, M. Comparison Study of Titania Pillared Interlayered Clays and Porous Clay Heterostructures Modified with Copper and Iron as Catalysts of the DeNOx Process. Appl. Clay Sci. 2011, 53, 164–173. [Google Scholar] [CrossRef]
- Marosz, M.; Kowalczyk, A.; Chmielarz, L. Modified vermiculites as effective catalysts for dehydration of methanol and ethanol. Catal. Today 2020, 355, 466–475. [Google Scholar] [CrossRef]
- Marosz, M.; Kowalczyk, A.; Gil, B.; Chmielarz, L. Acid-treated Clay Minerals as Catalysts for Dehydration of Methanol and Ethanol. Clays Clay Miner. 2020, 68, 23–371. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Piwowarska, Z.; Dudek, B.; Gil, B.; Michalik, M. Montmorillonite, vermiculite and saponite based porous clay heterostructures modified with transition metals as catalysts for the DeNOx process. Appl. Catal. B Environ. 2009, 88, 331–340. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kowalczyk, A.; Skoczek, M.; Rutkowska, M.; Gil, B.; Natkański, P.; Radko, M.; Motak, M.; Dębek, R.; Ryczkowski, J. Porous clay heterostructures intercalated with multicomponent pillars as catalysts for dehydration of alcohols. Appl. Clay Sci. 2018, 160, 116–125. [Google Scholar] [CrossRef]
- Kikuchi, E. Palladium/ceramic membranes for selective hydrogen permeation and their application to membrane reactor. Catal. Today 1995, 25, 333–337. [Google Scholar] [CrossRef]
- Lina, Y.M.; Leeb, G.L.; Rei, M.H. An integrated purification and production of hydrogen with a palladium membrane–catalytic reactor. Catal. Today 1998, 44, 343–349. [Google Scholar] [CrossRef]
- Shu, J.; Grandjean, B.P.A.; Kaliaguine, S. Methane steam reforming in asymmetric Pd–Ag and Pd–Ag/porous SS membrane reactors. Appl. Catal. 1994, 119, 305–325. [Google Scholar] [CrossRef]
- Uemiya, S.; Sato, N.; Ando, H.; Matsuda, T.; Kikuchi, E. Steam reforming of methane in a hydrogen-permeable membrane reactor. Appl. Catal. 1991, 67, 223–230. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Lotfinejad, M. Enhancement of Methanol Production in a Membrane Dual-Type Reactor. Chem. Eng. Technol. 2007, 30, 1062–1076. [Google Scholar] [CrossRef]
- Khassin, A.A.; Sipatrov, A.G.; Yurieva, T.M.; Chermashentseva, G.K.; Rudina, N.A.; Parmon, V.N. Performance of a catalytic membrane reactor for the Fischer–Tropsch synthesis. Catal. Today 2005, 105, 362–366. [Google Scholar] [CrossRef]
- Mohammed, M.B.A.; Wagialla, K.M.E. Effect of In-situ Membrane Removal of H2O on Methanol Conversion during Dimethyl Ether Synthesis Reaction. Int. J. Sci. Basic Appl. Res. 2016, 27, 42–52. [Google Scholar]
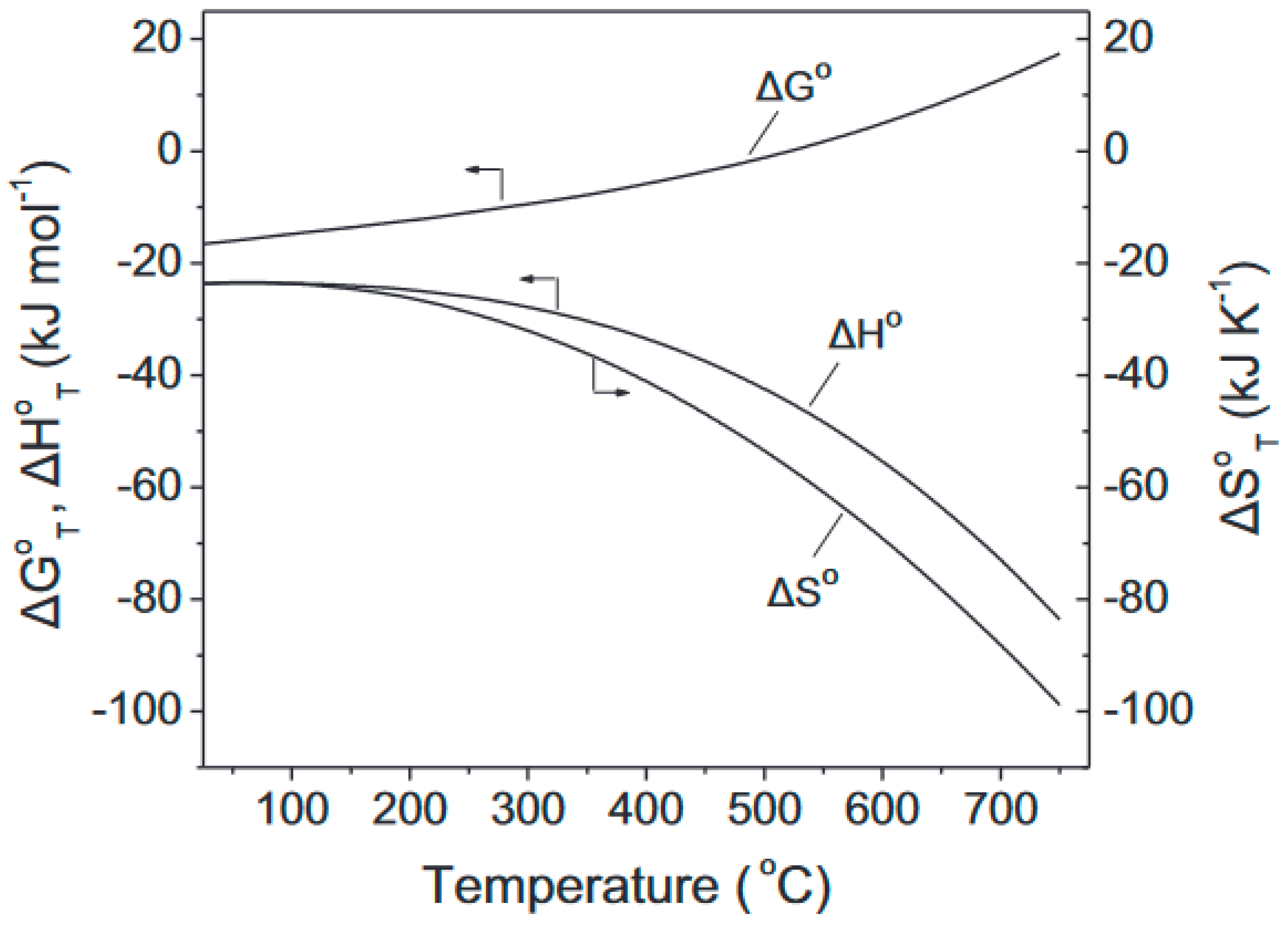
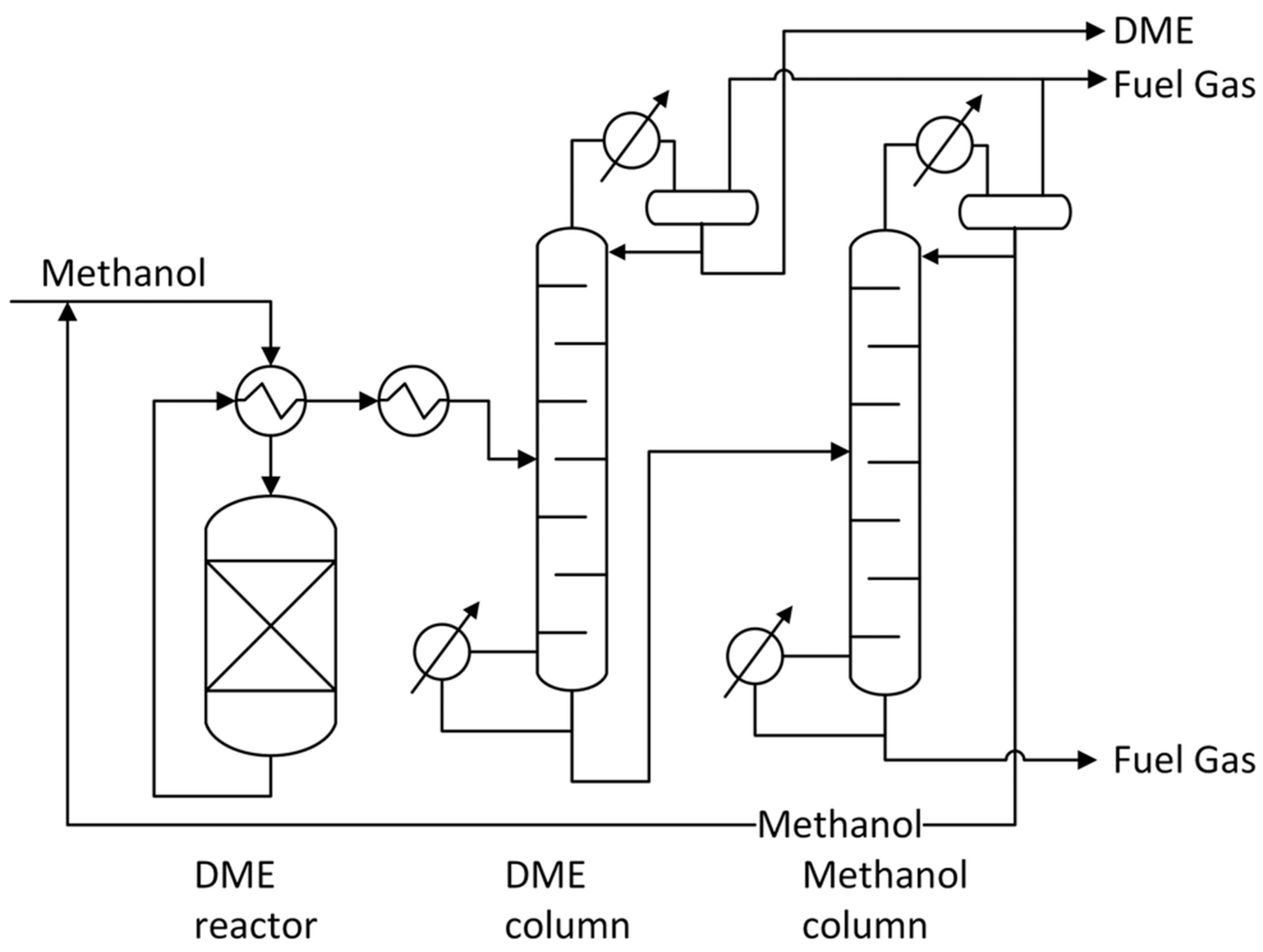
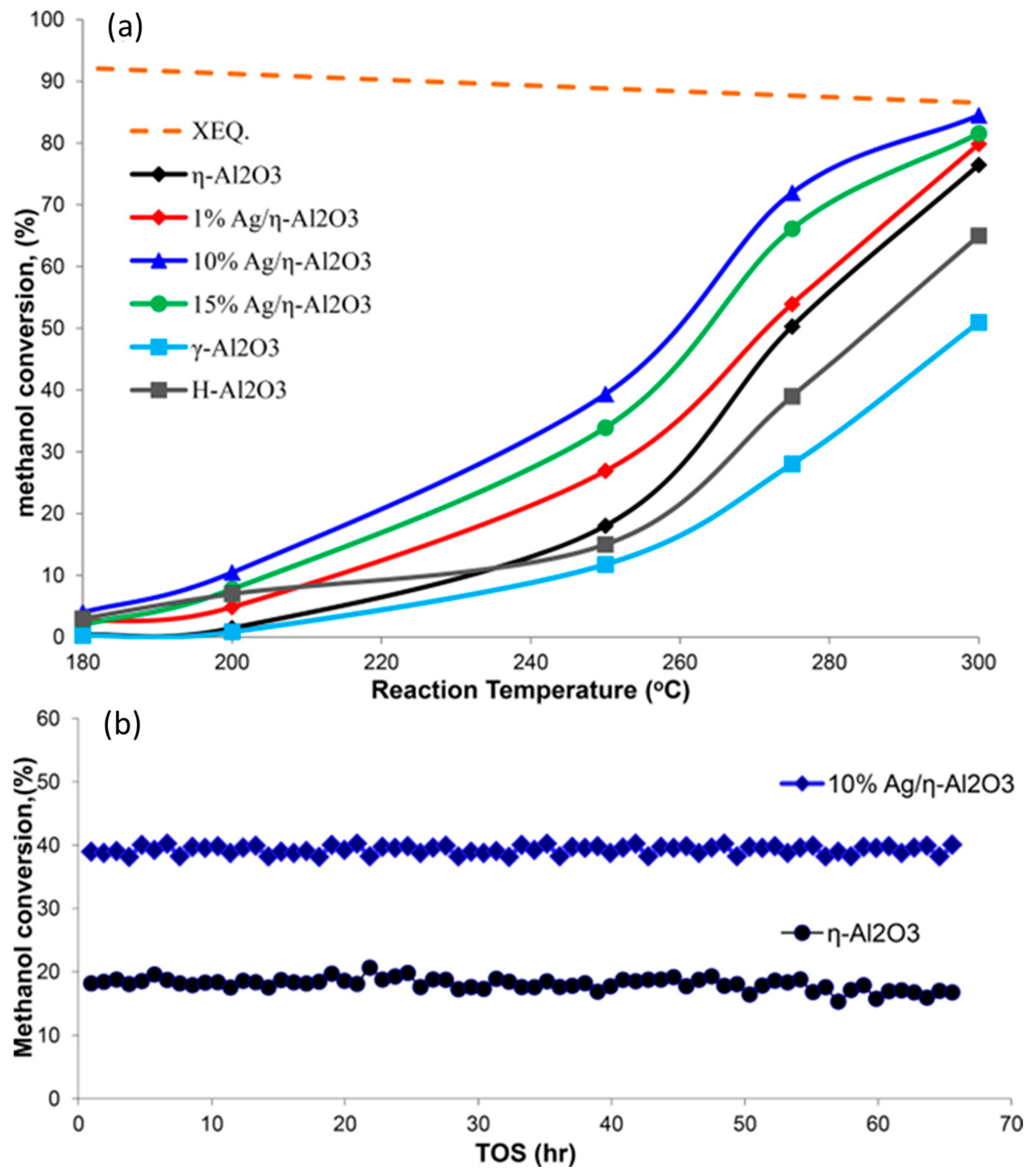
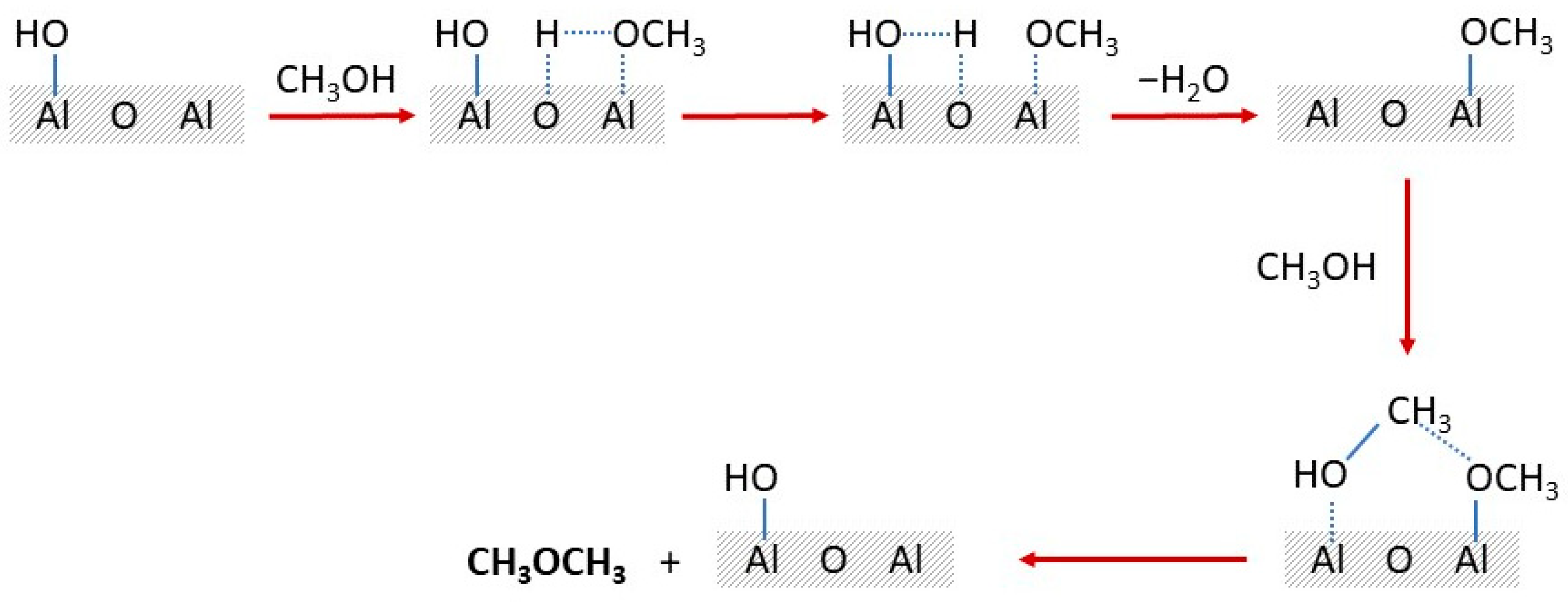


| Catalyst | Modifications/Properties | Catalytic Properties in MTD Process | Ref. |
|---|---|---|---|
| γ-Al2O3 | High surface concentration of acid sites and catalytic activity. | [37] | |
| η-Al2O3 | Lower concentration of acid sites compared to γ-Al2O3, but better catalytic activity. | [37] | |
| α-Al2O3 | Low concentration of acid sites and low catalytic activity. | [37] | |
| κ-Al2O3 | Low concentration of acid sites and low catalytic activity. | [37] | |
| γ-Al2O3 | Nano-crystallites; increased contribution of weak and medium acid sites. | Relatively high catalytic activity and resistance for coking. | [38] |
| γ-Al2O3 | High catalytic activity in the range of 150–325 °C. Formation of CO and CH4 at higher temperatures | [30] | |
| γ-Al2O3 | Various calcination temperatures of alumina precursors. | High contribution of weak and medium acid sites of the Lewis type active in the MTD process. | [40] |
| η-Al2O3 | Various calcination temperatures of alumina precursors. | High contribution of weak and medium acid sites of the Lewis type, but lower activity compared to γ-Al2O3. | [40] |
| γ-Al2O3 | Nano-crystalline (1–2 nm) obtained by ultrasonic-assisted precipitation. | Catalyst operating in the range of 270–380 °C. Optimum activity at 320 °C and a WHSV of 15 h−1. | [44] |
| γ-Al2O3 | Synthesised by a modified sol–gel method using cationic surfactants, resulting in nano-crystalline (about 3.9 nm), mesoporous material. | Small crystallites possess a higher concentration of medium acidic sites. Nano-crystalline alumina was more active than conventional alumina in the broad range of the WHSV (1.75–9.62 h−1). | [45] |
| γ-Al2O3 | Synthesised by a modified sol–gel method in non-aqueous solvent. | Improved catalytic performance compared to the classical sol–gel method. | [46] |
| γ-Al2O3 | Synthesised in the form of nano-size material (1–2 nm) using precipitation method under ultrasonic vibration. | About 90% of weak and medium acid sites active in the MTD process. | [44] |
| η-Al2O3 | Synthesis started from aluminium nitrate solution precipitated by ammonia solution resulted in fine particles of about 5.5 nm. | Improved catalytic performance compared to γ-Al2O3. Sensitive for water presence below 250 °C. | [42,52,54] |
| Ag/η-Al2O3 | Silver deposited by impregnation. | Increase in methanol conversion by about 10% at 300 °C for η-Al2O3 modified with 10 wt.% Ag. | [55] |
| Cu/γ-Al2O3 | Deposition of copper by wet-impregnation method with the aid of sonication. | Introduction of 6 wt.% Cu increased the acid site concentrations by nearly 34% and catalytically activated alumina. | [55] |
| SiO2/Al2O3 | Obtained by the deposition of silica on η-Al2O3 by the impregnation method. | The best catalytic results for the samples doped with 0.5 wt.% SiO2, explained by the increased surface area and Brønsted-type acidity. | [56] |
| Al2O3-SiO2 | Obtained by the precipitation method in the form of fine particles. | The best catalytic results were obtained for the sample doped with 2 wt.% SiO2. | [56] |
| TiO2/γ-Al2O3 | Deposition of TiO2 (3–20 wt.%) on γ-Al2O3. | The best catalytic results were obtained for the sample doped with 3 wt.% TiO2. Generation of additional weak acid sites by Ti4+. | [57] |
| Nb2O5//γ-Al2O3 | Deposition of Nb2O5 (1–10 wt.%) on γ-Al2O3 by the impregnation method. | The best catalytic results were obtained for the sample doped with 10 wt.% Nb2O5, which generated weak acid sites. | [58] |
| ZSM-5 | Better catalytic activity than γ-Al2O3 at 230 °C. Above 270 °C, lower selectivity and stability (coke formation). | [60] | |
| ZSM-5 | Zeolites with Si/Al in the range of 25,250. | The highest acid site concentrations and catalytic activity for Si/Al = 125. | [61] |
| ZSM-5 | Nano-crystalline material (about 120 nm). | Improved activity in comparison to classical zeolite. Better accessibility of acid sites in nano-crystalline ZSM-5. | [61] |
| ZSM-5 | Zeolite composed of loosely sticked nano-size crystals (10–20 nm) with the enhanced internal diffusion in inter-crystal spaces. | Improved reaction to DME in relation to conventional ZSM-5. | [78] |
| ZSM-5 | Nano-zeolites (Si/Al = 125, crystallite size about 27 nm) synthesised by the hydrothermal method. | Higher catalytic activity compared to conventional ZSM-5, explained by the increased concentration of acid sites. | [79] |
| ZSM-5 | Modification ZSM-5 with sodium. | Improved selectivity to DME in the range of 230–340 °C. | [60] |
| ZSM-5 | Modification ZSM-5 with sodium. | Mainly strong acid sites were neutralised by sodium. Limited coke formation. | [63] |
| ZSM-5 | Modification of H-ZSM-5 with a KNO3 solution by the wet-impregnation method. | Increased selectivity (nearly 100%) to DME. | [63] |
| ZSM-5 | Alkaline treatment resulted in mesopore formation. | In comparison to classical ZSM-5, improved catalytic properties and limited carbon deposit formation. | [66] |
| ZSM-5 | Alkaline treatment resulted in mesopore formation and modification of acidic properties. | In comparison to classical ZSM-5, improved catalytic properties and limited carbon deposit formation. | [67] |
| ZSM-5 Mordenite Y zeolite | Zeolites in protonic forms were impregnated with ammonium chloride or ammonium fluoride and ultrasonically treated. | Fluorination of H-ZSM-5 and H-MOR resulted in their activation. Chlorination activated H-Y. Ultrasonic treatment of chlorinated zeolites additionally improved their catalytic activity, while the opposite effect was observed for fluorinated zeolites. | [80] |
| Beta zeolite | Strong acid sites promoted higher hydrocarbon formation. Increase in reaction temperature (280–450 °C) increased methanol conversion and decreased DME selectivity. | [65] | |
| Y zeolite | Lower activity and stability than ZSM-5. | [69] | |
| Y zeolite | Introduction of Zr and Ni (1.8–2.0 wt.%) by the ion-exchange method. | Increased stability due to limited coke formation compared to non-modified Y zeolite. | [70] |
| Y zeolite | Introduction of Fe, Co, or Cr by the ion-exchange method. | Decreased stability due to the increased coke formation compared to non-modified Y zeolite. Explained by the formation of additional strong acid sites. | [70] |
| Mordenite | Catalytic activity at low temperature (220 °C) but limited stability due to coke formation. | [72,73] | |
| Ferrierite | Catalytic activity at low temperature (220 °C) and high stability. | [72,73] | |
| Ferrierite | Zeolites (Si/Al = 11) prepared in the form of nano- (0.1–0.3 µm) and micro (3–10 µm)-crystallites. | Increased activity of nano-zeolites compared to micro-zeolites. Explained by the improved internal diffusion rate. | [74] |
| Beta Mordenite | Composite material consisting of beta zeolite and mordenite. | More active than the mechanical mixture of beta and mordenite. Over 90% of methanol conversion with almost 100% selectivity to DME in range of 200–275 °C. Limited coking of the catalyst. | [74] |
| ZSM-5-MCM-41 | Composite material consisting of ZSM-5 and MCM-41, which combined the advantages of microporous zeolite. | Methanol conversion, like in the case of ZSM-5 (170–310 °C), but higher selectivity to DME and improved stability. | [75] |
| MCM-22 | Microporous MCM-22 as well as its delaminated (ITQ-2) and silica intercalated (MCM-36) forms were prepared. | It was postulated that the surface concentration and strength of the acid sites are dominant factors determining the catalytic activity, while the porous structure is less important. On the other hand, the open structures of ITQ-2 and MCM-36 were more resistant for coking. | [84] |
| Ferrierite | Microporous ferrierite as well as its delaminated (ITQ-6) and silica intercalated (ITQ-36) forms were prepared. | The surface acidity of zeolite materials is crucial for their catalytic performance, while a porous structure is significantly less important. | [87] |
| Vermiculite | Acid treatment (HNO3—2, 8, 24 h) resulted in development of its porosity and the decrease in surface acidity. | Activation effect was observed for acid-treated vermiculites. | [92] |
| Allophane Palygorskite Sepiolite | Minerals were treated with a solution of HNO3 for 2, 8, and 24 h. | Acid treatment increased catalytic activity of palygorskite and sepiolite, and slightly decreased the activity of allophane. | [93] |
| PCHs | PCHs were obtained by the intercalation of montmorillonite with SiO2, SiO2-Al2O3, SiO2-TiO2, and SiO2-ZrO2 pillars by the surfactant-directed method. | The best results were obtained for the PCH sample intercalated with SiO2-Al2O3 pillars, as well as the PCH sample intercalated with SiO2 and deposited with alumina. | [95] |
| PILCs | Vermiculite was intercalated with Al2O3 pillars by the ion-exchange method. | For the best catalysts of this series, the methanol conversion above 80% at 275 °C with selectivity to DME of about 98% was obtained. | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmielarz, L. Dehydration of Methanol to Dimethyl Ether—Current State and Perspectives. Catalysts 2024, 14, 308. https://doi.org/10.3390/catal14050308
Chmielarz L. Dehydration of Methanol to Dimethyl Ether—Current State and Perspectives. Catalysts. 2024; 14(5):308. https://doi.org/10.3390/catal14050308
Chicago/Turabian StyleChmielarz, Lucjan. 2024. "Dehydration of Methanol to Dimethyl Ether—Current State and Perspectives" Catalysts 14, no. 5: 308. https://doi.org/10.3390/catal14050308







