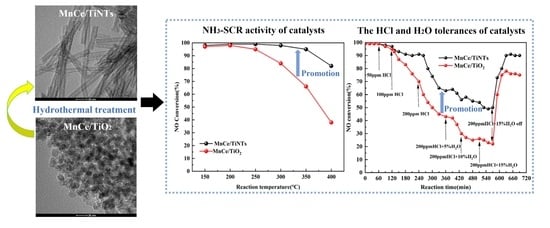
Study on NH3-SCR Activity and HCl/H2O Tolerance of Titanate-Nanotube-Supported MnOx-CeO2 Catalyst at Low Temperature
Abstract
:1. Introduction
2. Results and Discussion
2.1. NH3-SCR Activity of the MnCe/TiNTs and MnCe/TiO2 Catalysts
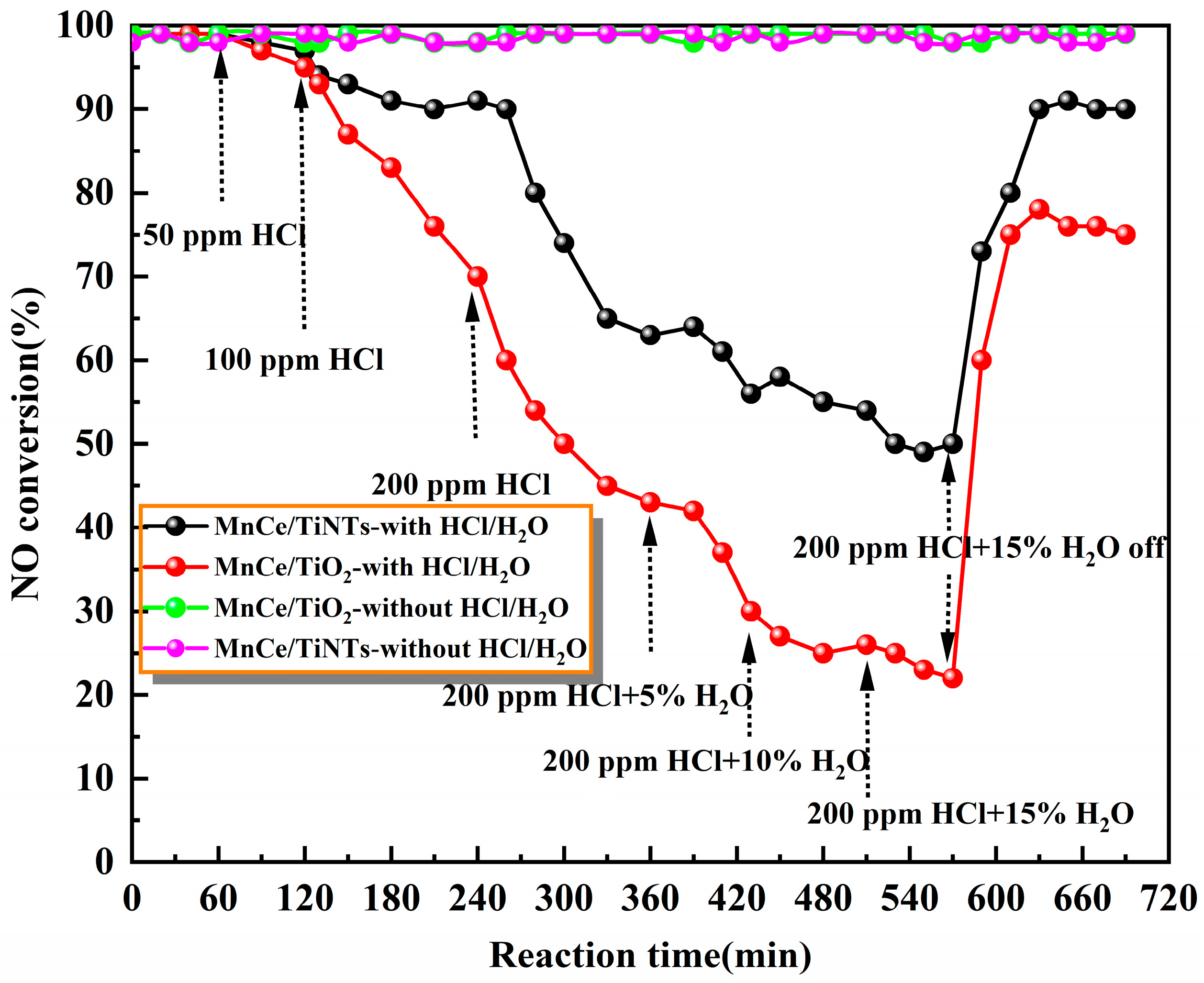
2.2. HCl/H2O Tolerance of the MnCe/TiNTs and MnCe/TiO2 Catalysts
2.3. Discussion Regarding the Promotion Effect of TiNTs on Catalytic Performance
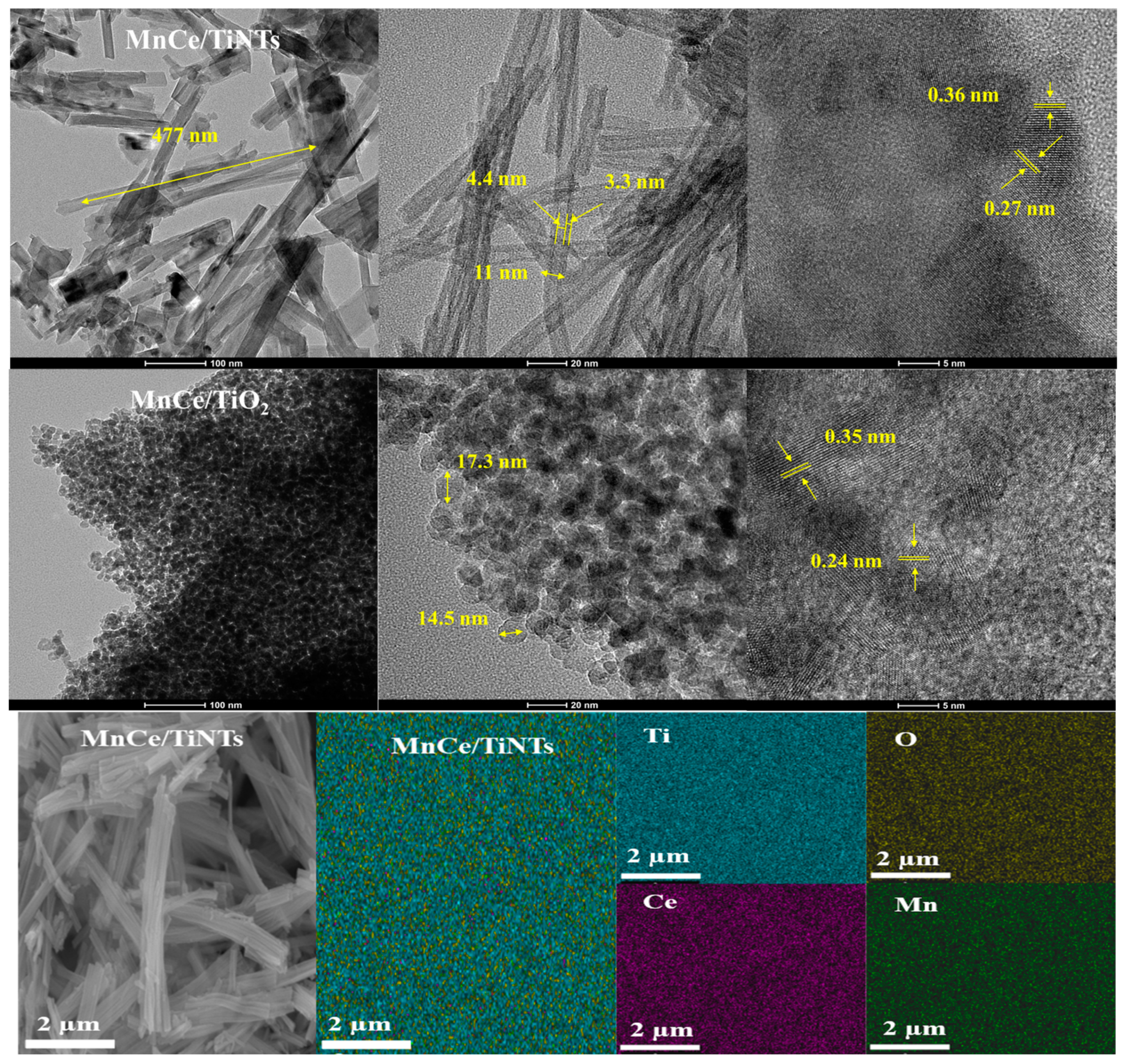
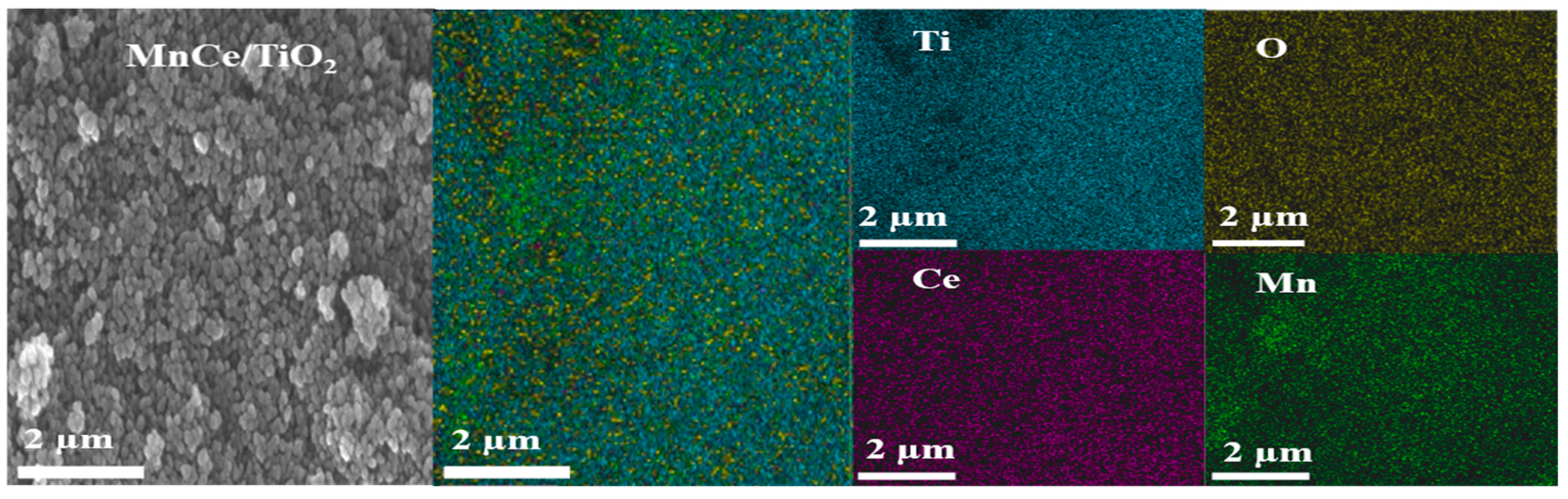
2.3.1. TEM and SEM Analysis
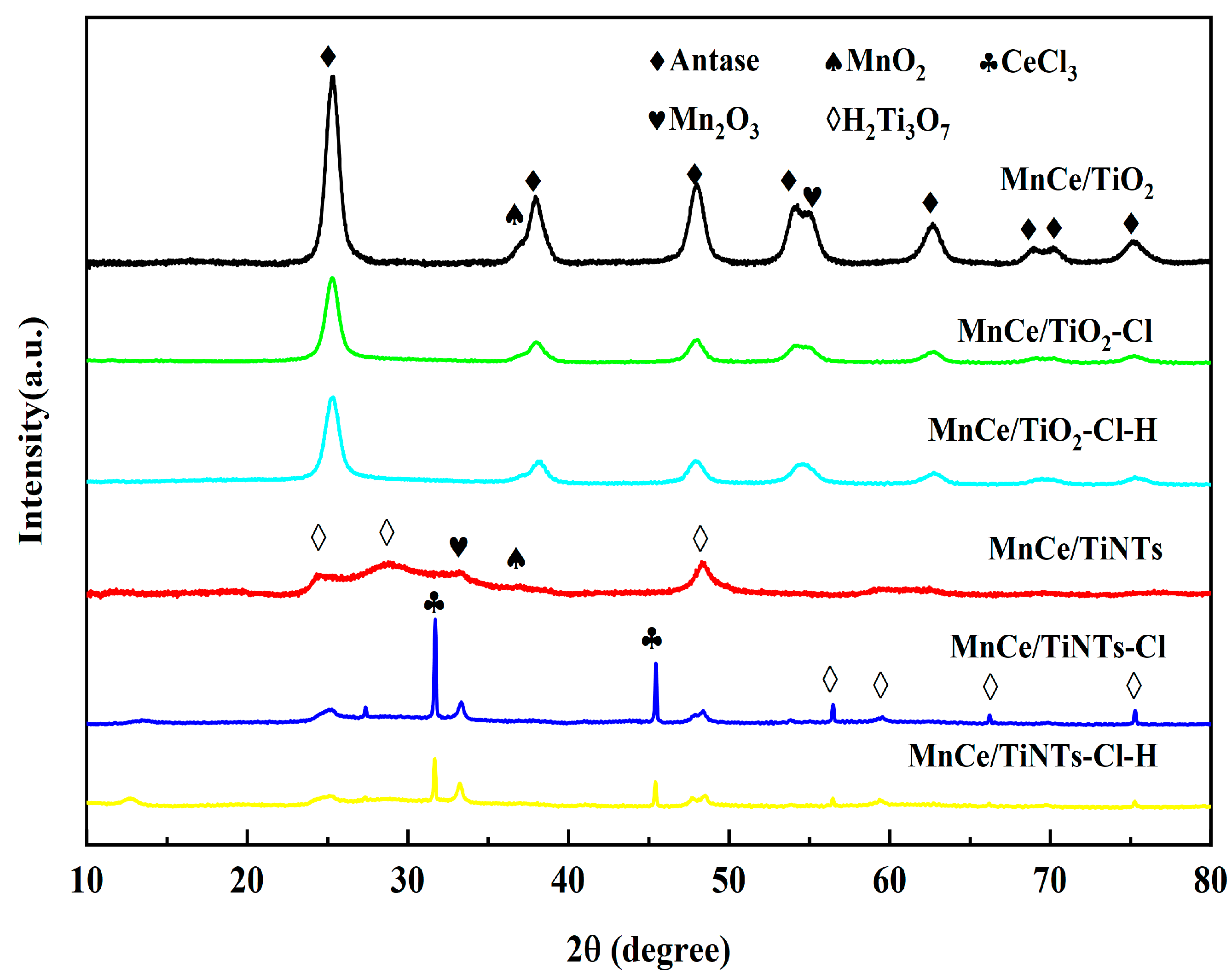
2.3.2. XRD and BET Analysis
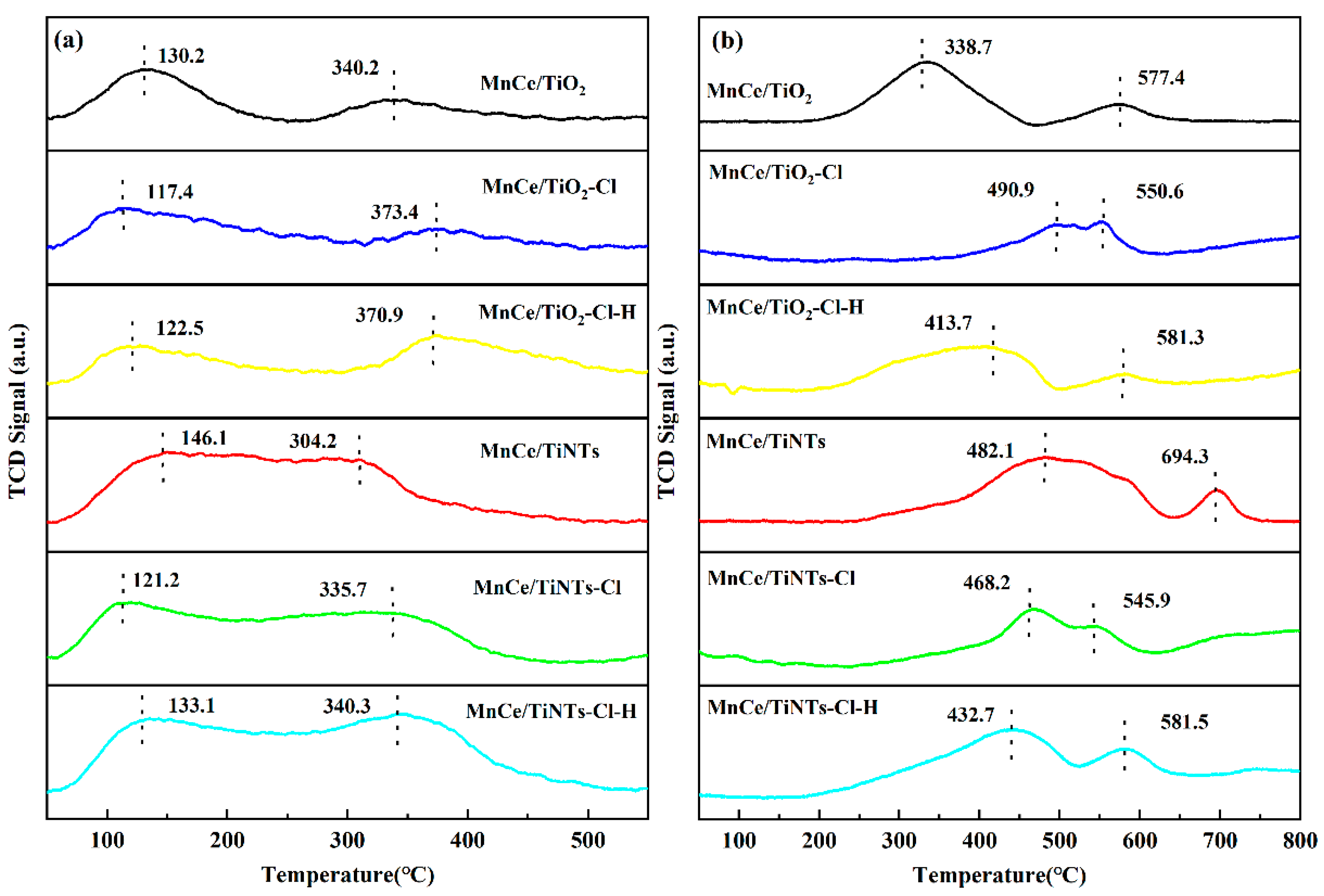
2.3.3. NH3-TPD and H2-TPR Analysis
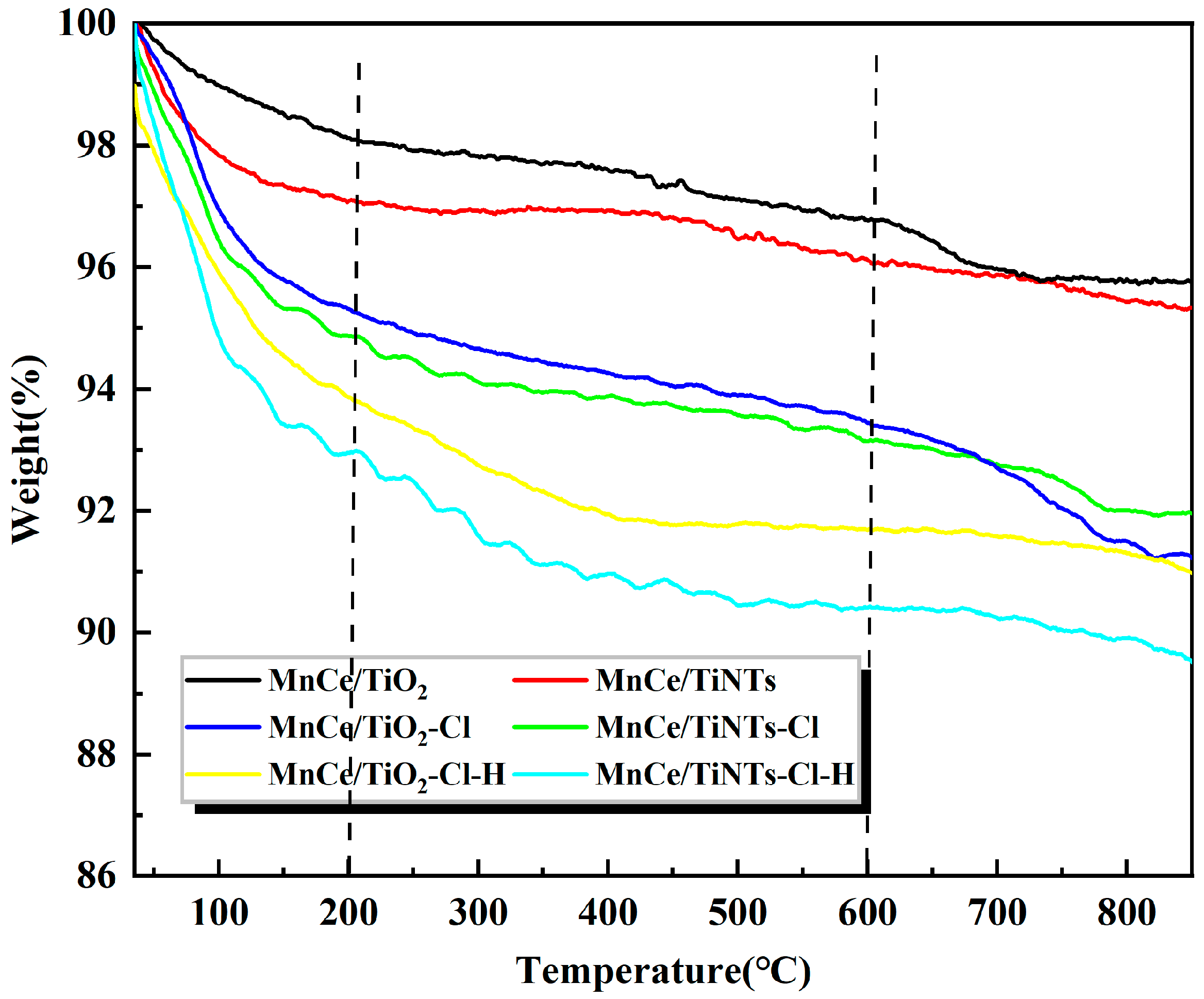
2.3.4. TG Analysis
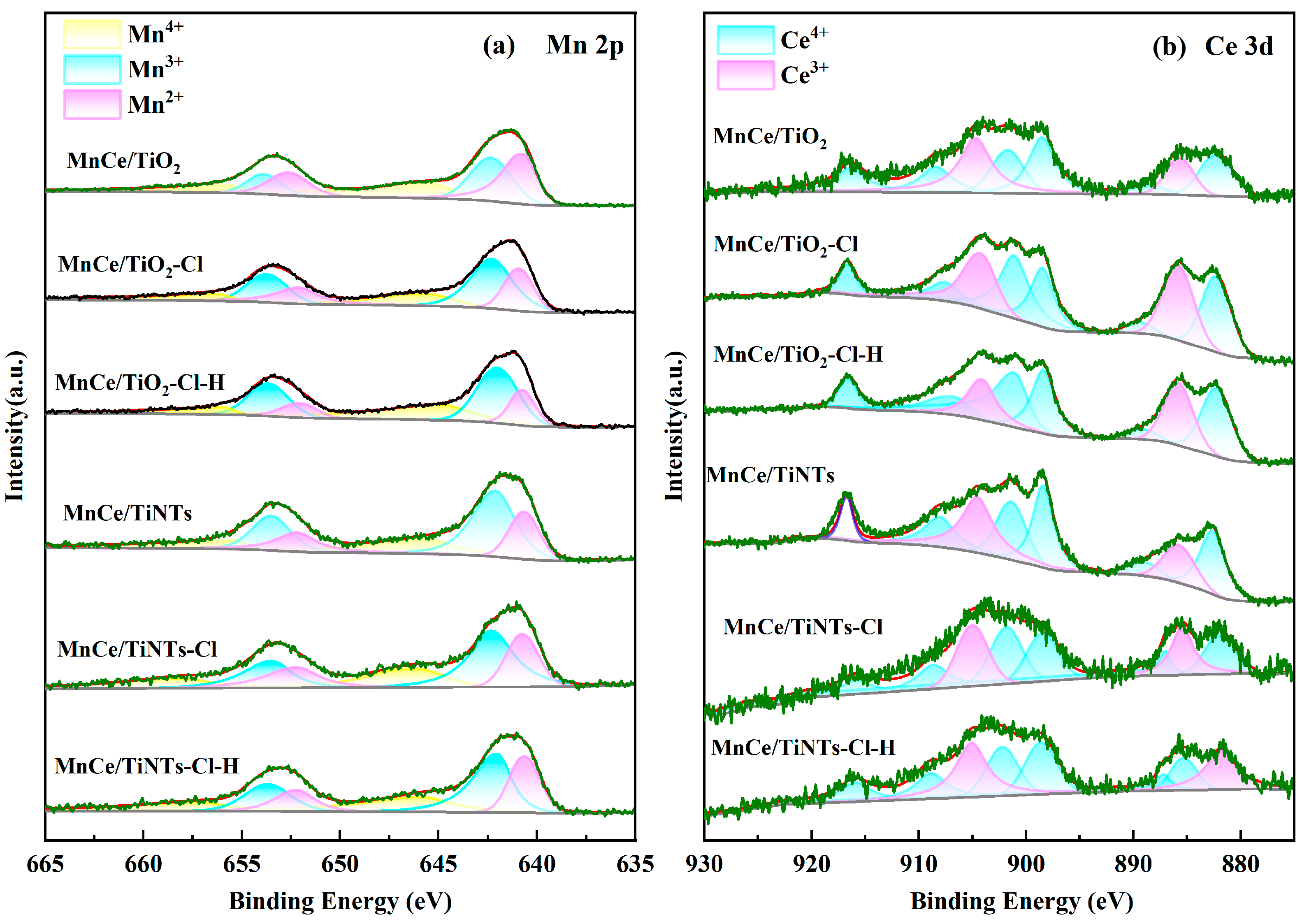
2.3.5. XPS Analysis
3. Methods and Materials
3.1. Catalyst Preparation and Characterization
3.2. Catalytic Activity Measurement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.H.; Chang, H.Z.; Ma, L.; Hao, J.M.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts-A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Zengel, D.; Stehle, M.; Deutschmann, O.; Casapu, M.; Grunwaldt, J.D. Impact of gas phase reactions and catalyst poisons on the NH3-SCR activity of a V2O5-WO3/TiO2 catalyst at pre-turbine position. Appl. Catal. B Environ. 2021, 288, 119991. [Google Scholar] [CrossRef]
- Yates, M.; Martín, J.A.; Martín, L.; Suárez, S.; Blanco, J. N2O formation in the ammonia oxidation and in the SCR process with V2O5-WO3 catalysts. Catal. Today 2005, 107–108, 120–125. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, H.; Li, D.Y.; Qin, Y.S.; Xie, Y.M.; Wang, S.D. WO3/CeO2—ZrO2, a Promising Catalyst for Selective Catalytic Reduction (SCR) of NOx with NH3 in diesel exhaust. Chem. Commun. 2008, 39, 1470–1472. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Huang, B.C.; Yu, C.L.; Lu, M.J.; Huang, H.; Zhou, Y.L. Research progress, challenges and perspectives on the sulfur and water resistance of catalysts for low temperature selective catalytic reduction of NOx by NH3. Appl. Catal. A Gen. 2019, 588, 117207. [Google Scholar] [CrossRef]
- Song, H.; Zhang, M.L.; Yu, J.P.; Wu, W.H.; Qu, R.Y.; Zheng, C.H.; Gao, X. The Effect of Cr Addition on Hg0 Oxidation and NO Reduction over V2O5/TiO2 Catalyst. Aerosol Air Qual. Res. 2017, 18, 803–810. [Google Scholar] [CrossRef]
- Lin, F.; Wang, Q.L.; Zhang, J.C.; Jin, J.; Lu, S.Y.; Yan, J.H. Mechanism and Kinetics Study on Low-Temperature NH3-SCR Over Manganese–Cerium Composite Oxide Catalysts. Ind. Eng. Chem. Res. 2019, 58, 22763–22770. [Google Scholar] [CrossRef]
- Wang, H.S.; Liang, H.S.; Chang, M.B. Chlorobenzene oxidation using ozone over iron oxide and manganese oxide catalysts. J. Catal. 2011, 186, 1781–1787. [Google Scholar] [CrossRef]
- Zhan, M.X.; Ji, L.J.; Ma, Y.F.; Chen, W.R.; Lu, S.Y. The impact of hydrochloric acid on the catalytic destruction behavior of 1,2-dichlorbenzene and PCDD/Fs in the presence of VWTi catalysts. Waste. Manage. 2018, 78, 249–257. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Amiridis, M.D. Kinetic and in situ FTIR studies of the catalytic oxidation of 1,2-dichlorobenzene over V2O5/Al2O3 catalysts. Catal. Today 1999, 51, 203–214. [Google Scholar] [CrossRef]
- Amrute, A.P.; Mondelli, C.; Moser, M. Performance, structure, and mechanism of CeO2 in HCl oxidation to Cl2. J. Catal. 2012, 286, 287–297. [Google Scholar] [CrossRef]
- Wang, Q.L.; Zhou, J.J.; Zhang, J.C.; Zhu, H.; Feng, Y.H.; Jin, J. Effect of Ceria Doping on the Catalytic Activity and SO2 Resistance of MnOx/TiO2 Catalysts for the Selective Catalytic Reduction of NO with NH3 at Low Temperatures. Aerosol Air Qual. Res. 2020, 20, 477–488. [Google Scholar] [CrossRef]
- Fang, X.; Liu, Y.J.; Cheng, Y.; Cen, W.L. Mechanism of Ce-Modified Birnessite-MnO2 in Promoting SO2 Poisoning Resistance for Low-Temperature NH3-SCR. ACS Catal. 2021, 11, 4125–4135. [Google Scholar] [CrossRef]
- Wang, Q.L.; Lin, F.; Zhou, J.J.; Zhang, J.C.; Jin, J. Effect of HCl and o-DCBz on NH3-SCR of NO over MnOx/TiO2 and MnOx-CeO2/TiO2 catalysts. Appl. Catal. A Gen. 2020, 605, 117801. [Google Scholar] [CrossRef]
- Wu, Z.B.; Jin, R.B.; Wang, H.Q.; Liu, Y. Effect of ceria doping on SO2 resistance of Mn/TiO2 for selective catalytic reduction of NO with NH3 at low temperature. Catal. Commun. 2009, 10, 935–939. [Google Scholar] [CrossRef]
- Nam, K.B.; Kwon, D.W.; Hong, S.C. DRIFT study on promotion effects of tungsten-modified Mn/Ce/Ti catalysts for the SCR reaction at low-temperature. Appl. Catal. A Gen. 2017, 542, 55–62. [Google Scholar] [CrossRef]
- Wang, P.L.; Wang, H.Q.; Chen, X.B.; Liu, Y.; Weng, X.L.; Wu, Z.B. Novel SCR catalyst with superior alkaline resistance performance: Enhanced self-protection originated from modifying protonated titanate nanotubes. J. Mater. Chem. A. 2014, 3, 680–690. [Google Scholar] [CrossRef]
- Xiong, L.Y.; Zhong, Q.; Chen, Q.Q.; Zhang, S.L. TIO2 nanotube-supported V2O5 catalysts for selective NO reduction by NH3. Korean J. Chem. Eng. 2013, 30, 836–841. [Google Scholar] [CrossRef]
- Jiang, Z.P.; Wang, Q.L.; Cai, Y.Z. Enhanced Catalytic Activity and SO2/H2O Tolerance for Selective Catalytic Reduction of NOx with NH3 over Titanate Nanotubes Supported MnOx–CeO2 Catalyst at Low Temperature. Catal. Surv. Asia. 2022, 26, 161–173. [Google Scholar] [CrossRef]
- Wang, Z.H.; Jiao, M.Y.; Chen, Z.P.; He, H.; Liu, L.C. Effects of montmorillonite and anatase TiO2 support on CeO2 catalysts during NH3-SCR reaction. Micropor Mesopor Mat. 2021, 320, 111072. [Google Scholar] [CrossRef]
- Andreoli, S.; Deorsola, F.A.; Pirone, R. MnO-CeO2 catalysts synthesized by solution combustion synthesis for the low-temperature NH3-SCR. Catal. Today. 2015, 253, 199–206. [Google Scholar] [CrossRef]
- Tang, C.J.; Li, J.C.; Yao, X.J.; Sun, J.F.; Cao, Y. Mesoporous NiO-CeO2 catalysts for CO oxidation: Nickel content effect and mechanism aspec. Appl. Catal. A Gen. 2015, 494, 77–86. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Ma, S.B.; Li, Z.B.; Yuan, F.L.; Niu, X.Y.; Zhu, Y.J. Synthesis of CenTiOx flakes with hierarchical structure and its enhanced activity for selective catalytic reduction of NOx with NH3. Chem. Eng. J. 2020, 392, 123801. [Google Scholar] [CrossRef]
- Li, C.; Hess, F.; Djerdj, I.; Chai, G.; Sun, Y.; Guo, Y.; Smarsly, B.M.; Over, H. The stabilizing effect of water and high reaction temperatures on the CeO2-catalyst in the harsh HCl oxidation reaction. J. Catal. 2018, 357, 257–262. [Google Scholar] [CrossRef]
- Xiong, S.C.; Liao, Y.; Xiao, X.; Dang, H.; Yang, S.J. The mechanism of the effect of H2O on the low temperature selective catalytic reduction of NO with NH3 over Mn–Fe spinel. Catal. Sci. Technol. 2015, 5, 2132–2140. [Google Scholar] [CrossRef]
- Aguilar, R.M.; Camposeco, R.; Castillo, S.; Marín, J.; Rodríguez, G.V.; García, S.L.; Mejía, C.I. Acidity, surface species, and catalytic activity study on V2O5-WO3/TiO2 nanotube catalysts for selective NO reduction by NH3. Fuel 2017, 198, 123–133. [Google Scholar] [CrossRef]
- Ye, B.; Kim, J.; Lee, M.J.; Chun, S.Y.; Jeong, B.; Kim, T.; Lee, D.H.; Kim, H.D. Mn-Ce oxide nanoparticles supported on nitrogen-doped reduced graphene oxide as low-temperature catalysts for selective catalytic reduction of nitrogen oxides. Micropor. Mesopor. Mat. 2021, 310, 110588. [Google Scholar] [CrossRef]
- Yang, N.Z.; Guo, R.T.; Pan, W.G. The deactivation mechanism of Cl on Ce/TiO2 catalyst for selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2016, 378, 513–518. [Google Scholar] [CrossRef]
- Chen, X.B.; Wang, P.L.; Fang, P. Tuning the property of Mn-Ce composite oxides by titanate nanotubes to improve the activity, selectivity and SO2/H2O tolerance in middle temperature NH3-SCR reaction. Fuel Process Technol. 2017, 167, 221–228. [Google Scholar] [CrossRef]
- Maupin, I.; Pinard, L.; Mijoin, J.; Magnoux, P. Bifunctional mechanism of dichloromethane oxidation over Pt/Al2O3:CH2Cl2 disproportionation over alumina and oxidation over platinum. J. Catal. 2012, 291, 104–109. [Google Scholar] [CrossRef]
- Lopez, N.; Segura, J.G.; Marin, R.P.; Ramirez, J.P. Mechanism of HCl oxidation (Deacon process) over RuO2. J. Catal. 2008, 255, 29–39. [Google Scholar] [CrossRef]
- Wu, Z.B.; Jin, R.B.; Liu, Y.; Wang, H.Q. Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature. Catal. Commun. 2008, 9, 2217–2220. [Google Scholar] [CrossRef]
- Zuo, S.F.; Huang, Q.Q.; Li, J.; Zhou, R.X. Promoting effect of Ce added to metal oxide supported on Al pillared clays for deep benzene oxidation. Appl. Catal. B Environ. 2009, 91, 204–209. [Google Scholar] [CrossRef]
- Wu, X.; Lee, H.R.; Liu, S.; Weng, D. Sulfur poisoning and regeneration of MnOx-CeO2 Al2O3 catalyst for soot oxidation. J. Rare Earth. 2012, 30, 659–694. [Google Scholar] [CrossRef]
- Wang, Q.L.; Huang, X.N.; Feng, Y.H. Interaction Mechanism Study on Simultaneous Removal of 1,2-Dichlorobenzene and NO over MnOx−CeO2/TiO2 Catalysts at Low Temperatures. Ind. Eng. Chem. Res. 2021, 60, 4820–4830. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, X.Y.; Lu, G.Z. Low-temperature catalytic destruction of chlorinated VOCs over cerium oxide. Catal. Commun. 2007, 8, 1645–1649. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, X.Y.; Dai, Q.Y.; Li, D. Effect of Ce and La on the structure and activity of MnOx catalyst in catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2012, 111–112, 141–149. [Google Scholar] [CrossRef]
- Zhang, J.H.; Li, Y.B.; Wang, L.; Zhang, C.B.; He, H. Catalytic oxidation of formaldehyde over manganese oxides with different crystal structures. Catal. Sci. Technol. 2015, 5, 2305–2313. [Google Scholar] [CrossRef]
- Boningari, T.; Ettireddy, P.R.; Somogyvari, A.; Liu, Y.; Vorontsov, A.; Mcdonald, C.A.; Smirniotis, P.G. Influence of elevated surface texture hydrated titania on Ce-doped Mn/TiO2 catalysts for the low-temperature SCR of NOx under oxygen-rich conditions. J. Catal. 2015, 325, 145–155. [Google Scholar] [CrossRef]


| Notation | Component Molar Ratio | |
|---|---|---|
| Mn/Ti | Ce/Ti | |
| Mn0.15Ce0.01/TiNTs | 0.15 | 0.01 |
| Mn0.15Ce0.05/TiNTs | 0.05 | |
| Mn0.15Ce0.10/TiNTs | 0.10 | |
| Mn0.15Ce0.15/TiNTs | 0.15 | |
| Mn0.15Ce0.05/TiO2 | 0.15 | 0.05 |
| Catalyst | Treatment Conditions | SBET (m2/g) a | Vtot (cm3/g) a | Dave (nm) a | |||
|---|---|---|---|---|---|---|---|
| Time (h) | Temperature (°C) | HCl Concentration (ppm) | H2O Content (vol.%) | ||||
| MnCe/TiO2 | / | / | / | / | 143.5 | 0.28 | 9.20 |
| MnCe/TiO2-Cl | 2 | 150 | 200 | / | 79 | 0.22 | 11.2 |
| MnCe/TiO2-Cl-H | 2 | 150 | 200 | 15 | 85 | 0.23 | 10.2 |
| MnCe/TiNTs | / | / | / | / | 191.7 | 0.37 | 6.64 |
| MnCe/TiNTs-Cl | 2 | 150 | 200 | / | 165.5 | 0.32 | 7.7 |
| MnCe/TiNTs-Cl-H | 2 | 150 | 200 | 15 | 166.7 | 0.33 | 7.7 |
| Catalysts | Mn (at%) | Mn Valence Distribution (%) | Ce (at%) | Ce Valence Distribution (%) | Ti (at%) | O (at%) | Oα/(Oα+Oβ) | Cl (at%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn4+ | Mn3+ | Mn2+ | Ce4+ | Ce3+ | |||||||
| MnCe/TiO2 | 3.85 | 26.0 | 47.2 | 26.8 | 1.25 | 78.3 | 21.7 | 18.46 | 50.55 | 22.4 | |
| MnCe/TiO2-Cl | 2.73 | 21.8 | 46.4 | 32 | 0.97 | 83.8 | 16.2 | 18.4 | 51.78 | 18.4 | 7.0 |
| MnCe/TiO2-Cl-H | 2.84 | 24.4 | 47.9 | 27.7 | 0.7 | 81.9 | 18.1 | 19.2 | 54.3 | 19.2 | 5.6 |
| MnCe/TiNTs | 2.18 | 30.8 | 31.9 | 37.3 | 0.86 | 72.7 | 27.3 | 20.74 | 51.95 | 27.5 | |
| MnCe/TiNTs-Cl | 1.34 | 28.9 | 43.7 | 27.4 | 0.63 | 76 | 24 | 22.3 | 54.2 | 25.1 | 4.8 |
| MnCe/TiNTs-Cl-H | 2.03 | 29.9 | 41.4 | 28.7 | 0.45 | 74.5 | 25.5 | 23.1 | 59.6 | 26.1 | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Liu, F.; Wu, Z.; Jin, J.; Lin, X.; Lu, S.; Qiu, J. Study on NH3-SCR Activity and HCl/H2O Tolerance of Titanate-Nanotube-Supported MnOx-CeO2 Catalyst at Low Temperature. Catalysts 2024, 14, 306. https://doi.org/10.3390/catal14050306
Wang Q, Liu F, Wu Z, Jin J, Lin X, Lu S, Qiu J. Study on NH3-SCR Activity and HCl/H2O Tolerance of Titanate-Nanotube-Supported MnOx-CeO2 Catalyst at Low Temperature. Catalysts. 2024; 14(5):306. https://doi.org/10.3390/catal14050306
Chicago/Turabian StyleWang, Qiulin, Feng Liu, Zhihao Wu, Jing Jin, Xiaoqing Lin, Shengyong Lu, and Juan Qiu. 2024. "Study on NH3-SCR Activity and HCl/H2O Tolerance of Titanate-Nanotube-Supported MnOx-CeO2 Catalyst at Low Temperature" Catalysts 14, no. 5: 306. https://doi.org/10.3390/catal14050306