Three-Dimensional Mesoporous Ni-CeO2 Catalyst for Dry Reforming of Methane
Abstract
:1. Introduction
2. Results and Discussion
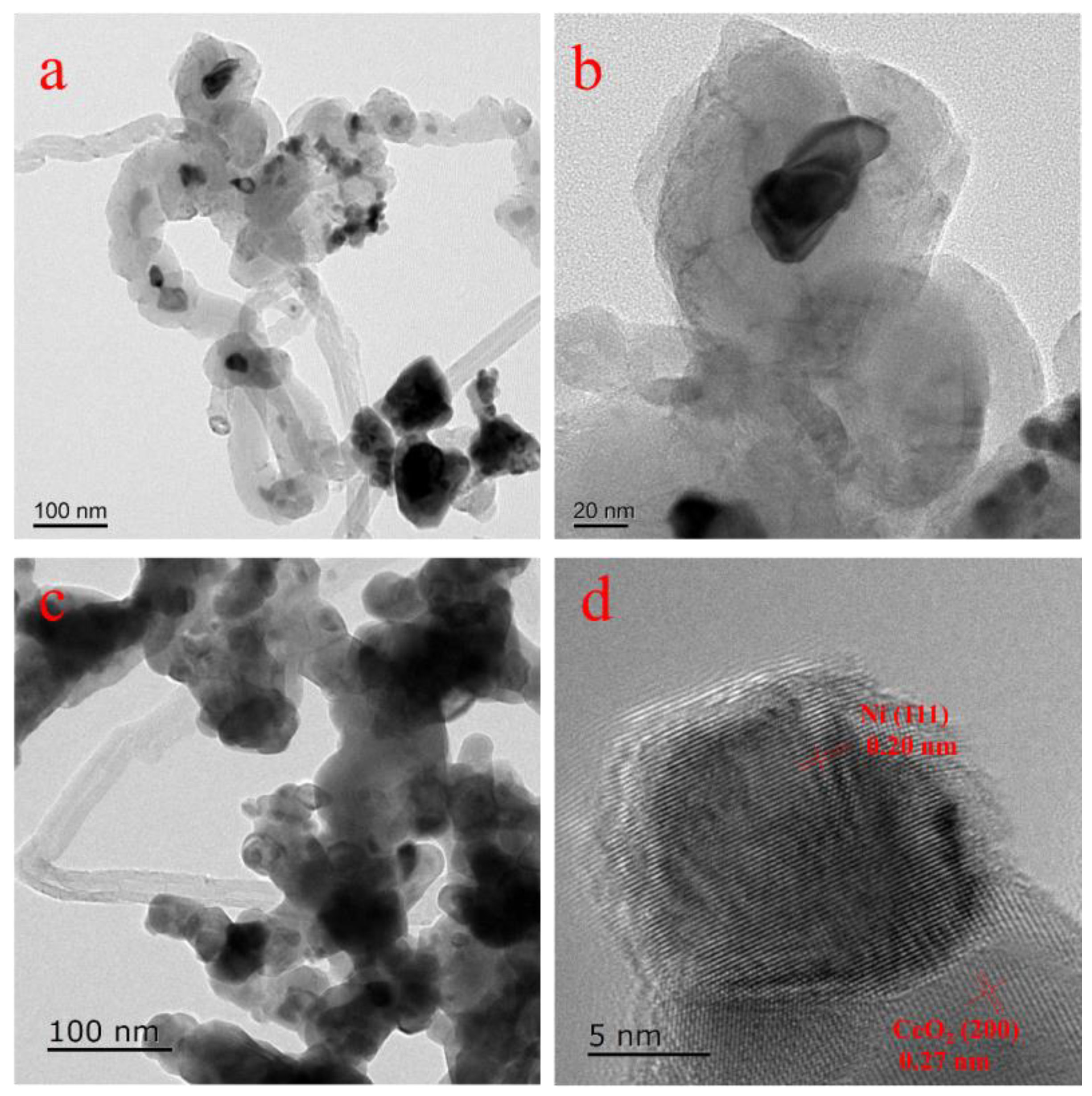
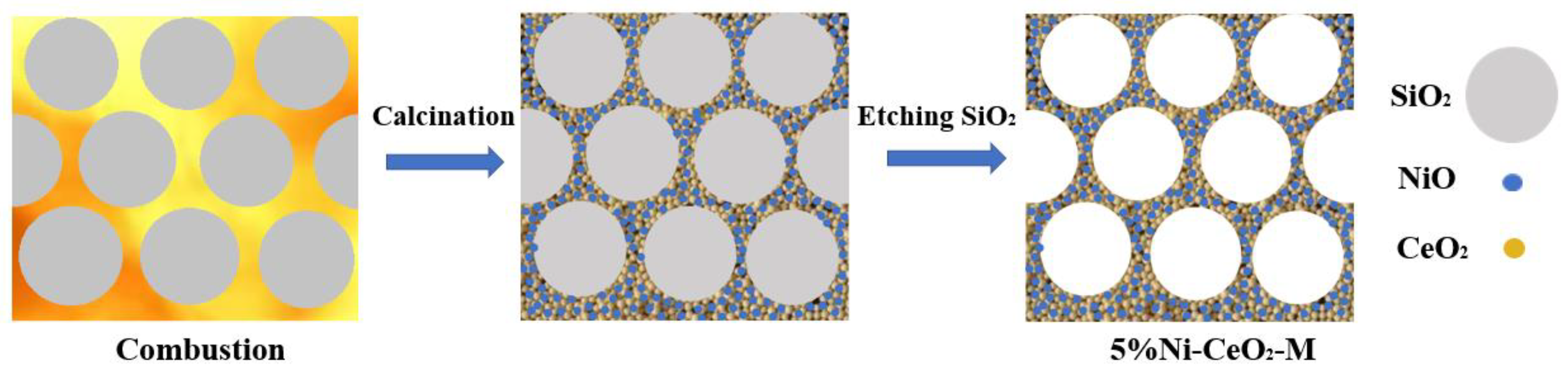
2.1. Characterization of Catalysts
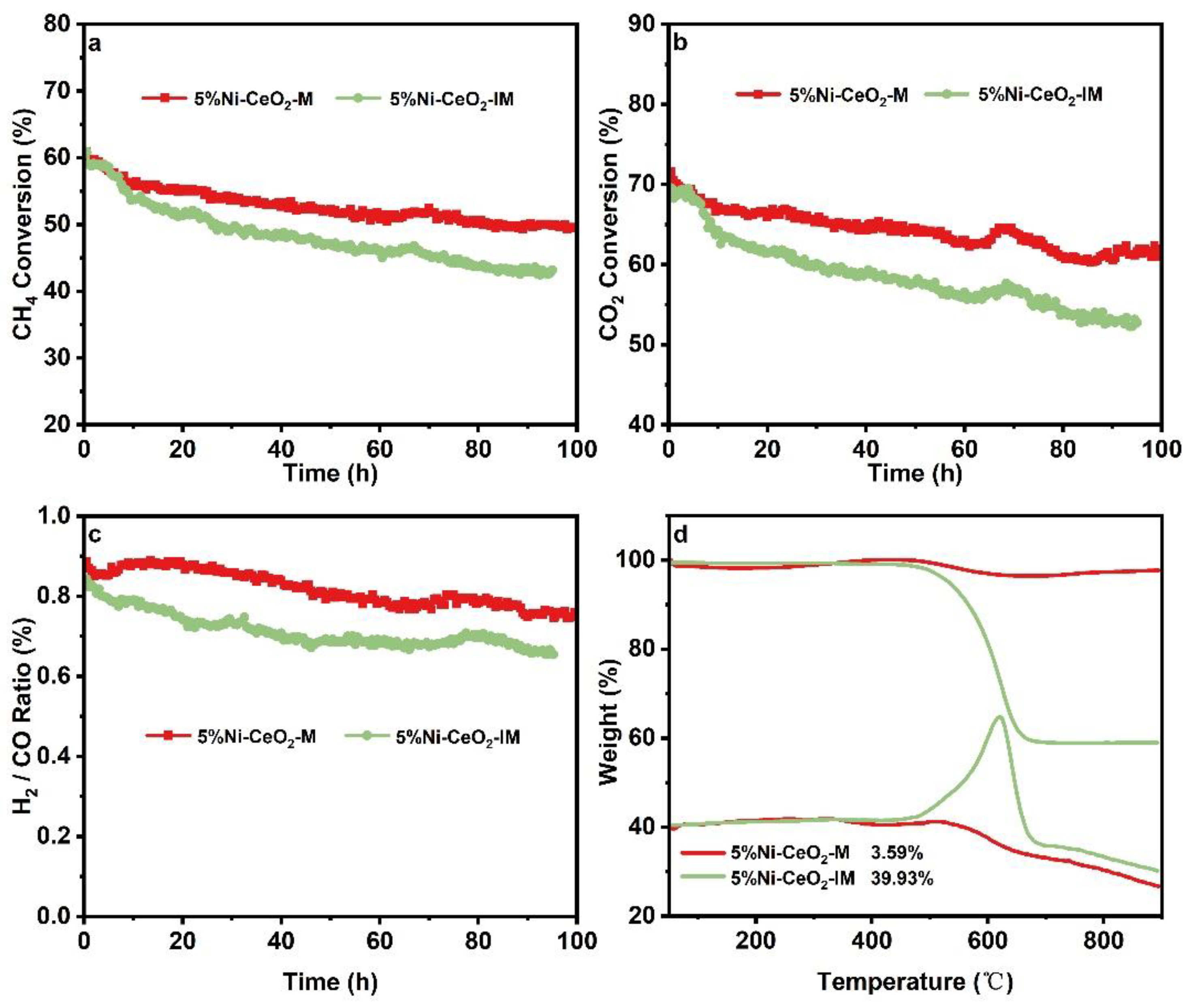
2.2. DRM Performances and Carbon Deposition over Catalysts
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alipour, Z.; Borugadda, V.B.; Wang, H.; Dalai, A.K. Syngas production through dry reforming: A review on catalysts and their materials, preparation methods and reactor type. Chem. Eng. J. 2023, 452, 139416. [Google Scholar] [CrossRef]
- Shen, D.; Wang, J.; Bai, Y.; Lyu, S.; Zhang, Y.; Li, J.; Li, L.; Wang, G. Carbon-confined Ni based catalyst by auto-reduction for low-temperature dry reforming of methane. Fuel 2023, 339, 10. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, H.; Cai, W.; Liang, W.; Xia, H.; Dang, C. Immobilization of Ni on MOF-derived CeO2 for promoting low-temperature dry reforming of methane. Fuel 2024, 363, 11. [Google Scholar] [CrossRef]
- Jang, W.J.; Shim, J.O.; Kim, H.M.; Yoo, S.Y.; Roh, H.S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.J.; Hou, X.L.; Mi, J.L.; Yuan, Z.Z.; Huang, J.; Stampfl, C. Active sites and mechanism of the direct conversion of methane and carbon dioxide to acetic acid over the zinc-modified H-ZSM-5 zeolite. Catal. Sci. Technol. 2019, 9, 6297–6307. [Google Scholar] [CrossRef]
- Ding, S.; Liu, Y.X. Adsorption of CO2 from flue gas by novel seaweed-based KOH-activated porous biochars. Fuel 2020, 260, 10. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Hamill, M.Y.S. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sust. Energ. Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Li, S.Q.; Fu, Y.; Kong, W.B.; Pan, B.R.; Yuan, C.K.; Cai, F.F.; Zhu, H.; Zhang, J.; Sun, Y.H. Dually confined Ni nanoparticles by room-temperature degradation of AlN for dry reforming of methane. Appl. Catal. B 2020, 277, 10. [Google Scholar] [CrossRef]
- Bai, Y.; Shen, D.; Yu, G.; Wang, J.; Lyu, S.; Zhang, Y.; Wang, G.; Li, J.; Li, L. Manufacture of highly loaded Ni catalysts by carbonization-oxidation-reduction for dry reforming of methane. New J. Chem. 2023, 47, 17186–17193. [Google Scholar] [CrossRef]
- He, D.; Wu, S.; Cao, X.; Chen, D.; Zhang, L.; Zhang, Y.; Luo, Y. Dynamic trap of Ni at elevated temperature for yielding high-efficiency methane dry reforming catalyst. Appl. Catal. B Environ. Energy 2024, 346, 11. [Google Scholar] [CrossRef]
- Song, Y.; Ozdemir, E.; Ramesh, S.; Adishev, A.; Subramanian, S.; Harale, A.; Albuali, M.; Fadhel, B.A.; Jamal, A.; Moon, D. Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science 2020, 367, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Shoji, S.; Peng, X.; Yamaguchi, A.; Watanabe, R.; Fukuhara, C.; Cho, Y.; Yamamoto, T.; Matsumura, S.; Yu, M.-W.; Ishii, S. Photocatalytic uphill conversion of natural gas beyond the limitation of thermal reaction systems. Nat. Catal. 2020, 3, 148–153. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhou, H.; Wang, L.; Wang, L.; Wang, C.; Wang, H.; Fang, W.; He, M.; Wu, Q.; Xiao, F.-S. Enhanced CO2 utilization in dry reforming of methane achieved through nickel-mediated hydrogen spillover in zeolite crystals. Nat. Catal. 2022, 5, 1030–1037. [Google Scholar] [CrossRef]
- Palmer, C.; Upham, D.C.; Smart, S.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Dry reforming of methane catalysed by molten metal alloys. Nat. Catal. 2020, 3, 83–89. [Google Scholar] [CrossRef]
- Niu, J.; Wang, Y.E.; Liland, S.K.; Regli, S.; Yang, J.; Rout, K.R.; Luo, J.; Rønning, M.; Ran, J.; Chen, D. Unraveling enhanced activity, selectivity, and coke resistance of Pt–Ni bimetallic clusters in dry reforming. ACS Catal. 2021, 11, 2398–2411. [Google Scholar] [CrossRef]
- Haug, L.; Thurner, C.; Bekheet, M.F.; Bischoff, B.; Gurlo, A.; Kunz, M.; Sartory, B.; Penner, S.; Klötzer, B. Zirconium Carbide Mediates Coke-Resistant Methane Dry Reforming on Nickel-Zirconium Catalysts. Angew. Chem. Int. Ed. 2022, 61, 7. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Littlewood, P.; Liu, Y.; Marks, T.J.; Stair, P.C. Stabilizing supported Ni catalysts for dry reforming of methane by combined La doping and Al overcoating using atomic layer deposition. ACS Catal. 2022, 12, 10522–10530. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, S.; Yang, B. Promoted coke resistance of Ni by surface carbon for the dry reforming of methane. Iscience 2023, 26, 106237. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.N.T.; Nguyen, H.H.; Pham, T.-P.T.; Le Phuong, D.H.; Nguyen, N.A.; Vo, D.-V.N.; Pham, P.T. Insight into the role of material basicity in the coke formation and performance of Ni/Al2O3 catalyst for the simulated-biogas dry reforming. J. Energy Inst. 2023, 108, 101252. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Y.; Liu, Y.; Zheng, J.; Deng, J.; Yan, T.; Cheng, D.; Zhang, D. Unraveling the unique promotion effects of a triple interface in Ni catalysts for methane dry reforming. Ind. Eng. Chem. Res. 2023, 62, 4965–4975. [Google Scholar] [CrossRef]
- Deng, J.; Bu, K.; Shen, Y.; Zhang, X.; Zhang, J.; Faungnawakij, K.; Zhang, D. Cooperatively enhanced coking resistance via boron nitride coating over Ni-based catalysts for dry reforming of methane. Appl. Catal. B Environ. 2022, 302, 10. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Fakeeha, A.H.; Ibrahim, A.A.; Abasaeed, A.E. Ni supported on La2O3 + ZrO2 for dry reforming of methane: The impact of surface adsorbed oxygen species. Int. J. Hydrog. Energy 2021, 46, 3780–3788. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Zhou, Z.; Chen, S.; Zhang, T.; Song, F.; Zhang, Q.; Tsubaki, N.; Tan, Y.; Han, Y. Effects of the surface adsorbed oxygen species tuned by rare-earth metal doping on dry reforming of methane over Ni/ZrO2 catalyst. Appl. Catal. B Environ. 2020, 264, 12. [Google Scholar] [CrossRef]
- Suchorski, Y.; Kozlov, S.M.; Bespalov, I.; Datler, M.; Vogel, D.; Budinska, Z.; Neyman, K.M.; Rupprechter, G. The role of metal/oxide interfaces for long-range metal particle activation during CO oxidation. Nat. Mater. 2018, 17, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Bao, X. Fundamental insights into interfacial catalysis. Chem. Soc. Rev. 2017, 46, 1770–1771. [Google Scholar] [CrossRef]
- Lovell, E.C.; Großman, H.; Horlyck, J.; Scott, J.; Mädler, L.; Amal, R. Asymmetrical double flame spray pyrolysis-designed SiO2/Ce0.7Zr0.3O2 for the dry reforming of methane. ACS Appl. Mater. Interfaces 2019, 11, 25766–25777. [Google Scholar] [CrossRef] [PubMed]
- Teh, L.; Setiabudi, H.; Timmiati, S.; Aziz, M.; Annuar, N.; Ruslan, N. Recent progress in ceria-based catalysts for the dry reforming of methane: A review. Chem. Eng. Sci. 2021, 242, 116606. [Google Scholar] [CrossRef]
- Yan, X.; Hu, T.; Liu, P.; Li, S.; Zhao, B.; Zhang, Q.; Jiao, W.; Chen, S.; Wang, P.; Lu, J. Highly efficient and stable Ni/CeO2-SiO2 catalyst for dry reforming of methane: Effect of interfacial structure of Ni/CeO2 on SiO2. Appl. Catal. B 2019, 246, 221–231. [Google Scholar] [CrossRef]
- Omoregbe, O.; Danh, H.T.; Abidin, S.; Setiabudi, H.; Abdullah, B.; Vu, K.B.; Vo, D.-V.N. Influence of lanthanide promoters on Ni/SBA-15 catalysts for syngas production by methane dry reforming. Procedia Eng. 2016, 148, 1388–1395. [Google Scholar] [CrossRef]
- Lustemberg, P.G.; Ramírez, P.J.; Liu, Z.; Gutierrez, R.A.; Grinter, D.G.; Carrasco, J.; Senanayake, S.D.; Rodriguez, J.A.; Ganduglia-Pirovano, M.V. Room-temperature activation of methane and dry re-forming with CO2 on Ni-CeO2(111) surfaces: Effect of Ce3+ sites and metal-support interactions on C-H bond cleavage. Acs Catal. 2016, 6, 8184–8191. [Google Scholar] [CrossRef]
- Liu, Z.; Grinter, D.C.; Lustemberg, P.G.; Nguyen-Phan, T.D.; Zhou, Y.; Luo, S.; Waluyo, I.; Crumlin, E.J.; Stacchiola, D.J.; Zhou, J. Dry reforming of methane on a highly-active Ni-CeO2 catalyst: Effects of metal-support interactions on C-H bond breaking. Angew. Chem. Int. Ed. 2016, 55, 7455–7459. [Google Scholar] [CrossRef] [PubMed]
- Rood, S.C.; Ahmet, H.B.; Gomez-Ramon, A.; Torrente-Murciano, L.; Reina, T.R.; Eslava, S. Enhanced ceria nanoflakes using graphene oxide as a sacrificial template for CO oxidation and dry reforming of methane. Appl. Catal. B Environ. 2019, 242, 358–368. [Google Scholar] [CrossRef]
- Tang, C.; Liping, L.; Zhang, L.; Tan, L.; Dong, L. High Carbon-Resistance Ni@ CeO2 Core-Shell Catalysts for Dry Reforming of Methane. Kinet. Catal. 2017, 58, 800–808. [Google Scholar] [CrossRef]
- Yahi, N.; Menad, S.; Rodríguez-Ramos, I. Dry reforming of methane over Ni/CeO2 catalysts prepared by three different methods. Green Process. Synth. 2015, 4, 479–486. [Google Scholar] [CrossRef]
- Zhou, R.F.; Mohamedali, M.; Ren, Y.X.; Lu, Q.Y.; Mahinpey, N. Facile synthesis of multi-layered nanostructured Ni/CeO2 catalyst plus in-situ pre-treatment for efficient dry reforming of methane. Appl. Catal. B Environ. 2022, 316, 14. [Google Scholar] [CrossRef]
- Kim, S.B.; Eissa, A.A.S.; Kim, M.J.; Goda, E.S.; Youn, J.R.; Lee, K. Sustainable Synthesis of a Highly Stable and Coke-Free Ni@CeO2 Catalyst for the Efficient Carbon Dioxide Reforming of Methane. Catalysts 2022, 12, 22. [Google Scholar] [CrossRef]
- Voskanyan, A.A.; Chan, K.-Y.; Li, C.-Y.V. Colloidal solution combustion synthesis: Toward mass production of a crystalline uniform mesoporous CeO2 catalyst with tunable porosity. Chem. Mater. 2016, 28, 2768–2775. [Google Scholar] [CrossRef]
- Voskanyan, A.A.; Ho, C.-K.; Chan, K.Y. 3D δ-MnO2 nanostructure with ultralarge mesopores as high-performance lithium-ion battery anode fabricated via colloidal solution combustion synthesis. J. Power Sources 2019, 421, 162–168. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H. Mesoporous Co-CeO2 catalyst prepared by colloidal solution combustion method for reverse water-gas shift reaction. Catal Today 2018, 316, 155–161. [Google Scholar] [CrossRef]
- de la Cruz-Flores, V.G.; Martinez-Hernandez, A.; Gracia-Pinilla, M.A. Deactivation of Ni-SiO2 catalysts that are synthetized via a modified direct synthesis method during the dry reforming of methane. Appl. Catal. A 2020, 594, 117455. [Google Scholar] [CrossRef]
- Hong, W.; Zhang, L.; Miao, L.; Yuan, L.; Xue, B. Co/CeO2 for ethanol steam reforming: Effect of ceria morphology. J. Rare Earths 2013, 31, 565–571. [Google Scholar]
- Zhang, Y.; Wang, Z.; Zhou, J.; Cen, K. Ceria as a catalyst for hydrogen iodide decomposition in sulfur-iodine cycle for hydrogen production. Int. J. Hydrog. Energy 2009, 34, 1688–1695. [Google Scholar] [CrossRef]
- Yisup, N.; Cao, Y.; Feng, W.-L.; Dai, W.-L.; Fan, K.-N. Catalytic oxidation of methane over novel Ce-Ni-O mixed oxide catalysts prepared by oxalate gel-coprecipitation. Catal. Lett. 2005, 99, 207–213. [Google Scholar] [CrossRef]
- Shan, W.; Luo, M.; Ying, P.; Shen, W.; Li, C. Reduction property and catalytic activity of Ce1-XNiXO2 mixed oxide catalysts for CH4 oxidation. Appl. Catal. A 2003, 246, 1–9. [Google Scholar] [CrossRef]
- Shan, W.; Fleys, M.; Lapicque, F.; Swierczynski, D.; Kiennemann, A.; Simon, Y.; Marquaire, P.-M. Syngas production from partial oxidation of methane over Ce1-XNiXOY catalysts prepared by complexation-combustion method. Appl. Catal. A Gen. 2006, 311, 24–33. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Yan, B. Ni/Ce0.9Eu0.1O1.95 with enhanced coke resistance for dry reforming of methane. J. Catal. 2022, 407, 77–89. [Google Scholar] [CrossRef]
- Kim, M.-J.; Youn, J.-R.; Kim, H.J.; Seo, M.W.; Lee, D.; Go, K.S.; Lee, K.B.; Jeon, S.G. Effect of surface properties controlled by Ce addition on CO2 methanation over Ni/Ce/Al2O3 catalyst. Int. J. Hydrog. Energy 2020, 45, 24595–24603. [Google Scholar] [CrossRef]
- Jang, W.-J.; Kim, H.-M.; Shim, J.-O.; Yoo, S.-Y.; Jeon, K.-W.; Na, H.-S.; Lee, Y.-L.; Jeong, D.-W.; Bae, J.W.; Nah, I.W. Key properties of Ni-MgO-CeO2, Ni-MgO-ZrO2, and Ni-MgO-Ce(1-X)Zr(X)O2 catalysts for the reforming of methane with carbon dioxide. Green Chem. 2018, 20, 1621–1633. [Google Scholar] [CrossRef]
- Das, S.; Ashok, J.; Bian, Z.; Dewangan, N.; Wai, M.; Du, Y.; Borgna, A.; Hidajat, K.; Kawi, S. Silica-Ceria sandwiched Ni core-shell catalyst for low temperature dry reforming of biogas: Coke resistance and mechanistic insights. Appl. Catal. B 2018, 230, 220–236. [Google Scholar] [CrossRef]
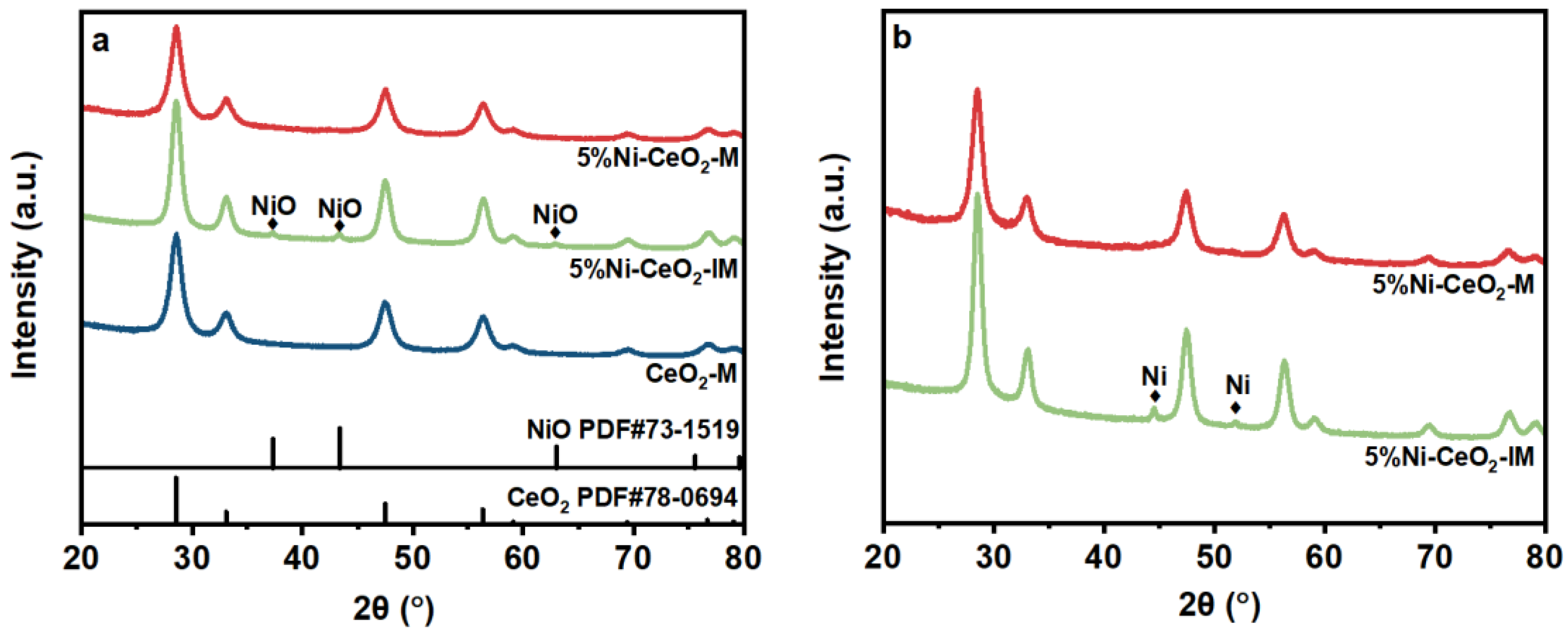
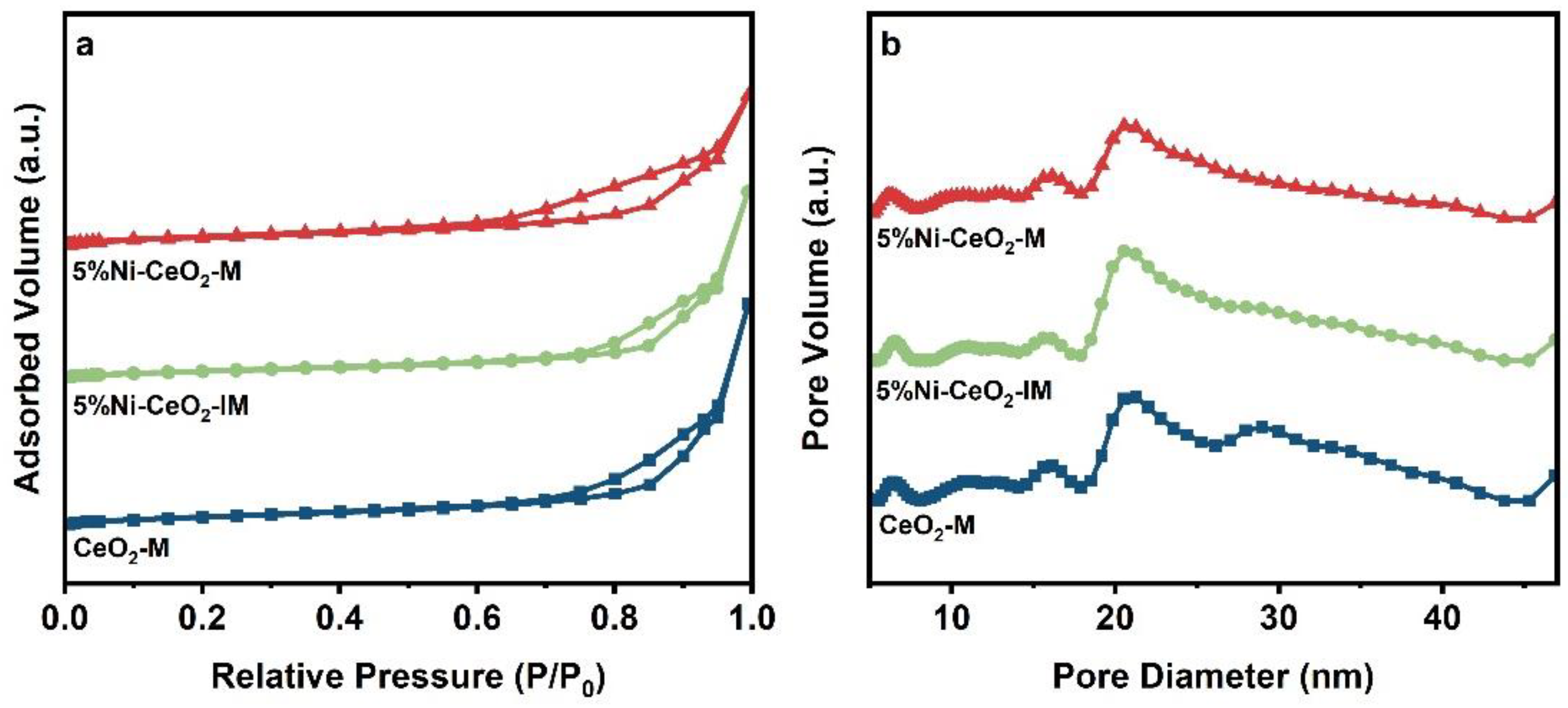
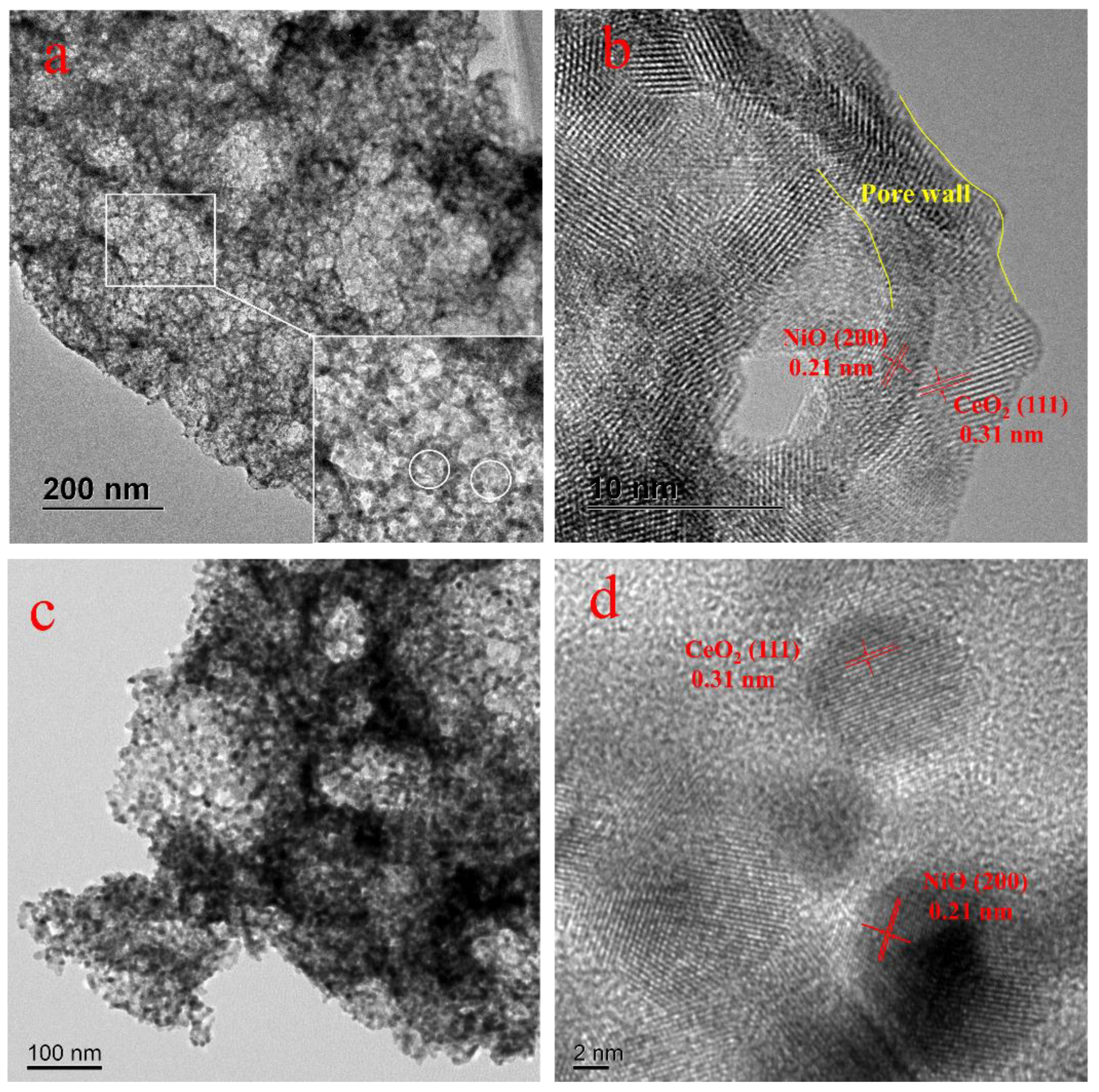

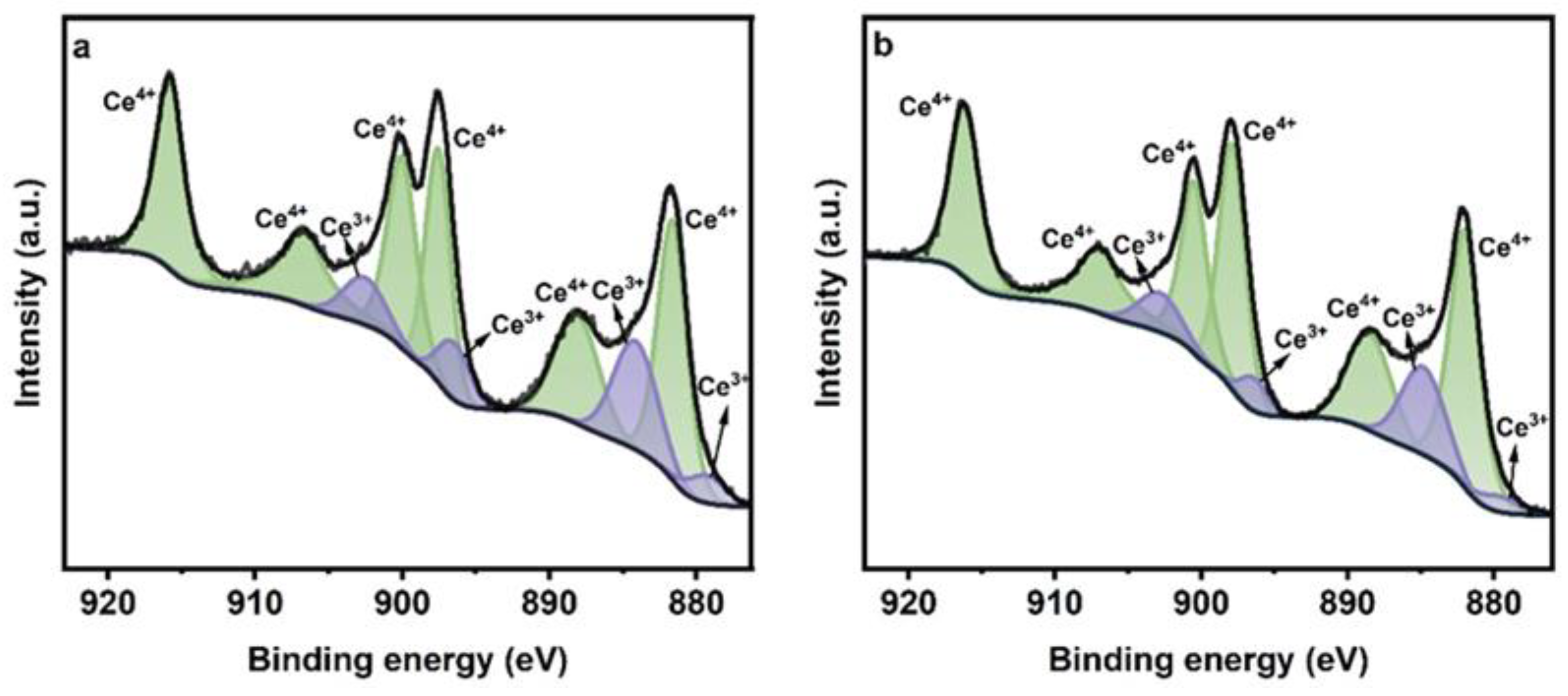
| Samples | SBET a (m2/g) | Pore Volume a (cm3/g) | CeO2 Crystal Size b (nm) |
|---|---|---|---|
| CeO2-M | 105 | 0.65 | 5.9 |
| 5%Ni-CeO2-M | 123 | 0.46 | 5.8 |
| 5%Ni-CeO2-IM | 80 | 0.55 | 6.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Liu, Y.; Huang, L.; Liu, Y.; Cui, S.; Liu, H.; Xu, J.; Wang, L. Three-Dimensional Mesoporous Ni-CeO2 Catalyst for Dry Reforming of Methane. Catalysts 2024, 14, 291. https://doi.org/10.3390/catal14050291
Jin H, Liu Y, Huang L, Liu Y, Cui S, Liu H, Xu J, Wang L. Three-Dimensional Mesoporous Ni-CeO2 Catalyst for Dry Reforming of Methane. Catalysts. 2024; 14(5):291. https://doi.org/10.3390/catal14050291
Chicago/Turabian StyleJin, Huiyao, Yuanqiao Liu, Lizhi Huang, Yali Liu, Sha Cui, Hui Liu, Jing Xu, and Luhui Wang. 2024. "Three-Dimensional Mesoporous Ni-CeO2 Catalyst for Dry Reforming of Methane" Catalysts 14, no. 5: 291. https://doi.org/10.3390/catal14050291





