Research Progress in Fuel Oil Production by Catalytic Pyrolysis Technologies of Waste Plastics
Abstract
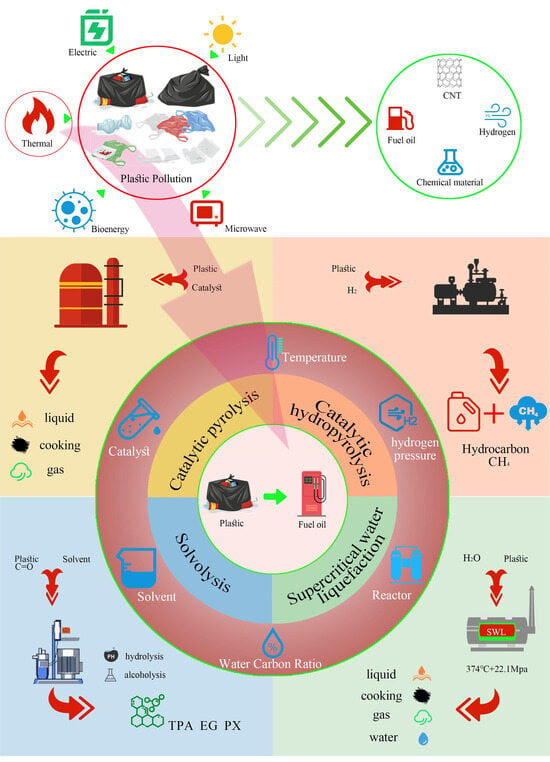
:1. Introduction
2. Plastic Pyrolysis Method for Oil Production
2.1. Thermal Pyrolysis
2.1.1. Pyrolysis
2.1.2. Catalytic Pyrolysis
2.1.3. Catalytic Reforming
2.2. Catalytic Hydropyrolysis
2.3. Solvolysis
2.4. Supercritical Water Liquefaction
2.5. Tandem Technology of Degradation
3. Optimization Paths of Technological Conditions for the Production of Fuel Oil
3.1. Co-Pyrolysis
3.2. Catalysts
3.3. Reactors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Awasthi, A.K.; Wei, F.; Tan, Q.; Li, J. Single-use plastics: Production, usage, disposal, and adverse impacts. Sci. Total Environ. 2020, 752, 141772. [Google Scholar] [CrossRef] [PubMed]
- Baca, D.; Monroy, R.; Castillo, M.; Elkhazraji, A.; Farooq, A.; Ahmad, R. Dioxins and plastic waste: A scientometric analysis and systematic literature review of the detection methods. Environ. Adv. 2023, 13, 100439. [Google Scholar] [CrossRef]
- Kalali, E.N.; Lotfian, S.; Shabestari, M.E.; Khayatzadeh, S.; Zhao, C.; Nezhad, H.Y. A critical review of the current progress of plastic waste recycling technology in structural materials. Curr. Opin. Green Sustain. Chem. 2023, 40, 100763. [Google Scholar] [CrossRef]
- Chen, H.; Wan, K.; Zhang, Y.; Wang, Y. Waste to Wealth: Chemical Recycling and Chemical Upcycling of Waste Plastics for a Great Future. ChemSusChem 2021, 14, 4123–4136. [Google Scholar] [CrossRef]
- Zhuo, C.; Levendis, Y.A. Upcycling waste plastics into carbon nanomaterials: A review. J. Appl. Polym. Sci. 2013, 131, 1001–1007. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, Y.; Williams, P.T.; Yang, H.; Chen, H. Co-production of hydrogen and carbon nanotubes from real-world waste plastics: Influence of catalyst composition and operational parameters. Appl. Catal. B Environ. 2018, 221, 584–597. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, Y.; Li, Z.; Xu, M.; Wang, Y.; Ge, R.; Duan, H. Electrocatalytic upcycling of polyethylene terephthalate to commodity chemicals and H2 fuel. Nat. Commun. 2021, 12, 4679. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Tiso, T.; Bertling, J.; O’Connor, K.; Blank, L.M.; Bornscheuer, U.T. Possibilities and limitations of biotechnological plastic degradation and recycling. Nat. Catal. 2020, 3, 867–871. [Google Scholar] [CrossRef]
- Sullivan, K.P.; Werner, A.Z.; Ramirez, K.J.; Ellis, L.D.; Bussard, J.R.; Black, B.A.; Brandner, D.G.; Bratti, F.; Buss, B.L.; Dong, X.; et al. Mixed plastics waste valorization through tandem chemical oxidation and biological funneling. Science 2022, 378, 207–211. [Google Scholar] [CrossRef]
- Huang, Z.; Shanmugam, M.; Liu, Z.; Brookfield, A.; Bennett, E.L.; Guan, R.; Herrera, D.E.V.; Lopez-Sanchez, J.A.; Slater, A.G.; McInnes, E.J.L.; et al. Chemical Recycling of Polystyrene to Valuable Chemicals via Selective Acid-Catalyzed Aerobic Oxidation under Visible Light. J. Am. Chem. Soc. 2022, 144, 6532–6542. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, M.-Q.; Hu, C.; Xiao, D.; Wang, M.; Ma, D. Catalytic oxidation of polystyrene to aromatic oxygenates over a graphitic carbon nitride catalyst. Nat. Commun. 2022, 13, 4809. [Google Scholar] [CrossRef] [PubMed]
- Uekert, T.; Kuehnel, M.F.; Wakerley, D.W.; Reisner, E. Plastic waste as a feedstock for solar-driven H2 generation. Energy Environ. Sci. 2018, 11, 2853–2857. [Google Scholar] [CrossRef]
- Ding, K.; Liu, S.; Huang, Y.; Liu, S.; Zhou, N.; Peng, P.; Ruan, R. Catalytic microwave-assisted pyrolysis of plastic waste over NiO and HY for gasoline-range hydrocarbons production. Energy Convers. Manag. 2019, 196, 1316–1325. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of plastic waste: Opportunities and challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
- Williams, P.T.; Slaney, E. Analysis of products from the pyrolysis and liquefaction of single plastics and waste plastic mixtures. Resour. Conserv. Recycl. 2007, 51, 754–769. [Google Scholar] [CrossRef]
- Ali, S.S.; Elsamahy, T.; Koutra, E.; Kornaros, M.; El-Sheekh, M.; Abdelkarim, E.A.; Sun, J. Degradation of conventional plastic wastes in the environment: A review on current status of knowledge and future perspectives of disposal. Sci. Total Environ. 2021, 771, 144719. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, X.; Miller, J.B.; Huber, G.W. The Chemistry and Kinetics of Polyethylene Pyrolysis: A Process to Produce Fuels and Chemicals. ChemSusChem 2020, 13, 1764–1774. [Google Scholar] [CrossRef]
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.T.; Inuwa, I.M. Conversion of low density polyethylene (LDPE) over ZSM-5 zeolite to liquid fuel. Fuel 2017, 192, 71–82. [Google Scholar] [CrossRef]
- Elordi, G.; Olazar, M.; Lopez, G.; Amutio, M.; Artetxe, M.; Aguado, R.; Bilbao, J. Catalytic pyrolysis of HDPE in continuous mode over zeolite catalysts in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis 2009, 85, 345–351. [Google Scholar] [CrossRef]
- Calero, M.; Solís, R.R.; Muñoz-Batista, M.J.; Pérez, A.; Blázquez, G.; Martín-Lara, M. Oil and gas production from the pyrolytic transformation of recycled plastic waste: An integral study by polymer families. Chem. Eng. Sci. 2023, 271, 118569. [Google Scholar] [CrossRef]
- Ding, W.; Liang, J.; Anderson, L.L. Thermal and catalytic degradation of high density polyethylene and commingled post-consumer plastic waste. Fuel Process. Technol. 1997, 51, 47–62. [Google Scholar] [CrossRef]
- Vasile, C.; Pakdel, H.; Mihai, B.; Onu, P.; Darie, H.; Ciocâlteu, S. Thermal and catalytic decomposition of mixed plastics. J. Anal. Appl. Pyrolysis 2001, 57, 287–303. [Google Scholar] [CrossRef]
- Lerici, L.C.; Renzini, M.S.; Pierella, L.B. Chemical Catalyzed Recycling of Polymers: Catalytic Conversion of PE, PP and PS into Fuels and Chemicals over H-Y. Procedia Mater. Sci. 2015, 8, 297–303. [Google Scholar] [CrossRef]
- Artetxe, M.; Lopez, G.; Amutio, M.; Elordi, G.; Bilbao, J.; Olazar, M. Cracking of High Density Polyethylene Pyrolysis Waxes on HZSM-5 Catalysts of Different Acidity. Ind. Eng. Chem. Res. 2013, 52, 10637–10645. [Google Scholar] [CrossRef]
- Neves, I.C.; Botelho, G.; Machado, A.V.; Rebelo, P. The effect of acidity behaviour of Y zeolites on the catalytic degradation of polyethylene. Eur. Polym. J. 2006, 42, 1541–1547. [Google Scholar] [CrossRef]
- Dwivedi, U.; Naik, S.N.; Pant, K.K. High quality liquid fuel production from waste plastics via two-step cracking route in a bottom-up approach using bi-functional Fe/HZSM-5 catalyst. Waste Manag. 2021, 132, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Park, J.W.; Kim, J.-H.; Sugi, Y.; Seo, G. Liquid-phase degradation of HDPE over alkali-treated MFI zeolites with mesopores. Polym. Degrad. Stab. 2006, 91, 2860–2866. [Google Scholar] [CrossRef]
- Lee, H.; Park, Y.-K. Catalytic Pyrolysis of Polyethylene and Polypropylene over Desilicated Beta and Al-MSU-F. Catalysts 2018, 8, 501. [Google Scholar] [CrossRef]
- Santos, B.P.S.; Almeida, D.; Marques, M.d.F.V.; Henriques, C.A. Petrochemical feedstock from pyrolysis of waste polyethylene and polypropylene using different catalysts. Fuel 2018, 215, 515–521. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, S.M.; Saha, S.K.; Cho, S.J.; Seo, G. Liquid-phase degradation of polyethylene (PE) over MFI zeolites with mesopores: Effects of the structure of PE and the characteristics of mesopores. Appl. Catal. B 2011, 108–109, 61–71. [Google Scholar] [CrossRef]
- Zhou, Q.; Zheng, L.; Wang, Y.-Z.; Zhao, G.-M.; Wang, B. Catalytic degradation of low-density polyethylene and polypropylene using modified ZSM-5 zeolites. Polym. Degrad. Stab. 2004, 84, 493–497. [Google Scholar] [CrossRef]
- Murata, K.; Brebu, M.; Sakata, Y. The effect of silica–alumina catalysts on degradation of polyolefins by a continuous flow reactor. J. Anal. Appl. Pyrolysis 2010, 89, 30–38. [Google Scholar] [CrossRef]
- Nwankwor, P.E.; Onuigbo, I.O.; Chukwuneke, C.E.; Yahaya, M.F.; Agboola, B.O.; Jahng, W.J. Synthesis of gasoline range fuels by the catalytic cracking of waste plastics using titanium dioxide and zeolite. Int. J. Energy Environ. Eng. 2020, 12, 77–86. [Google Scholar] [CrossRef]
- Singh, M.V. Waste and virgin high-density poly(ethylene) into renewable hydrocarbons fuel by pyrolysis-catalytic cracking with a CoCO3 catalyst. J. Anal. Appl. Pyrolysis 2018, 134, 150–161. [Google Scholar] [CrossRef]
- Singh, M.V.; Kumar, S.; Sarker, M. Waste HD-PE plastic, deformation into liquid hydrocarbon fuel using pyrolysis-catalytic cracking with a CuCO3 catalyst. Sustain. Energy Fuels 2018, 2, 1057–1068. [Google Scholar] [CrossRef]
- Habyarimana, J.B.; Njiemon, M.; Abdulnasir, R.; Neksumi, M.; Yahaya, M.; Sylvester, O.D.; Jahng, W.J. Synthesis of Hydrocarbon Fuel by Thermal Catalytic Cracking of Polypropylene. Int. J. Sci. Eng. Res. 2017, 8, 1193–1202. [Google Scholar] [CrossRef]
- Sun, K.; Huang, Q.; Ali, M.; Chi, Y.; Yan, J. Producing Aromatic-Enriched Oil from Mixed Plastics Using Activated Biochar as Catalyst. Energy Fuels 2018, 32, 5471–5479. [Google Scholar] [CrossRef]
- Lovás, P.; Hudec, P.; Jambor, B.; Hájeková, E.; Horňáček, M. Catalytic cracking of heavy fractions from the pyrolysis of waste HDPE and PP. Fuel 2017, 203, 244–252. [Google Scholar] [CrossRef]
- Artetxe, M.; Lopez, G.; Elordi, G.; Amutio, M.; Bilbao, J.; Olazar, M. Production of Light Olefins from Polyethylene in a Two-Step Process: Pyrolysis in a Conical Spouted Bed and Downstream High-Temperature Thermal Cracking. Ind. Eng. Chem. Res. 2012, 51, 13915–13923. [Google Scholar] [CrossRef]
- Park, K.-B.; Jeong, Y.-S.; Kim, J.-S. Activator-assisted pyrolysis of polypropylene. Appl. Energy 2019, 253, 113558. [Google Scholar] [CrossRef]
- Miandad, R.; Nizami, A.S.; Rehan, M.; Barakat, M.A.; Khan, M.I.; Mustafa, A.; Murphy, J.D. Influence of temperature and reaction time on the conversion of polystyrene waste to pyrolysis liquid oil. Waste Manag. 2016, 58, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Nisar, J.; Ali, G.; Shah, A.; Iqbal, M.; Khan, R.A.; Akhter, M.S. Fuel production from waste polystyrene via pyrolysis: Kinetics and products distribution. Waste Manag. 2019, 88, 236–247. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.I.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K.; Ahmad, W. Pyrolysis Study of Polypropylene and Polyethylene Into Premium Oil Products. Int. J. Green Energy 2014, 12, 663–671. [Google Scholar] [CrossRef]
- Budsaereechai, S.; Hunt, A.J.; Ngernyen, Y. Catalytic pyrolysis of plastic waste for the production of liquid fuels for engines. RSC Adv. 2019, 9, 5844–5857. [Google Scholar] [CrossRef] [PubMed]
- Auxilio, A.R.; Choo, W.-L.; Kohli, I.; Chakravartula Srivatsa, S.; Bhattacharya, S. An experimental study on thermo-catalytic pyrolysis of plastic waste using a continuous pyrolyser. Waste Manag. 2017, 67, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Nizami, A.-S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef]
- Chaianansutcharit, S.; Katsutath, R.; Chaisuwan, A.; Bhaskar, T.; Nigo, A.; Muto, A.; Sakata, Y. Catalytic degradation of polyolefins over hexagonal mesoporous silica: Effect of aluminum addition. J. Anal. Appl. Pyrolysis 2007, 80, 360–368. [Google Scholar] [CrossRef]
- Almustapha, M.N.; Andrésen, J.M. Recovery of valuable chemicals from high density polyethylene (HDPE) polymer: A catalytic approach for plastic waste recycling. Int. J. Environ. Sci. Dev. 2012, 3, 263–267. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A.; Aranzabal, A. Catalytic pyrolysis of plastic wastes with two different types of catalysts: ZSM-5 zeolite and Red Mud. Appl. Catal. B 2011, 104, 211–219. [Google Scholar] [CrossRef]
- Li, K.; Lei, J.; Yuan, G.; Weerachanchai, P.; Wang, J.-Y.; Zhao, J.; Yang, Y. Fe-, Ti-, Zr- and Al-pillared clays for efficient catalytic pyrolysis of mixed plastics. Chem. Eng. J. 2017, 317, 800–809. [Google Scholar] [CrossRef]
- Rodríguez, E.; Gutiérrez, A.; Palos, R.; Vela, F.J.; Arandes, J.M.; Bilbao, J. Fuel production by cracking of polyolefins pyrolysis waxes under fluid catalytic cracking (FCC) operating conditions. Waste Manag. 2019, 93, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Munir, D.; Irfan, M.F.; Usman, M.R. Hydrocracking of virgin and waste plastics: A detailed review. Renew. Sustain. Energy Rev. 2018, 90, 490–515. [Google Scholar] [CrossRef]
- Lee, W.-T.; van Muyden, A.; Bobbink, F.D.; Mensi, M.D.; Carullo, J.R.; Dyson, P.J. Mechanistic classification and benchmarking of polyolefin depolymerization over silica-alumina-based catalysts. Nat. Commun. 2022, 13, 4850. [Google Scholar] [CrossRef] [PubMed]
- Kots, P.A.; Liu, S.; Vance, B.C.; Wang, C.; Sheehan, J.D.; Vlachos, D.G. Polypropylene Plastic Waste Conversion to Lubricants over Ru/TiO2 Catalysts. ACS Catal. 2021, 11, 8104–8115. [Google Scholar] [CrossRef]
- Kots, P.A.; Xie, T.; Vance, B.C.; Quinn, C.M.; de Mello, M.D.; Boscoboinik, J.A.; Vlachos, D.G. Electronic modulation of metal-support interactions improves polypropylene hydrogenolysis over ruthenium catalysts. Nat. Commun. 2022, 13, 5186. [Google Scholar] [CrossRef] [PubMed]
- Rorrer, J.E.; Beckham, G.T.; Román-Leshkov, Y. Conversion of Polyolefin Waste to Liquid Alkanes with Ru-Based Catalysts under Mild Conditions. JACS Au 2020, 1, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Celik, G.; Kennedy, R.M.; Hackler, R.A.; Ferrandon, M.; Tennakoon, A.; Patnaik, S.; Delferro, M. Upcycling Single-Use Polyethylene into High-Quality Liquid Products. ACS Cent. Sci. 2019, 5, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Tennakoon, A.; Wu, X.; Paterson, A.L.; Patnaik, S.; Pei, Y.; LaPointe, A.M.; Perras, F.A. Catalytic upcycling of high-density polyethylene via a processive mechanism. Nat. Catal. 2020, 3, 893–901. [Google Scholar] [CrossRef]
- Bin Jumah, A.; Anbumuthu, V.; Tedstone, A.A.; Garforth, A.A. Catalyzing the Hydrocracking of Low Density Polyethylene. Ind. Eng. Chem. Res. 2019, 58, 20601–20609. [Google Scholar] [CrossRef]
- Liu, S.; Kots, P.A.; Vance, B.C.; Danielson, A.; Vlachos, D.G. Plastic waste to fuels by hydrocracking at mild conditions. Sci. Adv. 2021, 7, eabf8283. [Google Scholar] [CrossRef]
- Escola, J.M.; Aguado, J.; Serrano, D.P.; Briones, L. Transportation fuel production by combination of LDPE thermal cracking and catalytic hydroreforming. Waste Manag. 2014, 34, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Mangesh, V.L.; Perumal, T.; Subramanian, S.; Padmanabhan, S. Clean Energy from Plastic: Production of Hydroprocessed Waste Polypropylene Pyrolysis Oil Utilizing a Ni–Mo/Laponite Catalyst. Energy Fuels 2020, 34, 8824–8836. [Google Scholar] [CrossRef]
- Escola, J.M.; Aguado, J.; Serrano, D.P.; García, A.; Peral, A.; Briones, L.; Fernandez, E. Catalytic hydroreforming of the polyethylene thermal cracking oil over Ni supported hierarchical zeolites and mesostructured aluminosilicates. Appl. Catal. B 2011, 106, 405–415. [Google Scholar] [CrossRef]
- Raheem, A.B.; Noor, Z.Z.; Hassan, A.; Abd Hamid, M.K.; Samsudin, S.A.; Sabeen, A.H. Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: A review. J. Clean. Prod. 2019, 225, 1052–1064. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, Y.; Furukawa, S.; Xia, J.; Sun, C.; Hülsey, M.J.; Yan, N. Towards the Circular Economy: Converting Aromatic Plastic Waste Back to Arenes over a Ru/Nb2O5 Catalyst. Angew. Chem. Int. Ed. 2021, 60, 5527–5535. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Feng, B.; Guo, Y.; Liu, X.; Wang, Y. H2-free Plastic Conversion: Converting PET back to BTX by Unlocking Hidden Hydrogen. ChemSusChem 2021, 14, 4242–4250. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Yu, H.J.; Jegal, J.; Kim, H.S.; Cha, H.G. Depolymerization of PET into terephthalic acid in neutral media catalyzed by the ZSM-5 acidic catalyst. Chem. Eng. J. 2020, 398, 125655. [Google Scholar] [CrossRef]
- Gao, Z.; Ma, B.; Chen, S.; Tian, J.; Zhao, C. Converting waste PET plastics into automobile fuels and antifreeze components. Nat. Commun. 2022, 13, 3343. [Google Scholar] [CrossRef]
- Tang, H.; Li, N.; Li, G.; Wang, A.; Cong, Y.; Xu, G.; Wang, X.; Zhang, T. Synthesis of gasoline and jet fuel range cycloalkanes and aromatics from poly (ethylene terephthalate) waste. Green Chem. 2019, 21, 2709–2719. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Su, X.-L.; Sun, D.-K.; Zhang, R.; Bi, J.-C. Investigation on degradation of polyethylene to oil in a continuous supercritical water reactor. J. Fuel Chem. Technol. 2007, 35, 487–491. [Google Scholar] [CrossRef]
- Jie, H.; Ke, H.; Qing, Z.; Lei, C.; Yongqiang, W.; Zibin, Z. Study on depolymerization of polycarbonate in supercritical ethanol. Polym. Degrad. Stab. 2006, 91, 2307–2314. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, C.; Bai, B.; Jin, H.; Wei, W. Study on the polystyrene plastic degradation in supercritical water/CO2 mixed environment and carbon fixation of polystyrene plastic in CO2 environment. J. Hazard. Mater. 2022, 421, 126763. [Google Scholar] [CrossRef]
- Bai, B.; Wang, W.; Jin, H. Experimental study on gasification performance of polypropylene (PP) plastics in supercritical water. Energy 2020, 191, 116527. [Google Scholar] [CrossRef]
- Arregi, A.; Seifali Abbas-Abadi, M.; Lopez, G.; Santamaria, L.; Artetxe, M.; Bilbao, J.; Olazar, M. CeO2 and La2O3 Promoters in the Steam Reforming of Polyolefinic Waste Plastic Pyrolysis Volatiles on Ni-Based Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 17307–17321. [Google Scholar] [CrossRef]
- Helmer Pedersen, T.; Conti, F. Improving the circular economy via hydrothermal processing of high-density waste plastics. Waste Manag. 2017, 68, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Onwudili, J.A.; Williams, P.T. Catalytic supercritical water gasification of plastics with supported RuO2: A potential solution to hydrocarbons–water pollution problem. Process Saf. Environ. Prot. 2016, 102, 140–149. [Google Scholar] [CrossRef]
- Chen, W.-T.; Jin, K.; Linda Wang, N.-H. Use of Supercritical Water for the Liquefaction of Polypropylene into Oil. ACS Sustain. Chem. Eng. 2019, 7, 3749–3758. [Google Scholar] [CrossRef]
- Xu, Z.R.; Zhu, W.; Gong, M.; Zhang, H.W. Direct gasification of dewatered sewage sludge in supercritical water. Part 1: Effects of alkali salts. Int. J. Hydrog. Energy 2013, 38, 3963–3972. [Google Scholar] [CrossRef]
- Seshasayee, M.S.; Savage, P.E. Oil from plastic via hydrothermal liquefaction: Production and characterization. Appl. Energy 2020, 278, 115673. [Google Scholar] [CrossRef]
- Su, X.; Zhao, Y.; Zhang, R.; Bi, J. Investigation on degradation of polyethylene to oils in supercritical water. Fuel Process. Technol. 2004, 85, 1249–1258. [Google Scholar] [CrossRef]
- Bai, B.; Jin, H.; Fan, C.; Cao, C.; Wei, W.; Cao, W. Experimental investigation on liquefaction of plastic waste to oil in supercritical water. Waste Manag. 2019, 89, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Kim, S.; Wahl, L.; Khare, R.; Hale, L.; Hu, J.; Lercher, J.A. Low-temperature upcycling of polyolefins into liquid alkanes via tandem cracking-alkylation. Science 2023, 379, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Qin, C.; Friedberger, T.; Guan, Z.; Huang, Z. Efficient and selective degradation of polyethylenes into liquid fuels and waxes under mild conditions. Sci. Adv. 2016, 2, e1501591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zeng, M.; Yappert, R.D.; Sun, J.; Lee, Y.-H.; LaPointe, A.M.; Peters, B.; Abu-Omar, M.M.; Scott, S.L. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 2020, 370, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Zhao, Y.; Wen, H.; Xu, Z. Interactions between Low-Density Polyethylene (LDPE) and Polypropylene (PP) during the Mild Cracking of Polyolefin Mixtures in a Closed-Batch Reactor. Energy Fuels 2013, 27, 5841–5851. [Google Scholar] [CrossRef]
- Wang, B.; Huang, Y.; Zhang, J. Hydrothermal liquefaction of lignite, wheat straw and plastic waste in sub-critical water for oil: Product distribution. J. Anal. Appl. Pyrolysis 2014, 110, 382–389. [Google Scholar] [CrossRef]
- Park, Y.-K.; Lee, B.; Watanabe, A.; Lee, H.W.; Lee, J.Y.; Kim, S.; Kim, Y.-M. Catalytic Copyrolysis of Cork Oak and Waste Plastic Films over HBeta. Catalysts 2018, 8, 318. [Google Scholar] [CrossRef]
- Sergeyev, N.S.; Sviridenko, N.N.; Urazov, K.K. Co-cracking of atmospheric residue and plastic waste. J. Anal. Appl. Pyrolysis 2024, 179, 106422. [Google Scholar] [CrossRef]
- Kasar, P.; Ahmaruzzaman, M. Characterization of liquid products obtained from catalytic binary co-cracking of residual fuel oil with various waste plastics. Sci. Rep. 2022, 12, 10987. [Google Scholar] [CrossRef]
- Serrano, D.P.; Aguado, J.; Escola, J.M. Developing Advanced Catalysts for the Conversion of Polyolefinic Waste Plastics into Fuels and Chemicals. ACS Catal. 2012, 2, 1924–1941. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, L.; Gu, J.; Yuan, H.; Chen, Y. Chemical recycling of plastic wastes via homogeneous catalysis: A review. Chem. Eng. J. 2023, 479, 147853. [Google Scholar] [CrossRef]
- Arabiourrutia, M.; Elordi, G.; Lopez, G.; Borsella, E.; Bilbao, J.; Olazar, M. Characterization of the waxes obtained by the pyrolysis of polyolefin plastics in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis 2012, 94, 230–237. [Google Scholar] [CrossRef]
- Kodera, Y.; Ishihara, Y.; Kuroki, T. Novel process for recycling waste plastics to fuel gas using a moving-bed reactor. Energy Fuels 2006, 20, 155–158. [Google Scholar] [CrossRef]






| Plastics | Methods | Catalysts | Conditions a | Reactors | Liquid Yield | References |
|---|---|---|---|---|---|---|
| PS | pyrolysis | — | 450 °C/75 min | batch reactor | 81% | [41] |
| PS | pyrolysis | — | 410 °C/70 min | fixed bed reactor | 85% | [42] |
| PP | pyrolysis | — | 300 °C | fixed bed reactor | 69% | [43] |
| PE | pyrolysis | — | 500 °C, | fluidized bed reactor | 81% | [11] |
| PS | catalytic reforming | pelletized bentonite | 700 °C/500 °C | fixed-bed pyrolysis stainless steel batch reactor | 88% | [44] |
| PP | catalytic reforming | pelletized bentonite | 700 °C/500 °C | fixed-bed pyrolysis stainless steel batch reactor | 91% | [40] |
| HDPE | catalytic reforming | CAT-1 | 425 °C/425 °C | continuous stirred tank reactor, reactive distillation column | 80% | [45] |
| mixed plastic | catalytic reforming | Fe/HZSM-5 | 350 °C/350 °C | batch reactor, fixed bed reactor | 76% | [22] |
| PS | catalytic pyrolysis | NZ zeolite | 450 °C/75 min | batch reactor | 70% | [46] |
| PS | catalytic pyrolysis | HY | 450 °C/44 min | batch reactor | 71% | [19] |
| PP | catalytic pyrolysis | HY | 500 °C/44 min | batch reactor | 44% | [19] |
| PP | catalytic pyrolysis | hexagonal mesoporous silica | 380 °C/1:10 b | fixed bed reactor | 65% | [47] |
| PE | catalytic pyrolysis | hexagonal mesoporous silica | 430 °C/1:10 b | fixed bed reactor | 75% | [43] |
| HDPE | catalytic pyrolysis | 7% SO3 | 400 °C | fixed bed reactor | 32% | [48] |
| HDPE | catalytic pyrolysis | HZSM-5 | 550 °C/6 h | conical spouted bed reactor | 20% | [13] |
| mixed plastic | catalytic pyrolysis | ZSM-5 zeolite and Red Mud | 440 °C/1:10 b | batch reactor | 57% | [49] |
| mixed plastic | catalytic pyrolysis | Al-pillared clays | 500 °C/30 min | tube reactor | 79.3% | [50] |
| Plastics | Catalysts | Conditions a | Yields | References |
|---|---|---|---|---|
| LDPEO | Ni/beta | 310 °C/20 bar/ 45 min | 81% gasoline fuel | [61] |
| PPO | Ni-Mo/Lap | 350 °C/70 bar | 95% diesel range of n-alkanes, isoalkanes, aromatics | [62] |
| LDPEO | Ni/beta | 310 °C/20 bar/ 45 min | 54% gasoline, 40% diesel | [63] |
| LDPE | Pt/WO3/ZrO2 and HY | 225 °C/30 bar/ 2 h | 85% diesel and jet fuel oils, gasoline range hydrocarbons | [56] |
| LDPE | Pt/USY and beta | 330 °C/20 bar/ 15 min | 95% liquid hydrocarbon | [55] |
| PP | Ru/TiO2 | 250 °C/30 bar/ | 66% liquid hydrocarbon, 25%C1-C2,4% C3-C6 | [51] |
| PE | Pt/SrTiO3 | 300 °C/11.7 bar/96 h | 100% lubricants, waxes | [53] |
| PE | Ru/C | 200 °C/20 bar/ 16 h | 45% liquid n-alkanes, C1-C6 | [52] |
| PP | Ru/TiO2 | 250 °C/40 bar/ 6 h | 74% liquid hydrocarbon | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, L.; Kou, Z.; Li, R.; Zhao, Z. Research Progress in Fuel Oil Production by Catalytic Pyrolysis Technologies of Waste Plastics. Catalysts 2024, 14, 212. https://doi.org/10.3390/catal14030212
An L, Kou Z, Li R, Zhao Z. Research Progress in Fuel Oil Production by Catalytic Pyrolysis Technologies of Waste Plastics. Catalysts. 2024; 14(3):212. https://doi.org/10.3390/catal14030212
Chicago/Turabian StyleAn, Liu, Zonglan Kou, Renjie Li, and Zhen Zhao. 2024. "Research Progress in Fuel Oil Production by Catalytic Pyrolysis Technologies of Waste Plastics" Catalysts 14, no. 3: 212. https://doi.org/10.3390/catal14030212