Synergistic Effect of Structure and Morphology of ZSM-5 Catalysts on the Transformation of Methanol to Propylene
Abstract
:1. Introduction
2. Results and Discussion
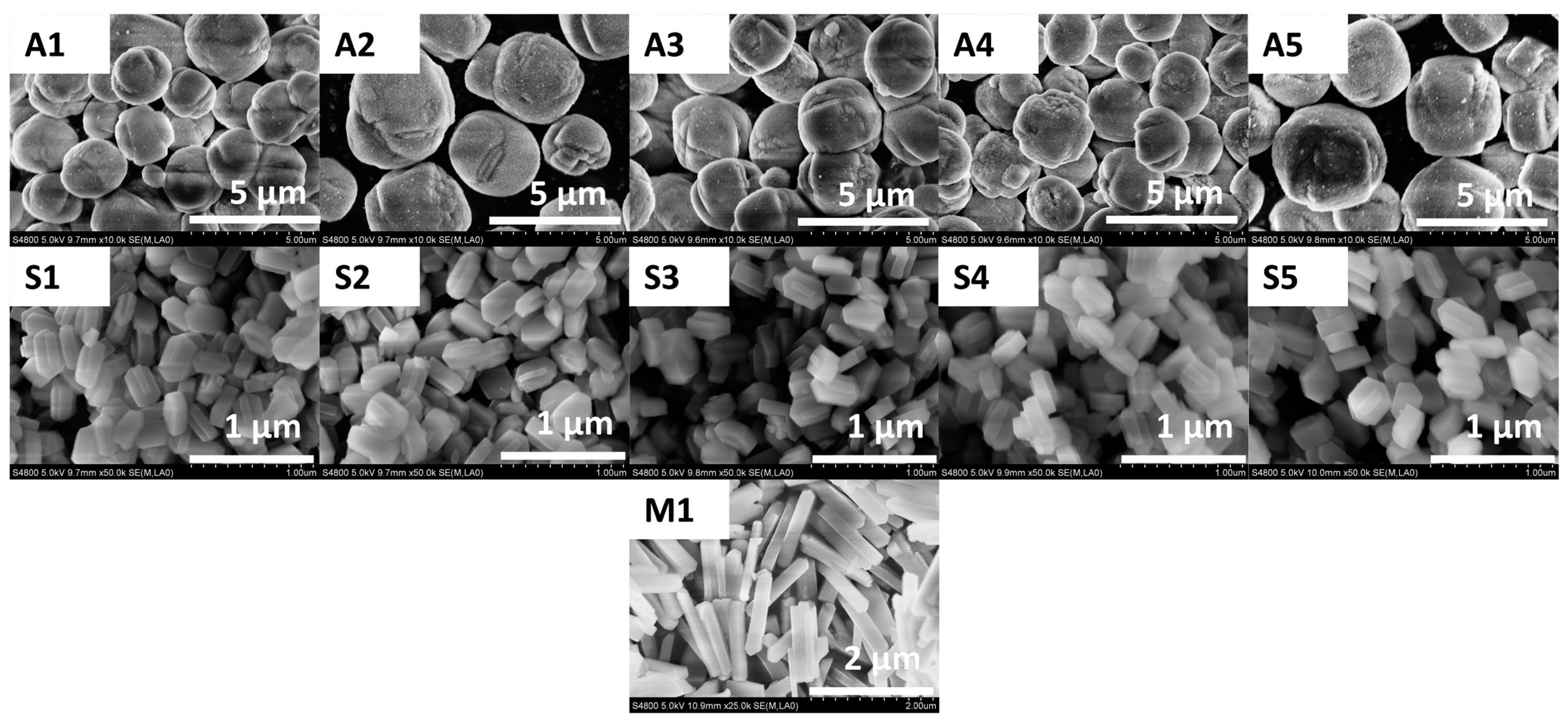
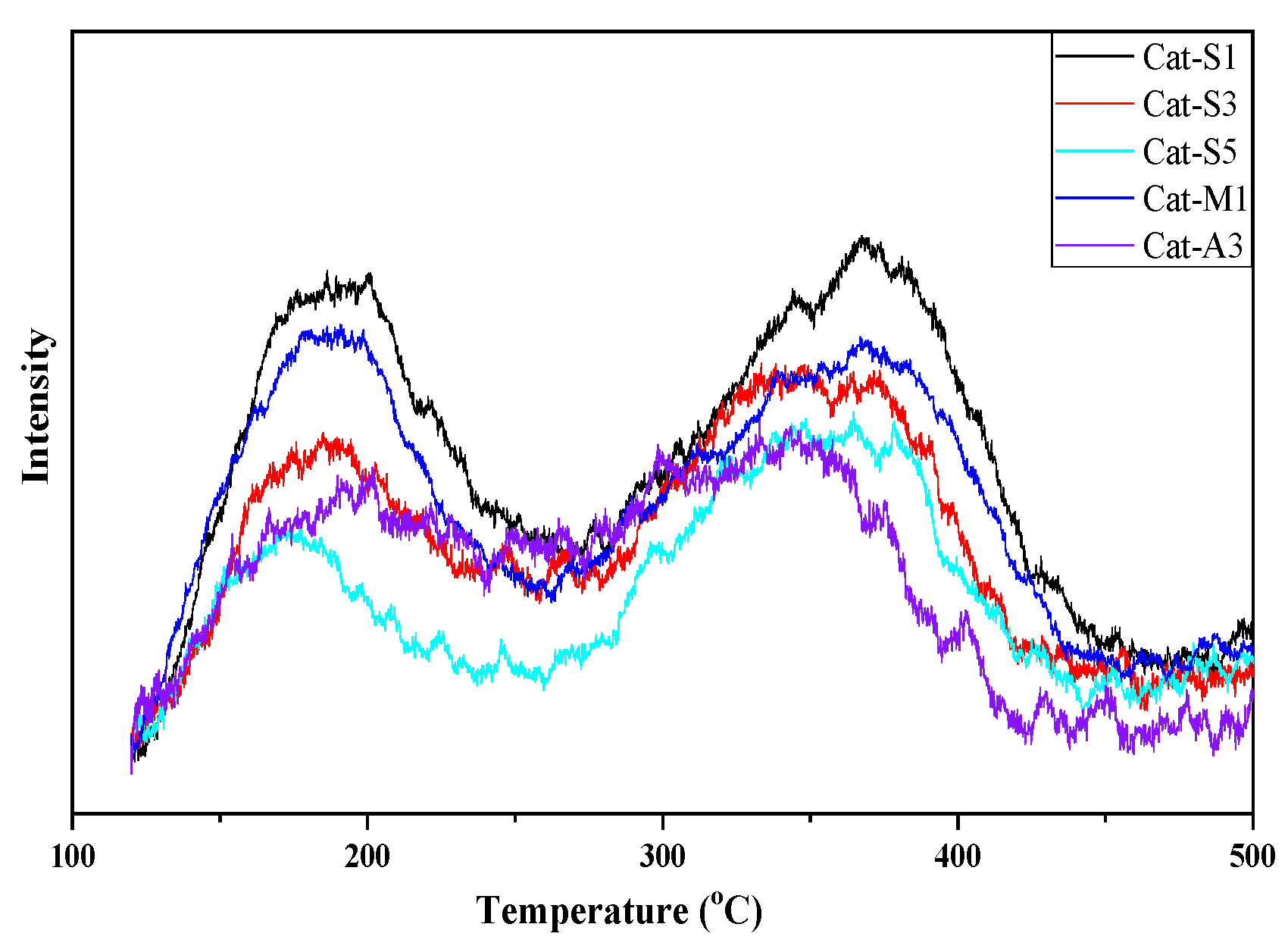
2.1. Structural and Morphology Analysis
2.2. Textural Properties
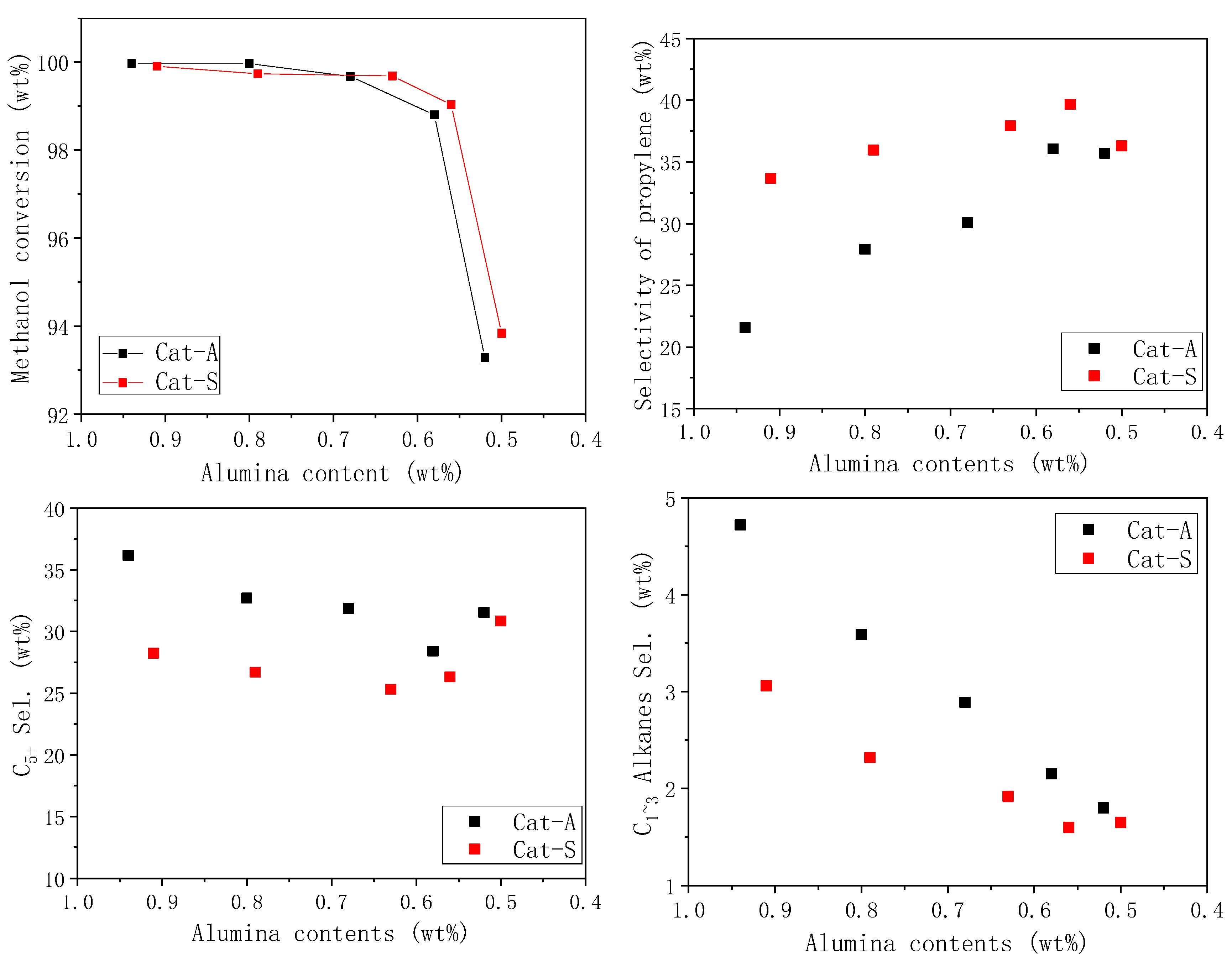
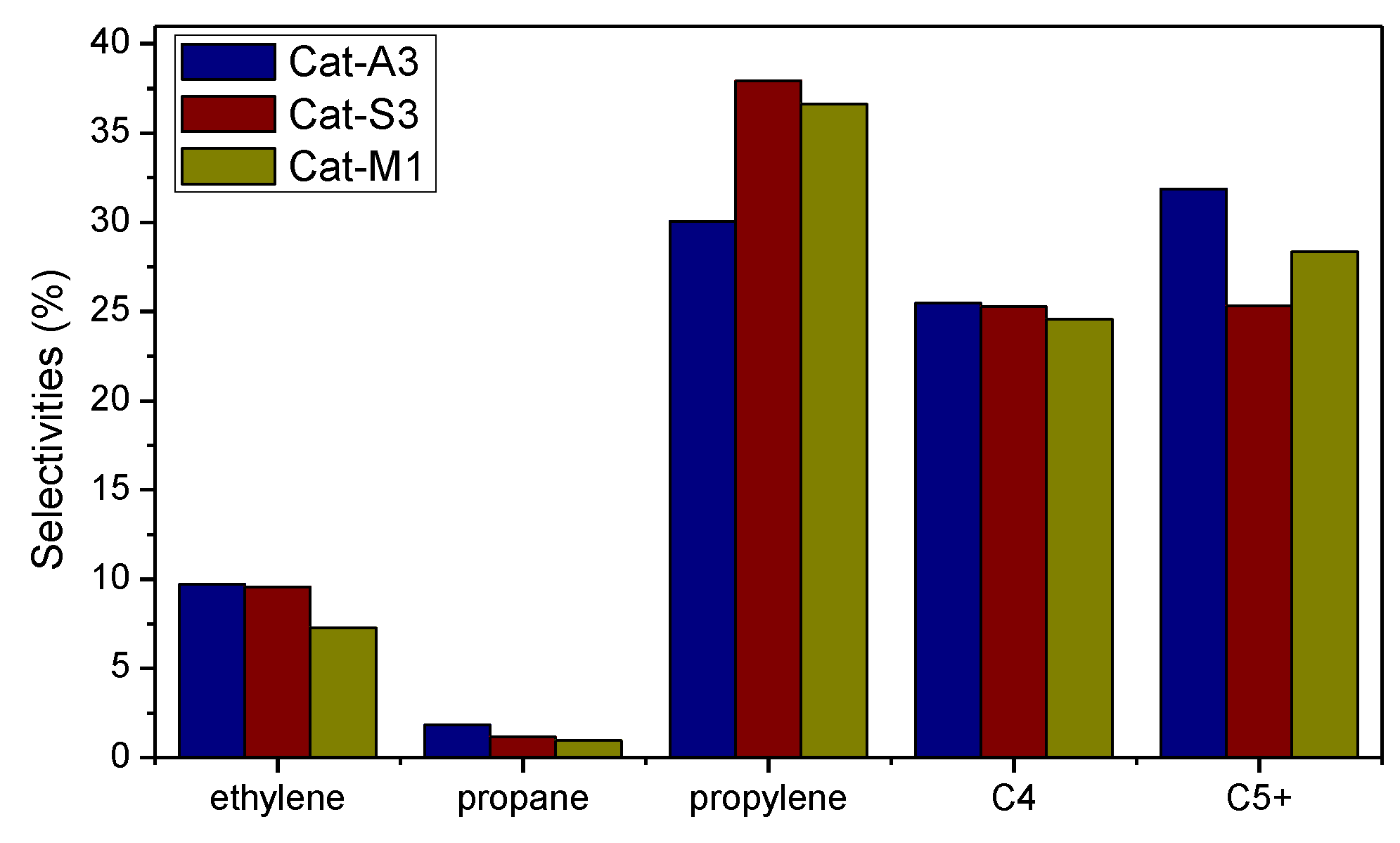
2.3. Catalytic Performance
2.4. Structure–Performance Relationship
3. Experiments
3.1. Catalyst Preparation
3.2. Characterization
3.3. Catalytic Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haw, J.F.; Song, W.; Marcus, D.M.; Nicholas, J.B. The mechanism of methanol to hydrocarbon catalysis. Acc. Chem. Res. 2003, 36, 317–326. [Google Scholar] [CrossRef]
- Ahmadi, S.M.A.; Askari, S.; Halladj, R. A review on kinetic modeling of deactivation of SAPO-34 catalyst during methanol to olefins (MTO) process. Afinidad 2013, 70, 130–138. [Google Scholar]
- Wragg, D.S.; Akporiaye, D.; Fjellvag, H. Direct observation of catalyst behaviour under real working conditions with X-ray diffraction: Comparing SAPO-18 and SAPO-34 methanol to olefin catalysts. J. Catal. 2011, 279, 397–402. [Google Scholar] [CrossRef]
- Dehertog, W.J.H.; Froment, G.F. Production of light alkenes from methanol on ZSM-5 catalysts. Appl. Catal. 1991, 71, 153–165. [Google Scholar] [CrossRef]
- Chang, C.D.; Chu, C.T.-W.; Socha, R.F. Methanol conversion to olefins over ZSM-5: I. Effect of temperature and zeolite SiO2/Al2O3. J. Catal. 1984, 86, 289–296. [Google Scholar] [CrossRef]
- Huang, H.-W.; Zhu, H.; Zhang, S.-H.; Zhang, Q.; Li, C.-Y. Effect of silicon to aluminum ratio on the selectivity to propene in methanol conversion over H-ZSM-5 zeolites. J. Fuel Chem. Technol. 2019, 47, 74–82. [Google Scholar] [CrossRef]
- Palčić, A.; Ordomsky, V.V.; Qin, Z.; Georgieva, V.; Valtchev, V. Tuning zeolite properties for highly efficient synthesis of propylene from methanol. Chem. A Eur. J. 2018, 24, 13136–13149. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246. [Google Scholar] [CrossRef]
- Dyballa, M.; Becker, P.; Trefz, D.; Klemm, E.; Fischer, A.; Jakob, H.; Hunger, M. Parameters influencing the selectivity to propene in the MTO conversion on 10-ring zeolites: Directly synthesized zeolites ZSM-5, ZSM-11, and ZSM-22. Appl. Catal. A Gen. 2016, 510, 233–243. [Google Scholar] [CrossRef]
- Müller, S.; Liu, Y.; Kirchberger, F.M.; Tonigold, M.; Sanchez-Sanchez, M.; Lercher, J.A. Hydrogen transfer pathways during zeolite catalyzed methanol conversion to hydrocarbons. J. Am. Chem. Soc. 2016, 138, 15994–16003. [Google Scholar] [CrossRef]
- Qiao, Q.; Wang, R.; Gou, M.; Yang, X. Catalytic performance of boron and aluminium incorporated ZSM-5 zeolites for isomerization of styrene oxide to phenylacetaldehyde. Microporous Mesoporous Mater. 2014, 195, 250–257. [Google Scholar] [CrossRef]
- Yarulina, I.; Wispelaere, K.D.; Bailleul, S. Structure–performance descriptors and the role of Lewis acidity in the methanol-to-propylene process. Nat. Chem. 2018, 10, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Sundaramurthy, V.; Lingappan, N. Isomorphic substitution of boron in ZSM-5 type zeolites using TBP as template. J. Mol. Catal. A Chem. 2000, 160, 367–375. [Google Scholar] [CrossRef]
- Saravi, F.Y.; Taghizadeh, M. Synergetic effect of Mn, Ce, Ba, and B modification and moderate desilication of nanostructured HZSM-5 catalyst on conversion of methanol to propylene. Turk. J. Chem. 2018, 42, 1640–1662. [Google Scholar] [CrossRef]
- Schulz, H. “Coking” of zeolites during methanol conversion: Basic reactions of the MTO-, MTP- and MTG processes. Catal. Today 2010, 154, 183–194. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, C.; Du, J.; Yue, Y.; Hua, W.; Zhang, C.; Shen, W.; Xu, H. The synthesis of endurable B-Al-ZSM-5 catalysts with tunable acidity for methanol to propylene reaction. Catal. Commun. 2012, 24, 44–47. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, Y.; Mokaya, R. Zeolite ZSM-5 with unique supermicropores synthesized using mesoporous carbon as a template. Adv. Mater. 2004, 16, 727–732. [Google Scholar] [CrossRef]
- Yaripour, F.; Shariatinia, Z.; Sahebdelfar, S.; Irandoukht, A. Effect of boron incorporation on the structure, products selectivities and lifetime of H-ZSM-5 nanocatalyst designed for application in methanol-to-olefins (MTO) reaction. Microporous Mesoporous Mater. 2015, 203, 41–53. [Google Scholar] [CrossRef]
- Almutairi, S.M.T.; Mezari, B.; Pidko, E.A.; Magusin, P.C.M.M.; Hensen, E.J.M. Influence of steaming on the acidity and the methanol conversion reaction of HZSM-5 zeolite. J. Catal. 2013, 307, 194–203. [Google Scholar] [CrossRef]
- Ding, C.; Wang, X.; Guo, X.; Zhang, S. Characterization and catalytic alkylation of hydrothermally dealuminated nanoscale ZSM-5 zeolite catalyst. Catal. Commun. 2008, 9, 487–493. [Google Scholar] [CrossRef]
- Vu, D.V.; Miyamoto, M.; Nishiyama, N.; Ichikawa, S.; Egashira, Y.; Ueyama, K. Catalytic activities and structures of silicalite-1/H-ZSM-5 zeolite composites. Microporous Mesoporous Mater. 2008, 115, 106–112. [Google Scholar] [CrossRef]
- Anderson, J.R.; Mole, T.; Christov, V. Mechanism of some conversion over ZSM-5 zeolite. J. Catal. 1980, 61, 477–484. [Google Scholar] [CrossRef]
- Öhrman, O.; Hedlund, J.; Msimang, V.; Möller, K.; Sterte, J. ZSM-5 structured catalysts coated with silicalite-1. Stud. Surf. Sci. Catal. 2004, 154, 677–684. [Google Scholar]
- Ono, Y.; Mori, T. Mechanism of methanol conversion into hydrocarbons over ZSM-5 zeolite. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1981, 77, 2209. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, L.; Wang, L.; Zhang, J. Synthesis of mesoporous ZSM-5 by one-pot method in the presence of polyethylene glycol. Microporous Mesoporous Mater. 2010, 134, 189–194. [Google Scholar] [CrossRef]
- Mikkelsen, Ø.; Rønning, P.O.; Kolboe, S. Use of isotopic labeling for mechanistic studies of the methanol-to-hydrocarbons reaction. Methylation of toluene with methanol over H-ZSM-5, H-mordenite and H-beta. Microporous Mesoporous Mater. 2000, 40, 95–113. [Google Scholar] [CrossRef]
- Cui, N.; Guo, H.; Zhou, J.; Li, L.; Guo, L.; Hua, Z. Regulation of framework Al distribution of high-silica hierarchically structured ZSM-5 zeolites by boron-modification and its effect on materials catalytic performance in methanol-to-propylene reaction. Microporous Mesoporous Mater. 2020, 306, 110411. [Google Scholar] [CrossRef]
- Shen, Y.; Liang, H.; Liao, Z.; Jiang, B.; Wang, J.; Yang, Y.; Li, M.; Luo, Y.; Shu, X. Pore plugging effects on the performance of ZSM-5 catalyst in MTP reaction using a discrete model. Chin. J. Chem. Eng. 2021, 32, 253–263. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C.; Meng, X.; Liu, H.; Chu, R.; Wu, G.; Li, W.; Jiang, X. Enhancement of catalytic and anti-carbon deposition performance of SAPO-34/ZSM-5/quartz films in MTA reaction by Si/Al ratio regulation. Chin. J. Chem. Eng. 2023, 56, 314–324. [Google Scholar] [CrossRef]


| Samples | Si/Al Ratio | Surface Area (BET), m2/g | Surface Area (T-plot), m2/g | Surface Area (Mesopore), m2/g | Pore Volume, cm3/g |
|---|---|---|---|---|---|
| A1 | 105 | 437 | 390 | 47 | 0.21 |
| A2 | 124 | 444 | 404 | 40 | 0.19 |
| A3 | 143 | 448 | 401 | 47 | 0.20 |
| A4 | 171 | 452 | 401 | 51 | 0.20 |
| A5 | 195 | 447 | 399 | 48 | 0.20 |
| S1 | 110 | 400 | 357 | 43 | 0.21 |
| S2 | 125 | 395 | 353 | 42 | 0.20 |
| S3 | 157 | 404 | 366 | 38 | 0.20 |
| S4 | 177 | 397 | 384 | 15 | 0.19 |
| S5 | 199 | 410 | 383 | 27 | 0.21 |
| M1 | 152 | 363 | 346 | 17 | 0.20 |
| Catalyst | Conv. /wt% | Selectivities/wt% | ||||||
|---|---|---|---|---|---|---|---|---|
| Methane | Ethylene | Ethane | Propylene | Propane | C4 | C5+ | ||
| Cat-A1 | 99.96 | 1.06 | 11.34 | 0.19 | 21.57 | 3.47 | 26.21 | 36.16 |
| Cat-A2 | 99.96 | 1.02 | 10.75 | 0.18 | 27.92 | 2.39 | 25.05 | 32.69 |
| Cat-A3 | 99.67 | 0.95 | 9.71 | 0.12 | 30.07 | 1.81 | 25.46 | 31.87 |
| Cat-A4 | 98.80 | 0.87 | 8.48 | 0.08 | 36.06 | 1.20 | 24.90 | 28.41 |
| Cat-A5 | 93.29 | 0.83 | 6.96 | 0.06 | 35.71 | 0.92 | 23.99 | 31.54 |
| Cat-S1 | 99.90 | 1.20 | 10.79 | 0.16 | 33.67 | 1.70 | 24.24 | 28.24 |
| Cat-S2 | 99.73 | 1.01 | 9.66 | 0.10 | 35.96 | 1.21 | 25.37 | 26.70 |
| Cat-S3 | 99.68 | 0.68 | 9.56 | 0.07 | 37.93 | 1.16 | 25.28 | 25.31 |
| Cat-S4 | 99.03 | 0.59 | 7.95 | 0.06 | 39.66 | 0.95 | 24.47 | 26.32 |
| Cat-S5 | 93.84 | 0.67 | 7.28 | 0.06 | 36.31 | 0.92 | 23.92 | 30.84 |
| Cat-M1 | 99.43 | 1.11 | 7.29 | 0.07 | 36.63 | 0.96 | 24.56 | 29.36 |
| Catalyst | Selectivities/wt% | ||||||
|---|---|---|---|---|---|---|---|
| Methane | Ethylene | Ethane | Propylene | Propane | C4 | C5+ | |
| Cat-S1 | 1.20 | 10.79 | 0.16 | 33.67 | 1.70 | 24.24 | 28.24 |
| Cat-A1 | 1.06 | 11.34 | 0.19 | 21.57 | 3.47 | 26.21 | 36.16 |
| Diff. | −0.16 | 0.55 | 0.03 | −12.10 | 1.77 | 1.97 | 7.92 |
| Relative Diff./% | −13.3 | 5.1 | 18.8 | 35.9 | 104.1% | 8.1% | 28.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Wang, K.; Gao, X.; Yong, X.; Gu, Y. Synergistic Effect of Structure and Morphology of ZSM-5 Catalysts on the Transformation of Methanol to Propylene. Catalysts 2024, 14, 67. https://doi.org/10.3390/catal14010067
Zhang W, Wang K, Gao X, Yong X, Gu Y. Synergistic Effect of Structure and Morphology of ZSM-5 Catalysts on the Transformation of Methanol to Propylene. Catalysts. 2024; 14(1):67. https://doi.org/10.3390/catal14010067
Chicago/Turabian StyleZhang, Wei, Kangzhou Wang, Xinhua Gao, Xiaojing Yong, and Yanlong Gu. 2024. "Synergistic Effect of Structure and Morphology of ZSM-5 Catalysts on the Transformation of Methanol to Propylene" Catalysts 14, no. 1: 67. https://doi.org/10.3390/catal14010067