RuNi/MMO Catalysts Derived from a NiAl-NO3-LDH Precursor for CO Selective Methanation in H2-Rich Gases
Abstract
:1. Introduction
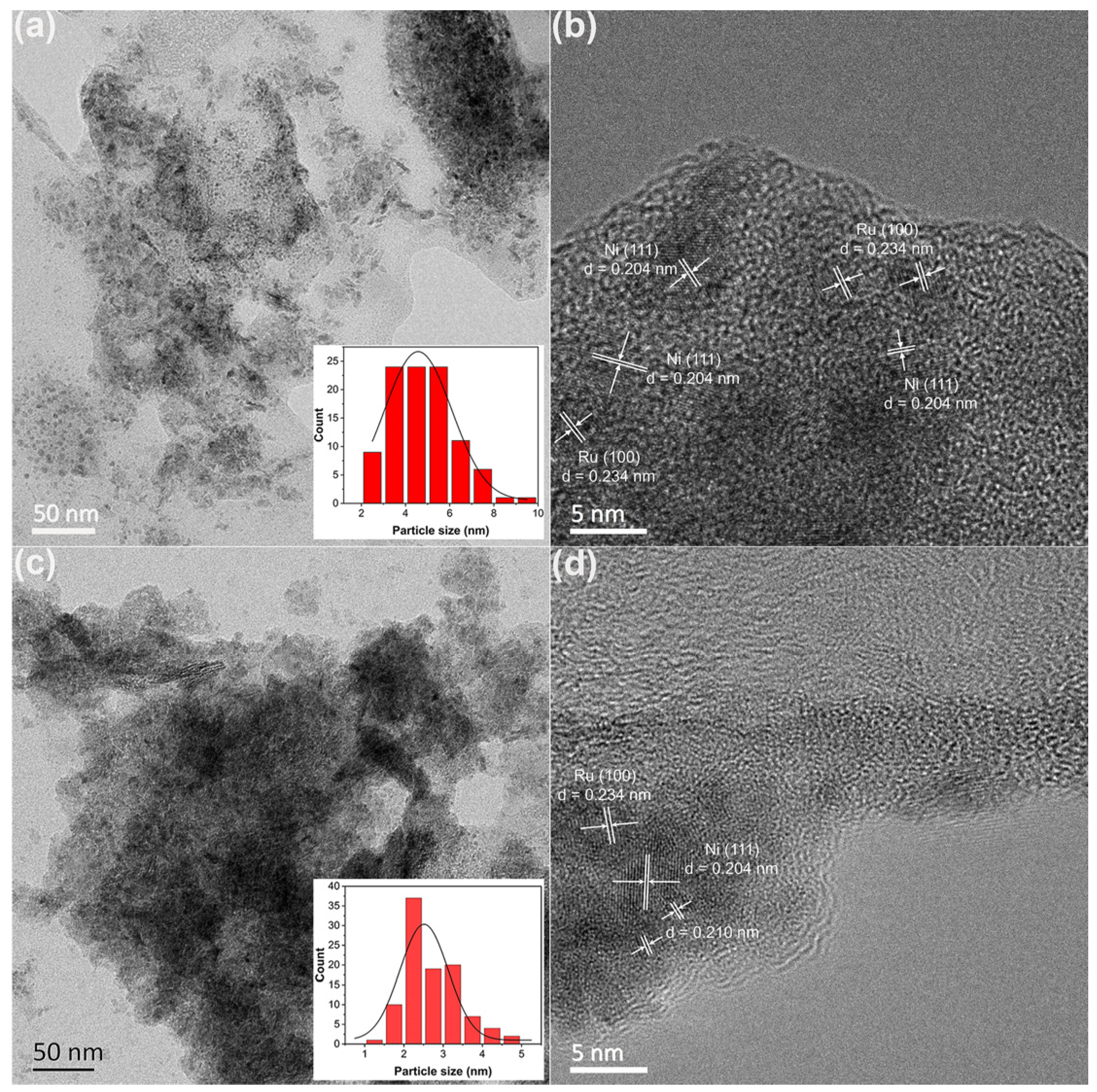
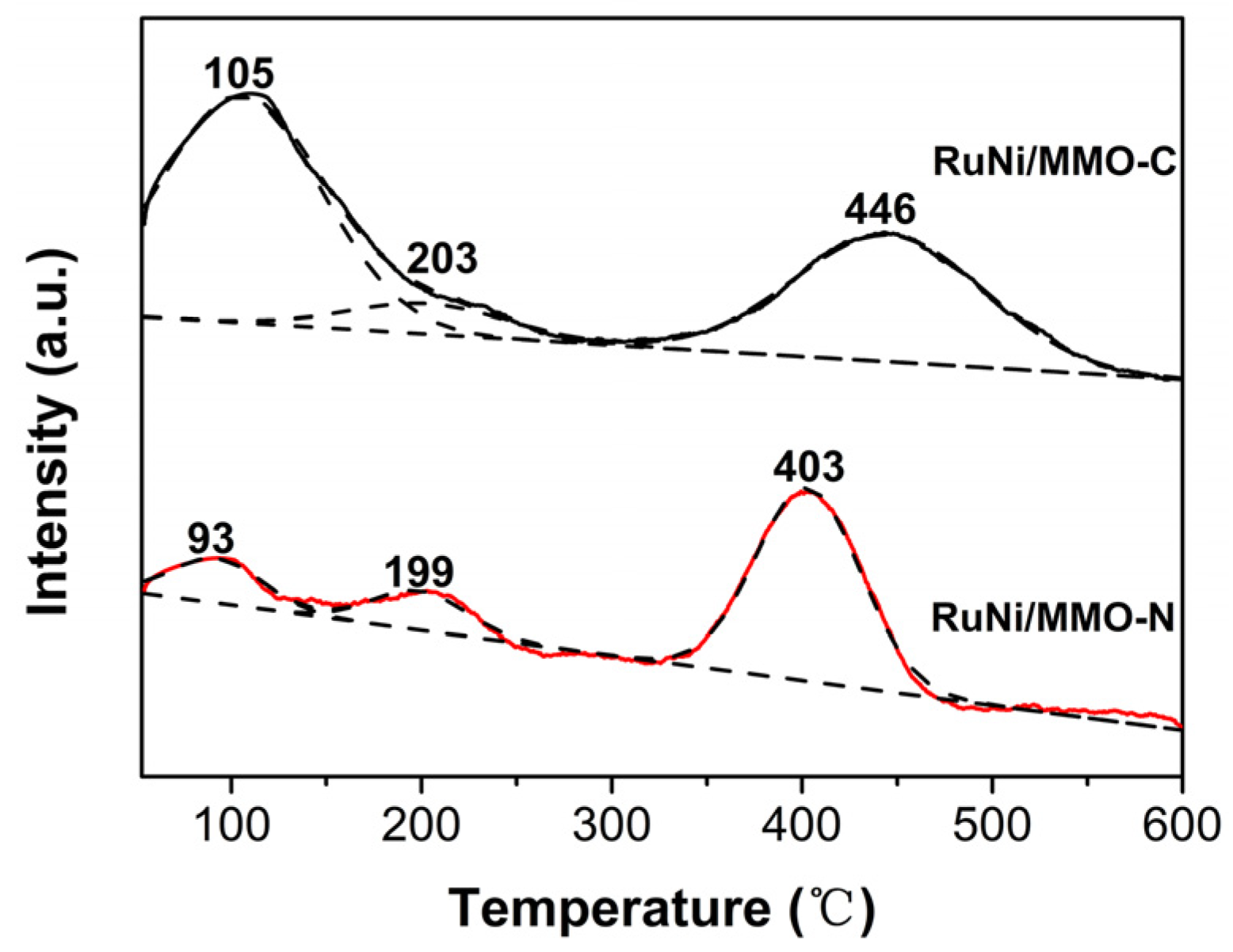
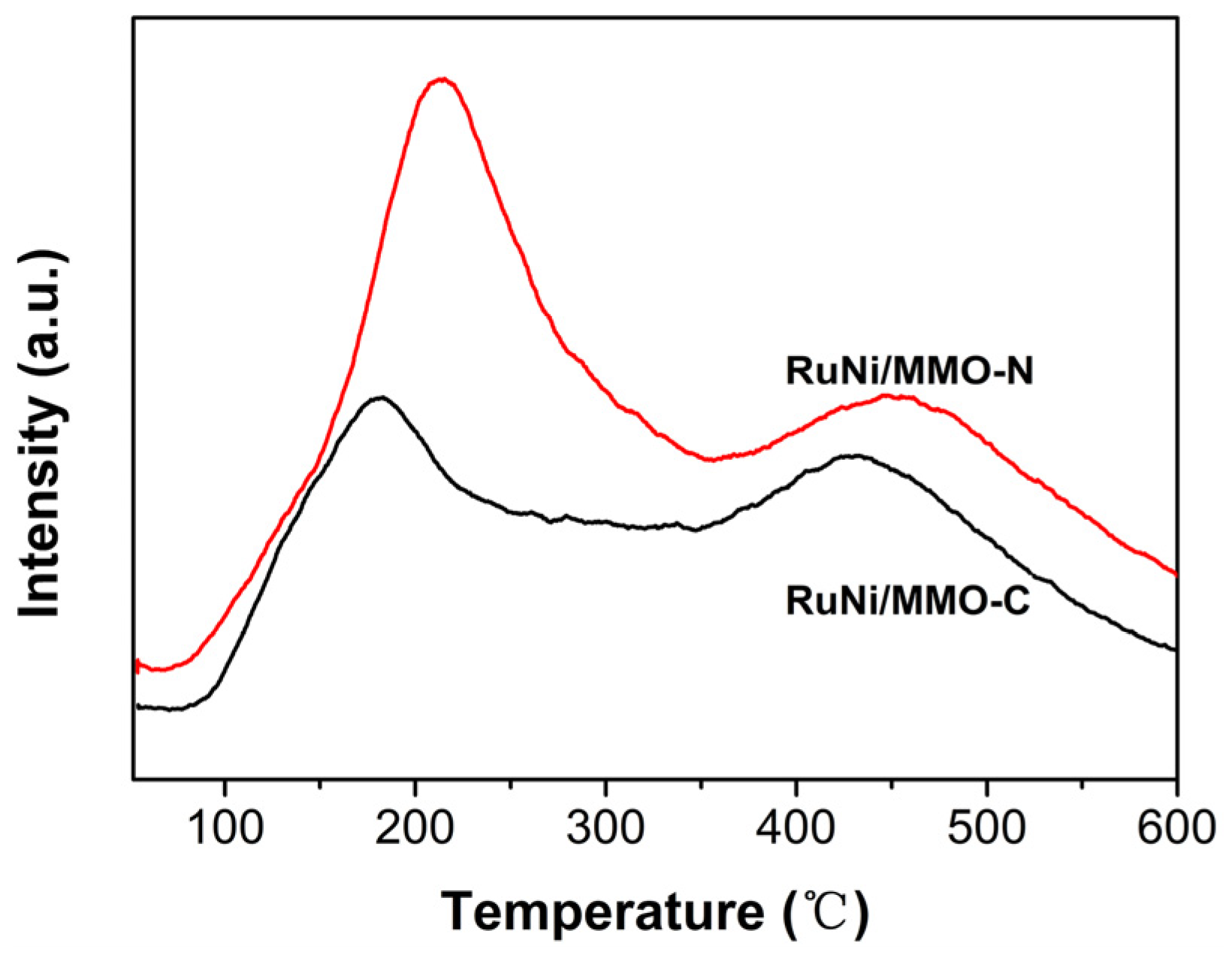
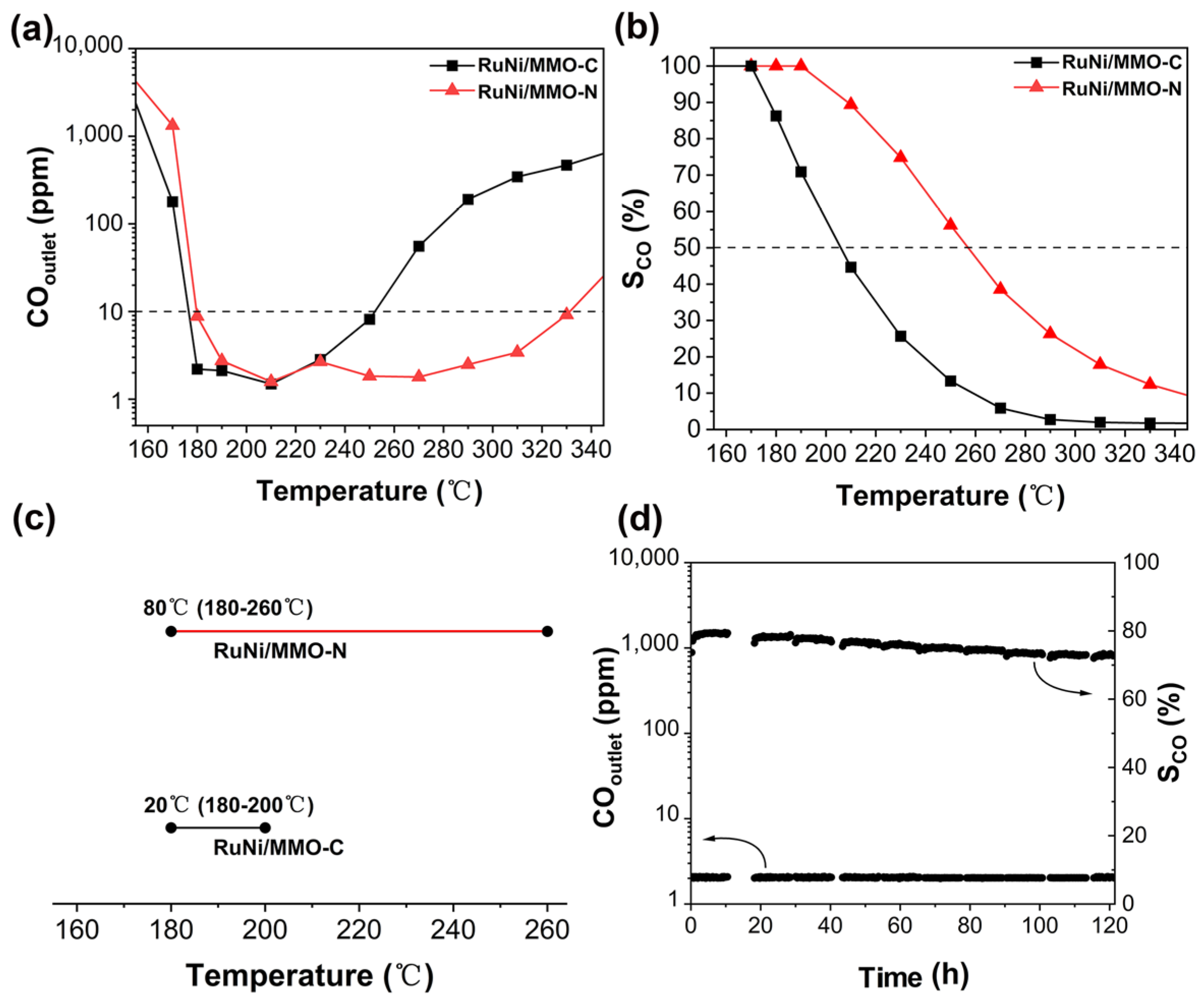
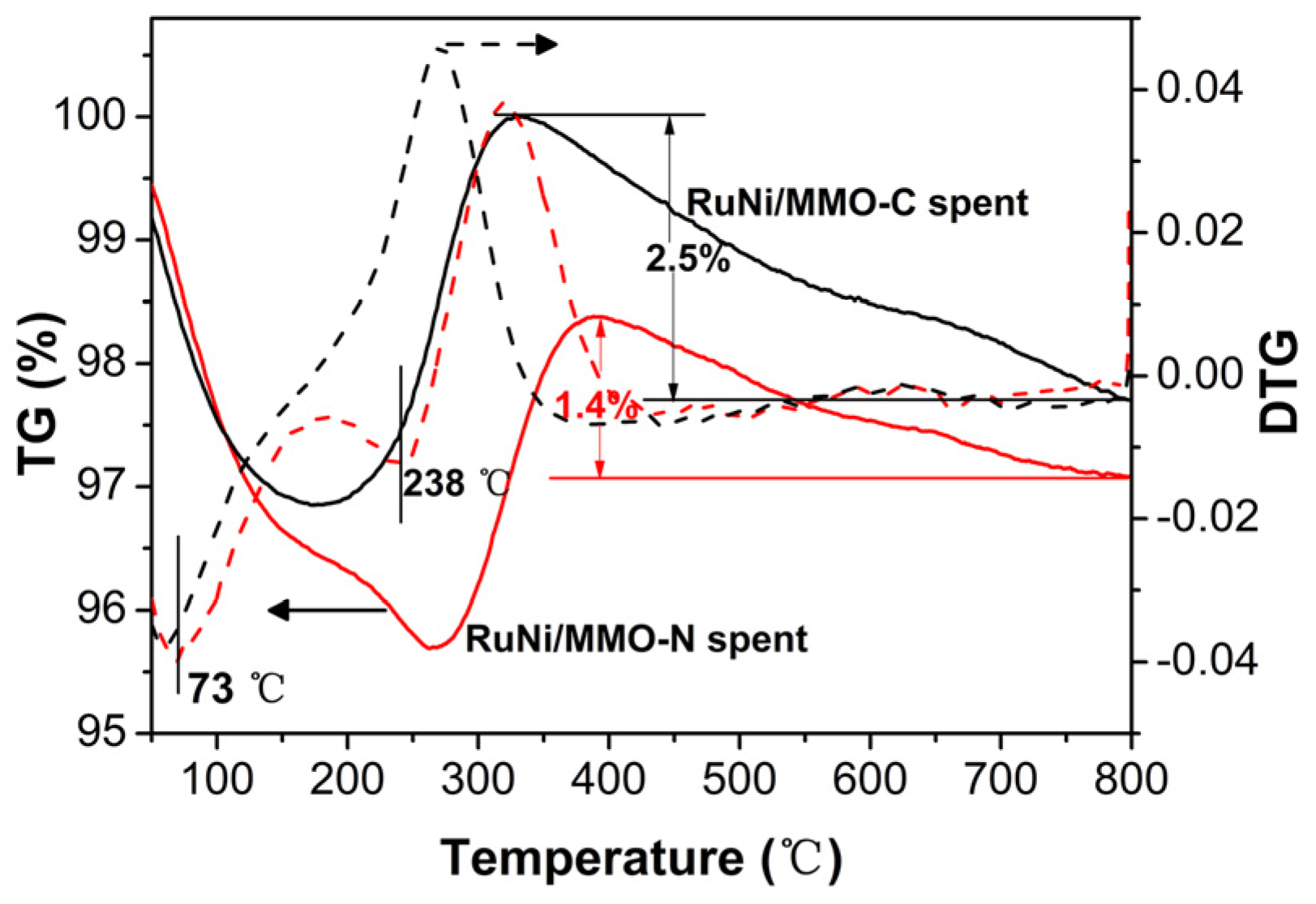
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Catalysts
3.3. Catalyst Characterization
3.4. Catalyst Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lan, Y.; Lu, J.; Mu, L.; Wang, S.; Zhai, H. Waste heat recovery from exhausted gas of a proton exchange membrane fuel cell to produce hydrogen using thermoelectric generator. Appl. Energy 2023, 334, 120687. [Google Scholar] [CrossRef]
- Carrette, L.P.L.; Friedrich, K.A.; Huber, M.; Stimming, U. Improvement of CO tolerance of proton exchange membrane (PEM) fuel cells by a pulsing technique. Phys. Chem. Chem. Phys. 2001, 3, 320–324. [Google Scholar] [CrossRef]
- Li, X.; Han, Y.; Huang, Y.; Lin, J.; Pan, X.; Zhao, Z.; Zhou, Y.; Wang, H.; Yang, X.; Wang, A.; et al. Hydrogenated TiO2 supported Ru for selective methanation of CO in practical conditions. Appl. Catal. B Environ. 2021, 298, 120597. [Google Scholar] [CrossRef]
- Fujita, S.I.; Takezawa, N. Difference in the selectivity of CO and CO2 methanation reactions. Chem. Eng. J. 1997, 68, 63–68. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Xu, G. Thermally differential methanation—A novel method to realize highly selective removal of CO from H2-rich reformates. Catal. Commun. 2007, 8, 1953–1956. [Google Scholar] [CrossRef]
- Ping, D.; Wan, Y.; Zhao, X.; Geng, J.; Dong, X. Zr-promoted nickel-rich spinel-supported Ni catalysts with enhanced performance for selective CO methanation. Int. J. Energy Res. 2022, 46, 9128–9137. [Google Scholar] [CrossRef]
- Takenaka, S.; Shimizu, T.; Otsuka, K. Complete removal of carbon monoxide in hydrogen-rich gas stream through methanation over supported metal catalysts. Int. J. Hydrogen Energy 2004, 29, 1065–1073. [Google Scholar] [CrossRef]
- Tada, S.; Kikuchi, R. Preparation of Ru nanoparticles on TiO2 using selective deposition method and their application to selective CO methanation. Catal. Sci. Technol. 2014, 4, 26–29. [Google Scholar] [CrossRef]
- Tada, S.; Kikuchi, R. Mechanistic study and catalyst development for selective carbon monoxide methanation. Catal. Sci. Technol. 2015, 5, 3061–3070. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Q.; Gu, F.; Liu, B.; Zhong, Z.; Su, F. Recent advances in methanation catalysts for the production of synthetic natural gas. RSC Adv. 2015, 5, 22759–22776. [Google Scholar] [CrossRef]
- Ping, D.; Dong, X.; Zang, Y.; Feng, X. Highly efficient Ru/TiO2-NiAl mixed oxide catalysts for CO selective methanation in hydrogen-rich gas. Int. J. Energy Res. 2017, 41, 2308–2317. [Google Scholar] [CrossRef]
- Krämer, M.; Stöwe, K.; Duisberg, M.; Müller, F.; Reiser, M.; Sticher, S.; Maier, W.F. The impact of dopants on the activity and selectivity of a Ni-based methanation catalyst. Appl. Catal. A Gen. 2009, 369, 42–52. [Google Scholar] [CrossRef]
- Chen, A.; Miyao, T.; Higashiyama, K.; Yamashita, H.; Watanabe, M. High Catalytic Performance of Ruthenium-Doped Mesoporous Nickel–Aluminum Oxides for Selective CO Methanation. Angew. Chew. Int. Ed. 2010, 122, 10091–10094. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, M.; Gao, Z.; Ma, H.; Bao, L.; Li, Z. CO removal via selective methanation over the catalysts Ni/ZrO2 prepared with reduction by the wet H2-rich gas. Int. J. Hydrogen Energy 2018, 43, 15985–15994. [Google Scholar] [CrossRef]
- Ping, D.; Dong, C.; Zhao, H.; Dong, X. A Novel Hierarchical RuNi/Al2O3–Carbon Nanotubes/Ni Foam Catalyst for Selective Removal of CO in H2-Rich Fuels. Ind. Eng. Chem. Res. 2018, 57, 5558–5567. [Google Scholar] [CrossRef]
- Kumi, D.O.; Dlamini, M.W.; Phaahlamohlaka, T.N.; Mhlanga, S.D.; Coville, N.J.; Scurrell, M.S. Selective CO Methanation Over Ru Supported on Carbon Spheres: The Effect of Carbon Functionalization on the Reverse Water Gas Shift Reaction. Catal. Lett. 2018, 148, 3502–3513. [Google Scholar] [CrossRef]
- Meng, X.; Wang, L.; Chen, L.; Xu, M.; Liu, N.; Zhang, J.; Yang, Y.; Wei, M. Charge-separated metal-couple-site in NiZn alloy catalysts towards furfural hydrodeoxygenation reaction. J. Catal. 2020, 392, 69–79. [Google Scholar] [CrossRef]
- Li, S.; Wang, D.; Wu, X.; Chen, Y. Recent advance on VOCs oxidation over layered double hydroxides derived mixed metal oxides. Chin. J. Catal. 2020, 41, 550–560. [Google Scholar] [CrossRef]
- Rosset, M.; Féris, L.A.; Perez-Lopez, O.W. Biogas dry reforming using Ni–Al-LDH catalysts reconstructed with Mg and Zn. Int. J. Hydrogen Energy 2021, 46, 20359–20376. [Google Scholar] [CrossRef]
- Mohaideen, K.K.; Kim, W.; Koo, K.Y.; Yoon, W.L. Highly dispersed Ni particles on Ru/NiAl catalyst derived from layered double hydroxide for selective CO methanation. Catal. Commun. 2015, 60, 8–13. [Google Scholar] [CrossRef]
- He, L.; Lin, Q.; Liu, Y.; Huang, Y. Unique catalysis of Ni-Al hydrotalcite derived catalyst in CO2 methanation: Cooperative effect between Ni nanoparticles and a basic support. J. Energy Chem. 2014, 23, 587–592. [Google Scholar] [CrossRef]
- Lima, D.d.S.; Dias, Y.R.; Perez-Lopez, O.W. CO2 methanation over Ni–Al and Co–Al LDH-derived catalysts: The role of basicity. Sustain. Energy Fuels 2020, 4, 5747–5756. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, J.; Jiang, X.; Evans, D.G.; Li, F. Influence of nature of precursors on the formation and structure of Cu–Ni–Cr mixed oxides from layered double hydroxides. J. Phys. Chem. Solids 2006, 67, 1678–1686. [Google Scholar] [CrossRef]
- Liu, H.-M.; Zhao, X.-J.; Zhu, Y.-Q.; Yan, H. DFT study on MgAl-layered double hydroxides with different interlayer anions: Structure, anion exchange, host-guest interaction and basic sites. Phys. Chem. Chem. Phys. 2020, 22, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, Z.; Tay, H.H.; Chen, L.; Liu, Y.; Chang, J.; Zhong, Z.; Luo, J.; Borgna, A. High temperature adsorption of CO2 on Mg–Al hydrotalcite: Effect of the charge compensating anions and the synthesis pH. Catal. Today 2011, 164, 198–203. [Google Scholar] [CrossRef]
- Meyer, O.; Roessner, F.; Rakoczy, R.A.; Fischer, R.W. Impact of Organic Interlayer Anions in Hydrotalcite Precursor on the Catalytic Activity of Hydrotalcite-Derived Mixed Oxides. ChemCatChem 2010, 2, 314–321. [Google Scholar] [CrossRef]
- Du, Y.; Feng, Y.; Zou, C.; Wu, X.; Huang, W. Kinetics and mechanism of acetalisation of furfural to furfural diethyl acetal with Ni–Al layered double hydroxides containing Lewis acid sites. Prog. React. Kinet. Mech. 2018, 43, 21–29. [Google Scholar] [CrossRef]
- Shi, Z.; Feng, J.; Dong, X. Ru–Ni/GA-MMO composites as highly active catalysts for CO selective methanation in H2-rich gases. Int. J. Hydrogen Energy 2023, 48, 24640–24651. [Google Scholar] [CrossRef]
- Iyi, N.; Yamada, H.; Sasaki, T. Deintercalation of carbonate ions from carbonate-type layered double hydroxides (LDHs) using acid–alcohol mixed solutions. Appl. Clay Sci. 2011, 54, 132–137. [Google Scholar] [CrossRef]
- Iyi, N.; Fujii, K.; Okamoto, K.; Sasaki, T. Factors influencing the hydration of layered double hydroxides (LDHs) and the appearance of an intermediate second staging phase. Appl. Clay Sci. 2007, 35, 218–227. [Google Scholar] [CrossRef]
- Liu, M.; Chang, J.; Sun, J.; Gao, L. A facile preparation of NiO/Ni composites as high-performance pseudocapacitor materials. RSC Adv. 2013, 3, 8003–8008. [Google Scholar] [CrossRef]
- Muttakin, M.; Mitra, S.; Thu, K.; Ito, K.; Saha, B. Theoretical framework to evaluate minimum desorption temperature for IUPAC classified adsorption isotherms. Int. J. Heat Mass Transf. 2018, 122, 795–805. [Google Scholar] [CrossRef]
- Zhu, L.; Cao, M.; Li, L.; Sun, H.; Tang, Y.; Zhang, N.; Zheng, J.; Zhou, H.; Li, Y.; Yang, L.; et al. Synthesis of Different Ruthenium Nickel Bimetallic Nanostructures and an Investigation of the Structure–Activity Relationship for Benzene Hydrogenation to Cyclohexane. ChemCatChem 2014, 6, 2039–2046. [Google Scholar] [CrossRef]
- Qin, Y.; Bai, X. Hydrogenation of N-ethylcarbazole over Ni-Ru alloy nanoparticles loaded on graphitized carbon prepared by carbothermal reduction. Fuel 2022, 307, 121921. [Google Scholar] [CrossRef]
- Wang, K.; Men, Y.; Liu, W.; Zhang, J. Recent progress in catalytical CO purification of H2-rich reformate for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2023, 48, 25100–25118. [Google Scholar] [CrossRef]
- Braos-García, P.; García-Sancho, C.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Jiménez-López, A. Bimetallic Ru/Ni supported catalysts for the gas phase hydrogenation of acetonitrile. Appl. Catal. A Gen. 2010, 381, 132–144. [Google Scholar] [CrossRef]
- Zheng, Z.; Du, X.; Wang, Y.; Li, C.M.; Qi, T. Efficient and Stable NiCo2O4/VN Nanoparticle Catalyst for Electrochemical Water Oxidation. ACS Sustain. Chem. Eng. 2018, 6, 11473–11479. [Google Scholar] [CrossRef]
- Sakai, M.; Imagawa, H.; Baba, N. Layered-double-hydroxide-based Ni catalyst for CO2 capture and methanation. Appl. Catal. A Gen. 2022, 647, 118904. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Ji, Y.; Yang, R.; Zhang, Z.; Wang, X.; Liu, H. Hybrid nanostructures of pit-rich TiO2 nanocrystals with Ru loading and N doping for enhanced solar water splitting. Chem. Commun. 2019, 55, 2781–2784. [Google Scholar] [CrossRef]
- Ramos-Fernández, E.V.; Silvestre-Albero, J.; Sepúlveda-Escribano, A.; Rodríguez-Reinoso, F. Effect of the metal precursor on the properties of Ru/ZnO catalysts. Appl. Catal. A Gen. 2010, 374, 221–227. [Google Scholar] [CrossRef]
- Luo, Z.; Zheng, Z.; Li, L.; Cui, Y.-T.; Zhao, C. Bimetallic Ru–Ni Catalyzed Aqueous-Phase Guaiacol Hydrogenolysis at Low H2 Pressures. ACS Catal. 2017, 7, 8304–8313. [Google Scholar] [CrossRef]
- Yang, M.; Feng, T.; Chen, Y.; Liu, J.; Zhao, X.; Yang, B. Synchronously integration of Co, Fe dual-metal doping in Ru@C and CDs for boosted water splitting performances in alkaline media. Appl. Catal. B Environ. 2020, 267, 118657. [Google Scholar] [CrossRef]
- Zhu, C.; Cao, J.-P.; Zhao, X.-Y.; Xie, T.; Zhao, M.; Wei, X.-Y. Bimetallic effects in the catalytic hydrogenolysis of lignin and its model compounds on Nickel-Ruthenium catalysts. Fuel Process. Technol. 2019, 194, 106126. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, Z.; Meng, X.; Lv, Y.; Tao, M. Effect of MoO3 on structures and properties of Ni-SiO2 methanation catalysts prepared by the hydrothermal synthesis method. Ind. Eng. Chem. Res. 2013, 52, 14533–14544. [Google Scholar] [CrossRef]
- Zhao, A.; Ying, W.; Zhang, H.; Ma, H.; Fang, D. Ni/Al2O3 catalysts for syngas methanation: Effect of Mn promoter. J. Nat. Gas Chem. 2012, 21, 170–177. [Google Scholar] [CrossRef]
- Chang, F.-W.; Kuo, M.-S.; Tsay, M.-T.; Hsieh, M.-C. Effect of calcination temperature on catalyst reducibility and hydrogenation reactivity in rice husk ash-alumina supported nickel systems. J. Chem. Technol. Biotechnol. 2004, 79, 691–699. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, J.; Dong, Q.; Zhao, Y.; Fan, B. Supported noble metal catalyst with a core-shell structure for enhancing hydro-genation performance. Mol. Catal. 2021, 506, 111543. [Google Scholar] [CrossRef]
- Shun, K.; Mori, K.; Masuda, S.; Hashimoto, N.; Hinuma, Y.; Kobayashi, H.; Yamashita, H. Revealing hydrogen spillover pathways in reducible metal oxides. Chem. Sci. 2022, 13, 8137–8147. [Google Scholar] [CrossRef]
- Li, M.; Yin, W.; Pan, J.; Zhu, Y.; Sun, N.; Zhang, X.; Wan, Y.; Luo, Z.; Yi, L.; Wang, L. Hydrogen spillover as a promising strategy for boosting heterogeneous catalysis and hydrogen storage. Chem. Eng. J. 2023, 471, 144691. [Google Scholar] [CrossRef]
- Santos, D.C.R.M.; Lisboa, J.S.; Passos, F.B.; Noronha, F.B. Characterization of steam-reforming catalysts. Braz. J. Chem. Eng. 2004, 21, 203–209. [Google Scholar] [CrossRef]
- Alstrup, I. On the Kinetics of Co Methanation on Nickel Surfaces. J. Catal. 1995, 151, 216–225. [Google Scholar] [CrossRef]
- Grad, O.; Kasza, A.M.; Turza, A.; Dan, M.; Barbu-Tudoran, L.; Lazar, M.D.; Mihet, M. Facile and efficient synthesis of ordered mesoporous MIL-53(Al)-derived Ni catalysts with improved activity in CO2 methanation. J. Environ. Chem. Eng. 2023, 11, 109456. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, R.; Liu, N.; Dai, C.; Yu, G.; Wang, N.; Chen, B. In situ Ce-doped catalyst derived from NiCeAl-LDHs with enhanced low-temperature performance for CO2 methanation. Appl. Surf. Sci. 2022, 579, 152204. [Google Scholar] [CrossRef]
- Barthos, R.; Lønyi, F.; Onyestyak, G.; Valyon, J. An NH3-TPD and -FR study on the acidity of sulfated zirconia. Solid State Ion. 2001, 141–142, 253–258. [Google Scholar] [CrossRef]
- Huang, L.; Shi, Y.; Xiong, W.; Ding, Y.; Zhang, Y. Facile design of highly effective Fe-modified bimetallic Fex–Ni1−x-MOFs catalysts with rodlike structures for low-temperature NO reduction by CO. J. Mater. Sci. 2021, 56, 9914–9928. [Google Scholar] [CrossRef]
- Al-Mubaddel, F.S.; Kumar, R.; Sofiu, M.L.; Frusteri, F.; Ibrahim, A.A.; Srivastava, V.K.; Kasim, S.O.; Fakeeha, A.H.; Abasaeed, A.E.; Osman, A.I.; et al. Optimizing acido-basic profile of support in Ni supported La2O3+Al2O3 catalyst for dry reforming of methane. Int. J. Hydrogen Energy 2021, 46, 14225–14235. [Google Scholar] [CrossRef]
- Tada, S.; Kikuchi, R.; Wada, K.; Osada, K.; Akiyama, K.; Satokawa, S.; Kawashima, Y. Long-term durability of Ni/TiO2 and Ru–Ni/TiO2 catalysts for selective CO methanation. J. Power Sources 2014, 264, 59–66. [Google Scholar] [CrossRef]
- Sengupta, S.; Ray, K.; Deo, G. Effects of modifying Ni/Al2O3 catalyst with cobalt on the reforming of CH4 with CO2 and cracking of CH4 reactions. Int. J. Hydrogen Energy 2014, 39, 11462–11472. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Z.; Gu, F.; Wang, X.; Lu, X.; Li, H.; Xu, G.; Su, F. CO methanation on ordered mesoporous Ni–Cr–Al catalysts: Effects of the catalyst structure and Cr promoter on the catalytic properties. J. Catal. 2016, 337, 221–232. [Google Scholar] [CrossRef]
- Gao, Z.; Cui, L.; Ma, H. Selective methanation of CO over Ni/Al2O3 catalyst: Effects of preparation method and Ru addition. Int. J. Hydrogen Energy 2016, 41, 5484–5493. [Google Scholar] [CrossRef]
- Dai, X.; Liang, J.; Ma, D.; Zhang, X.; Zhao, H.; Zhao, B.; Guo, Z.; Kleitz, F.; Qiao, S. Large-pore mesoporous RuNi-doped TiO2–Al2O3 nanocomposites for highly efficient selective CO methanation in hydrogen-rich reformate gases. Appl. Catal. B Environ. 2015, 165, 752–762. [Google Scholar] [CrossRef]
- Wang, C.; Ping, D.; Dong, X.; Dong, Y.; Zang, Y. Construction of Ru/Ni-Al-oxide/Ni-foam monolithic catalyst for deep-removing CO in hydrogen-rich gas via selective methanation. Fuel Process. Technol. 2016, 148, 367–371. [Google Scholar] [CrossRef]
- Tada, S.; Shoji, D.; Urasaki, K.; Shimoda, N.; Satokawa, S. Physical mixing of TiO2 with sponge nickel creates new active sites for selective CO methanation. Catal. Sci. Technol. 2016, 6, 3713–3717. [Google Scholar] [CrossRef]
- Guo, Q.; Li, S.; Li, J.; Hu, Y.; Li, Y. Effect of SiO2 on the CO Selective Methanation over SiO2/Ni-ZrO2 Catalysts. ChemCatChem 2022, 14, e202101281. [Google Scholar] [CrossRef]
- Feng, X.; Dong, C.; Ping, D.; Geng, J.; Zhang, J.; Dong, X. Zr-Modified SBA-15 Supported Ni Catalysts with Excellent Catalytic Performance of CO Selective Methanation in H2-Rich Fuels. Catal. Lett. 2018, 148, 2967–2973. [Google Scholar] [CrossRef]
- Zyryanova, M.M.; Snytnikov, P.V.; Gulyaev, R.V.; Amosov, Y.I.; Boronin, A.I.; Sobyanin, V.A. Performance of Ni/CeO2 catalysts for selective CO methanation in hydrogen-rich gas. Chem. Eng. J. 2014, 238, 189–197. [Google Scholar] [CrossRef]
- Ping, D.; Zhao, H.; Dong, X. Ni-doped TiO2 nanotubes supported Ru catalysts for CO selective methanation in H2-rich reformate gases. React. Kinet. Mech. Catal. 2018, 124, 619–631. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhao, X.; Ma, J.; Dong, X. RuNi/MMO Catalysts Derived from a NiAl-NO3-LDH Precursor for CO Selective Methanation in H2-Rich Gases. Catalysts 2023, 13, 1245. https://doi.org/10.3390/catal13091245
Li Z, Zhao X, Ma J, Dong X. RuNi/MMO Catalysts Derived from a NiAl-NO3-LDH Precursor for CO Selective Methanation in H2-Rich Gases. Catalysts. 2023; 13(9):1245. https://doi.org/10.3390/catal13091245
Chicago/Turabian StyleLi, Zhihui, Xinyuan Zhao, Jiteng Ma, and Xinfa Dong. 2023. "RuNi/MMO Catalysts Derived from a NiAl-NO3-LDH Precursor for CO Selective Methanation in H2-Rich Gases" Catalysts 13, no. 9: 1245. https://doi.org/10.3390/catal13091245





