A Cu-SiO2 Catalyst for Highly Efficient Hydrogenation of Methyl Formate to Methanol
Abstract
:1. Introduction
2. Results and Discussion
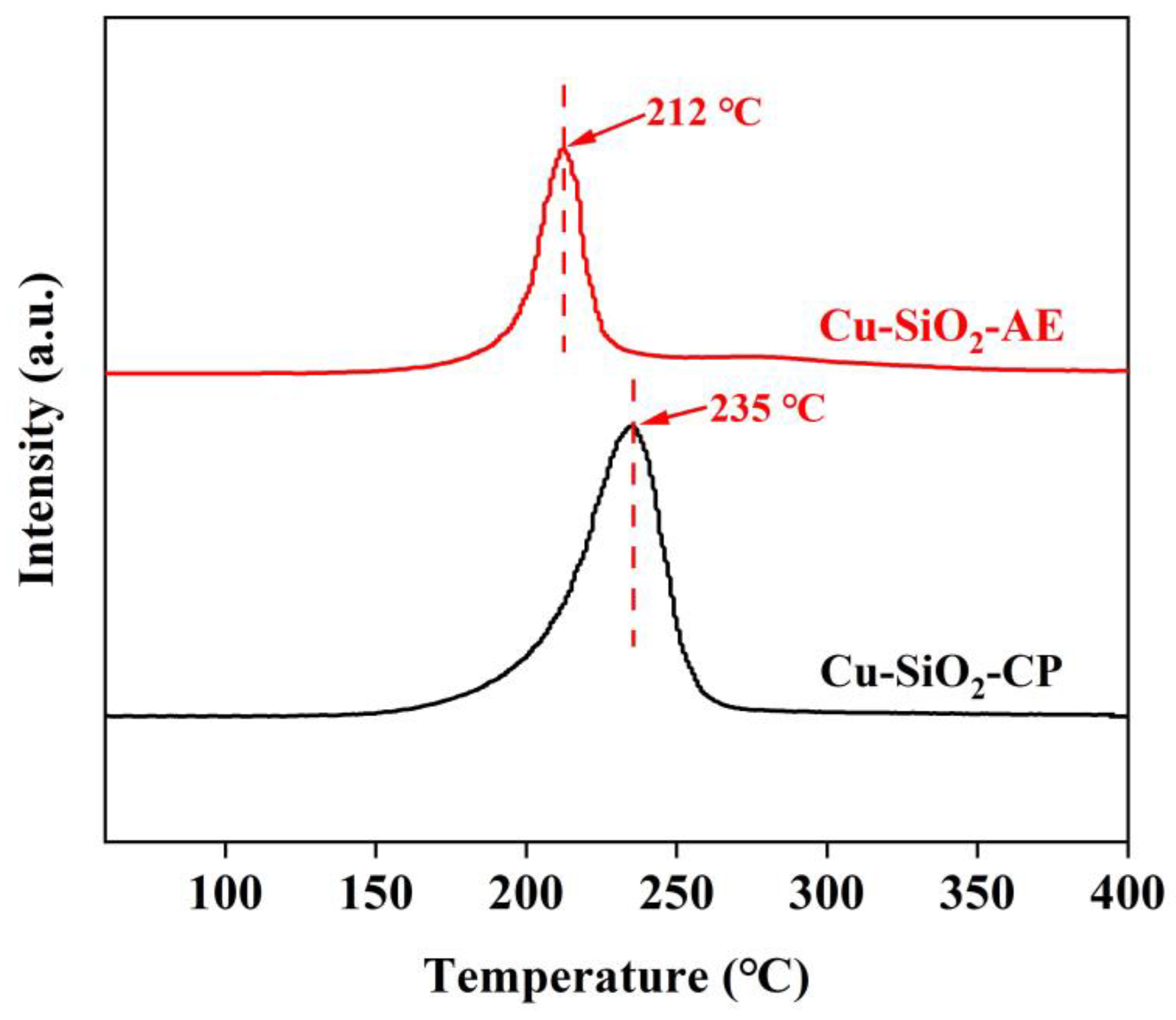
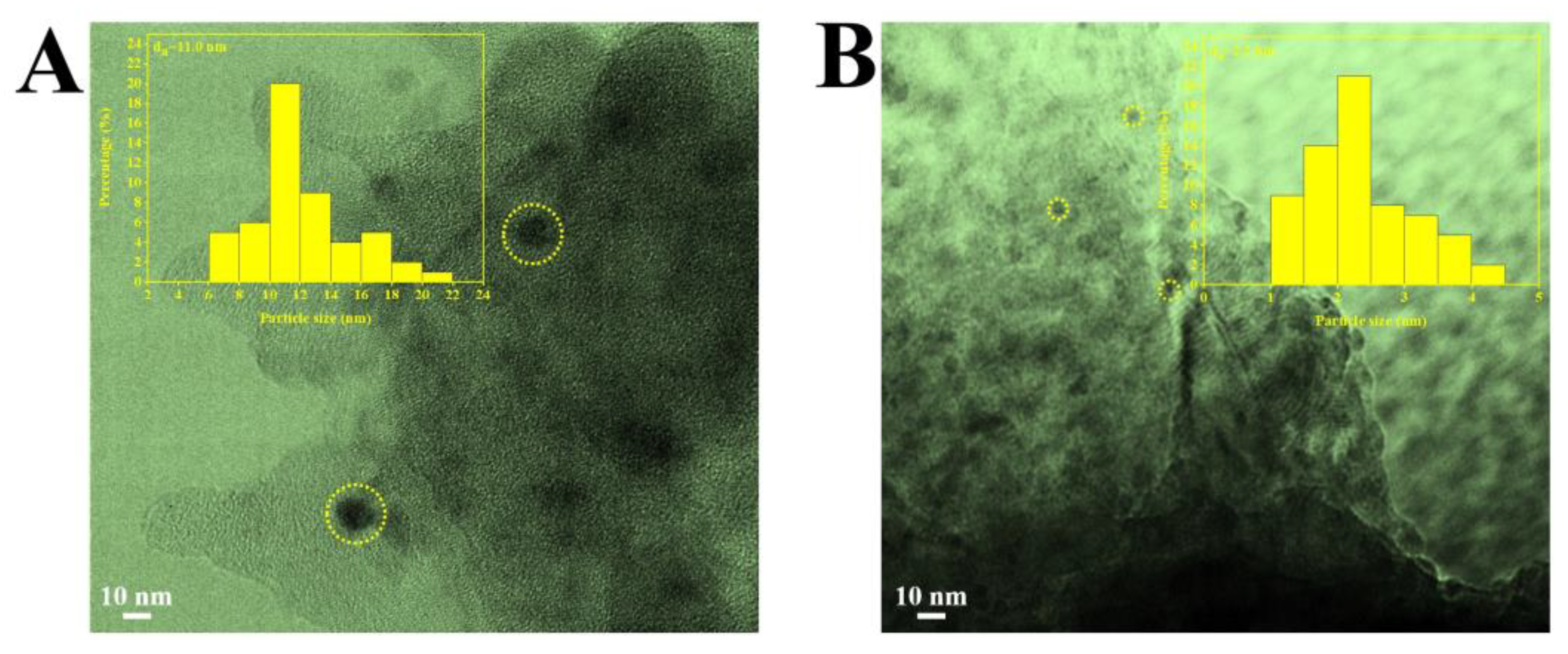
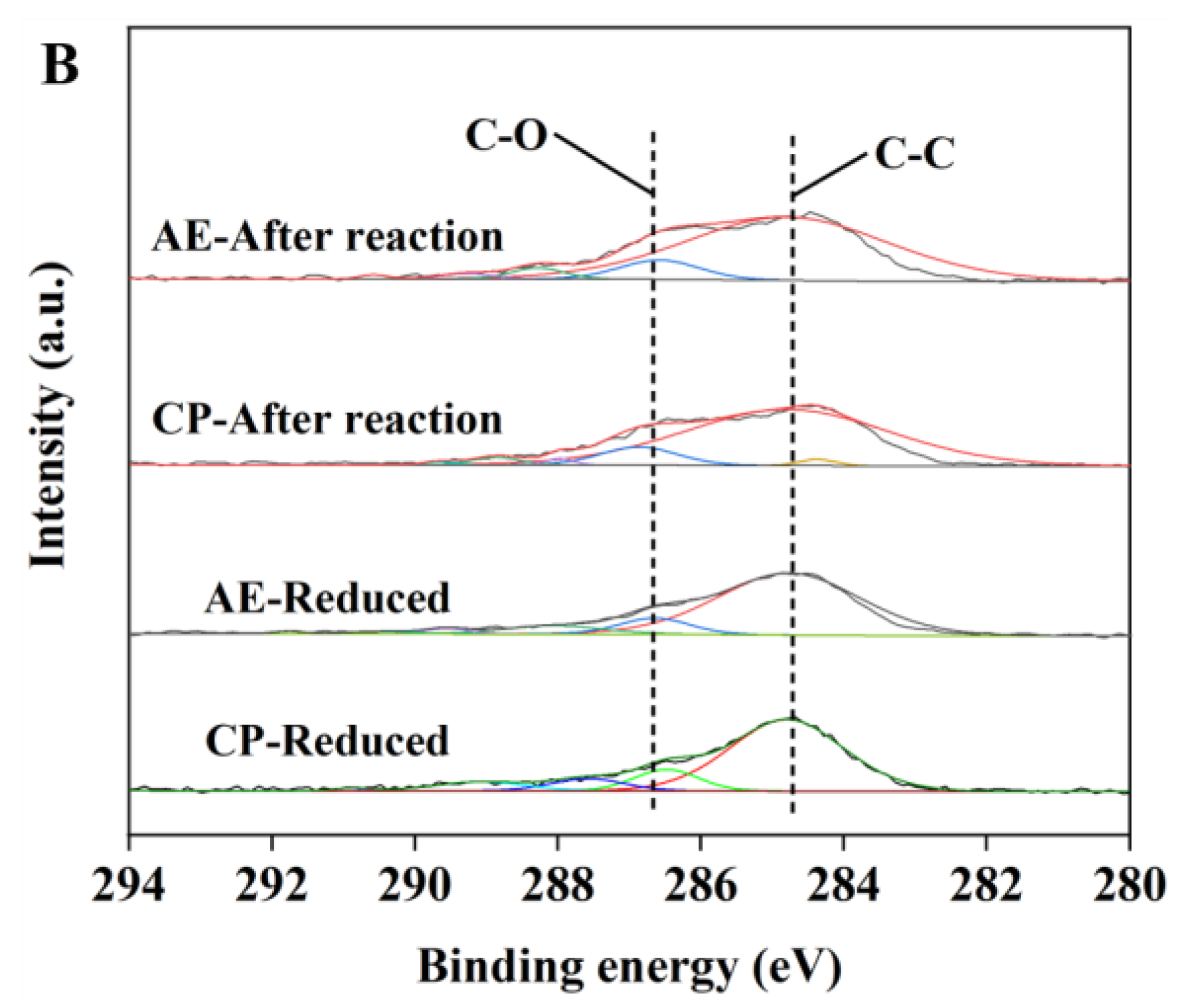
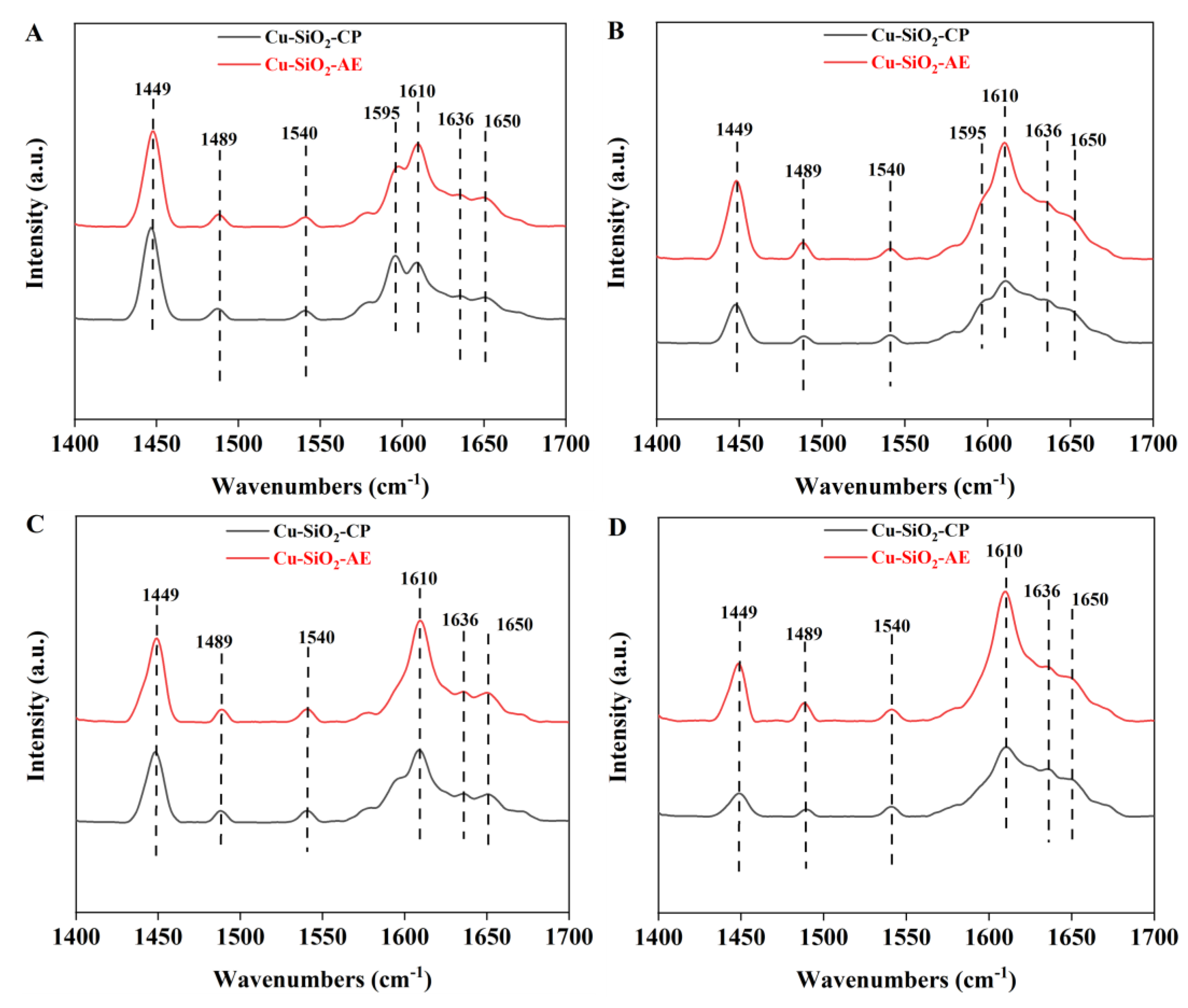
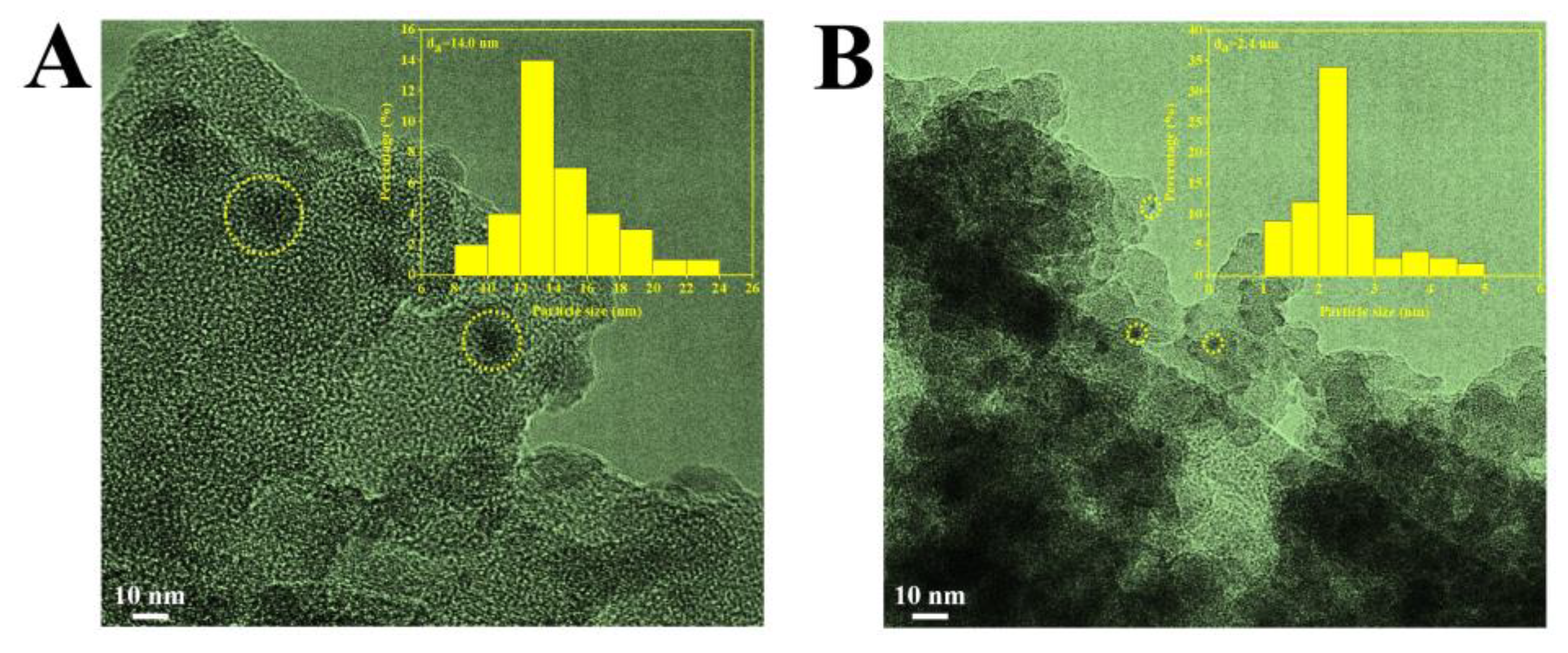
2.1. Characterization of the Catalysts
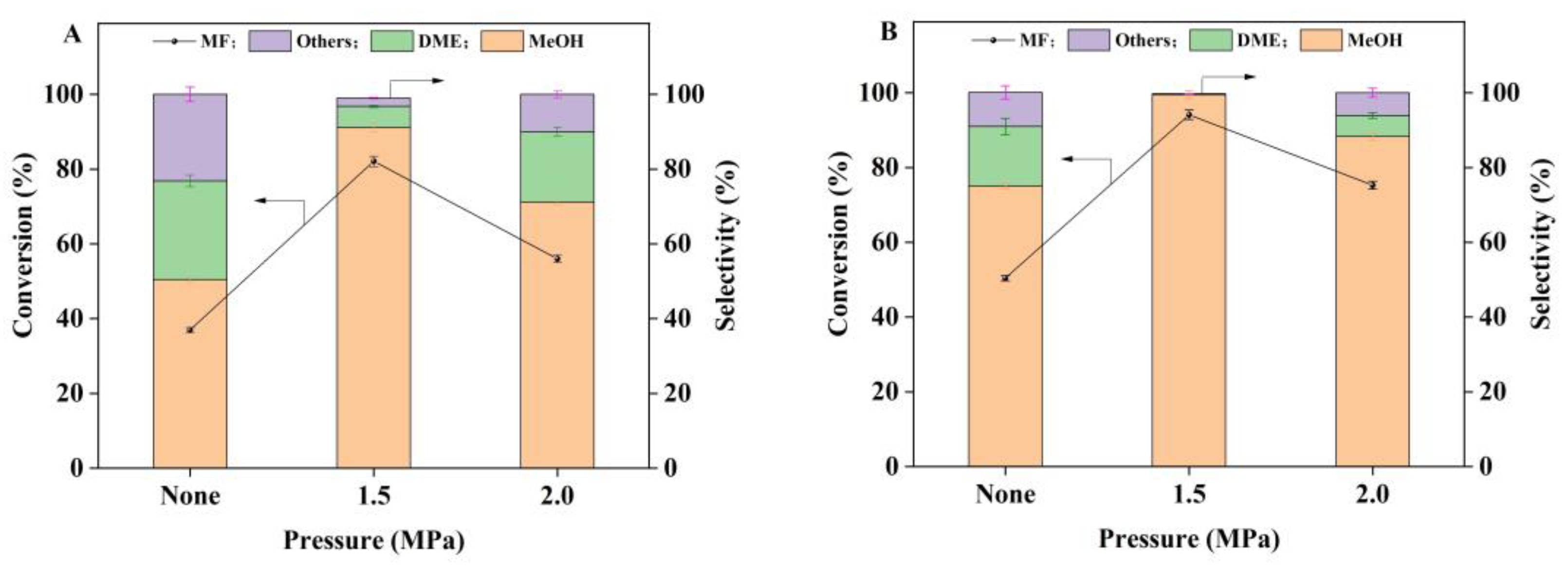
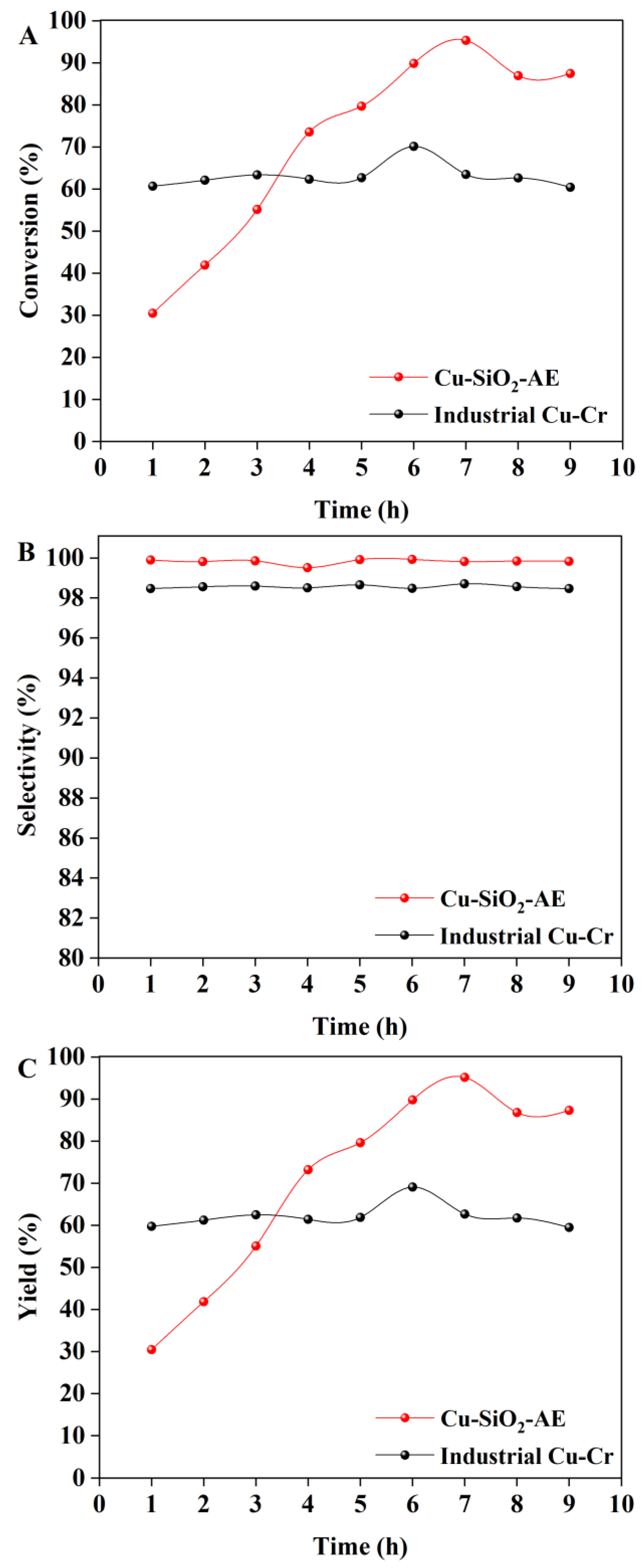
2.2. Catalytic Hydrogenation of Methyl Formate
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Cu-SiO2
3.3. Catalyst Characterization
3.4. Catalytic Reaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ke, S.; Yang, J.; Wende, X. Intrinsic kinetic research on vapor-phase catalytic coupling of CO and methyl nitrite to DMO. Guangdong Chem. Ind. 2007, 34, 12–14+24. [Google Scholar] [CrossRef]
- Fan, C.; Luo, M.; Xiao, W. Reaction mechanism of methyl nitrite dissociation during co catalytic coupling to dimethyl oxalate: A density functional theory study. Chin. J. Chem. Eng. 2016, 24, 132–139. [Google Scholar] [CrossRef]
- Chuanhao, S. Process optimization of CO coupling production of dimethyl oxalate in the process of ethylene glycol production. Yunnan Chem. Technol. 2017, 44, 36–39. [Google Scholar] [CrossRef]
- Xu, Q.S.; Guo, M.; Xia, L. Temperature gradient analyses of a tubular solid oxide fuel cell fueled by methanol. Trans. Tianjin Univ. 2023, 29, 14–30. [Google Scholar] [CrossRef]
- Qijun, G.; Yuxin, W.; Li, X. Proton-exchange sulfonated poly (ether ether ketone)(SPEEK)/SiOx-S composite membranes in direct methanol fuel cells. Chin. J. Chem. Eng. 2009, 17, 207–213. [Google Scholar] [CrossRef]
- Monti, D.M.; Cant, N.W.; Trimm, D.L. Hydrogenolysis of methyl formate over copper on silica: II. Study of the mechanism using labeled compounds. J. Catal. 1986, 100, 28–38. [Google Scholar] [CrossRef]
- Zhao, H.; Lin, M.; Fang, K. Preparation and evaluation of Cu-Mn/Ca-Zr catalyst for methyl formate synthesis from syngas. Appl. Catal. A: Gen. 2016, 514, 276–283. [Google Scholar] [CrossRef]
- Liu, X.Q.; Wu, Y.T.; Chen, W.K. Concurrent synthesis of methanol and methyl formate catalyzed by copper-based catalysts in a slurry phase. Stud. Surf. Sci. Catal. 1998, 119, 557–560. [Google Scholar] [CrossRef]
- Wang, K.; Chu, W.; He, C. High activity Cu-Cr-Si catalysts for lower temperature liquid phase synthesis of methanol I. Influence of preparation parameters on catalysts activities. Petrochem. Technol. 2001, 30, 686–688. [Google Scholar] [CrossRef]
- Yi, L.; Wei, C.; Yanghong, Y. Study of the effect of additives on Cu-Cr-Si catalyst for methanol synthesis from the hydrogenolysis of methyl formate. Chem. Peking 2003, 6, 663–666. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, S. Advances in copper-based catalyst for the methanol synthesis from CO2 hydrogenation. Chem. Indus. Engineer Pro. 2016, 35, 159–166. [Google Scholar] [CrossRef]
- Haiguang, G.; Wenfeng, H.; Huazhang, L. Study on copper-based catalysts for methanol synthesis. J. Zhejiang Univ. Technol. 2003, 31, 508–511. [Google Scholar] [CrossRef]
- Zu, Y.; Guo, Z.; Zheng, J. Investigation of Cu (I)-Y zeolites with different Cu/Al ratios towards the ultra-deep adsorption desulfurization: Discrimination and role of the specific adsorption active sites. Chem. Eng. J. 2020, 380, 122319. [Google Scholar] [CrossRef]
- Suzuki, N.; Asami, H.; Nakamura, T. Immobilization of a C2-symmetric ansa-zirconocene complex on silica surfaces using a Si-Cl Anchor: Catalysts for isospecific propene polymerization. Chem. Lett. 1999, 28, 341–342. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, H.R.; Jaenicke, S. Highly efficient and robust Cu catalyst for non-oxidative dehydrogenation of ethanol to acetaldehyde and hydrogen. J. Catal. 2020, 389, 19–28. [Google Scholar] [CrossRef]
- Wu, L.; Li, B.; Zhao, C. Direct synthesis of hydrogen and dimethoxylmethane from methanol on copper/silica catalysts with optimal Cu+/Cu0 sites. ChemCatChem 2018, 10, 1140–1147. [Google Scholar] [CrossRef]
- Jinyong, L.; Ming, M.; Yuqing, Z. Characterization on the fine structures of the copper species in the highly dispersed CuO/CeO2-Al2O3 catalysts. Chin. J. Inorg. Chem. 2006, 22, 861–866. [Google Scholar] [CrossRef] [Green Version]
- Weng, Z.; Wu, Y.; Wang, M. Active sites of copper-complex catalytic materials for electrochemical carbon dioxide reduction. Nat. Commun. 2018, 9, 415. [Google Scholar] [CrossRef] [Green Version]
- Li, G. Catalytic property and reactional mechanism of Cu-ZnO-ZrO2/CFG for reaction of methanol dehydrogenation to methyl formate. J. Fushun Petrochem. Inst. 1995, 1, 1–6. [Google Scholar]
- Kannapu, H.P.R.; Neeli, C.K.P.; Rao, K.S.R. Unusual effect of cobalt on Cu-MgO catalyst for the synthesis of γ-butyrolactone and aniline via coupling reaction. Catal. Sci. Technol. 2016, 6, 5494–5503. [Google Scholar] [CrossRef]
- Yu, J.; Suocai, R.; Liu, B. Advances in deactivation and solutions of Cu-based methanol catalysts. Nat. Gas Chem. Ind. 2019, 44, 118–122. [Google Scholar] [CrossRef]
- Cho, J.M.; Lee, S.R.; Sun, J.; Tsubaki, N.; Jang, E.J.; Bae, J.W. Highly Ordered Mesoporous Fe2O3-ZrO2 Bimetal Oxides for an Enhanced CO Hydrogenation Activity to Hydrocarbons with Their Structural Stability. ACS Catal. 2017, 7, 5955–5964. [Google Scholar] [CrossRef]
- Yuanzhi, L.; Ergan, C. Inverstigation on Co-Fe-Cu/SiO2 Catalyst by Unsingtemperature Programmed Reduction. J. Jingzhou Teach. Coll. 1998, 21, 60–62. [Google Scholar]
- Zhang, C.; Peiyi, L.; Zhibiao, S. Preparation of Cu/ZnO/MCM-41 catalyst with double-solvent impregnation method and catalytic performance in methanol synthesis by CO2 hydrogenation. J. Shanghai Univ. Nat. Sci. 2019, 25, 109–119. [Google Scholar] [CrossRef]
- Ding, D.; Wang, Q.; Jin, F. Influences of the ammonia evaporation pressure on the structure of Cu-SiO2 catalysts and catalytic performances for dimethyl oxalate hydrogenation to ethylene glycol. Chin. J. Appl. Chem. 2016, 33, 466. [Google Scholar] [CrossRef]
- Siqi, B.; Renjiang, L.; Lidi, G. Synthesis and electrochemical study of Sn-Ni core-shell nanoparticles modified carbon one-dimensional nanostructures. Chem. Res. Appl. 2022, 34, 2920–2926. [Google Scholar] [CrossRef]
- Cui, A.L.; Bai, Y.; Yu, H.Y. Electrocatalytic “Volcano-Type” effect of Nano-TiO2 (A)/(R) phase content in Pt/TiO2-CNx catalyst. J. Electrochem. 2022, 28, 2110021. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, X.; Mao, H. Photoluminescence and XPS investigations of Cu2+-doped ZnS quantum dots capped with polyvinylpyrrolidone. Phys. Status Solidi B 2009, 246, 2333–2336. [Google Scholar] [CrossRef]
- Hongfei, Y.; Yu, Z.; Guixian, L. Effect of carrier surface hydroxyl group on performance of Cu/SiO2 catalyst for DMO hydrogenation. Chem. Ind. Eng. Prog. 2022, 41, 6338–6349. [Google Scholar] [CrossRef]
- Bian, Z.; Zhong, W.; Yu, Y. Cu-SiO2 derived from copper phyllosilicate for low-temperature water-gas shift reaction: Role of Cu+ sites. Int. J. Hydrog. Energy 2020, 45, 27078–27088. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Zhong, B. H2 and CO desorption properties of raney Cu-Nd catalysts and correlation with their catalytic activity. Petrochem. Technol. 1999, 28, 219–222. [Google Scholar] [CrossRef]
- Haiyan, L.; Hongbin, Z.; Guodong, L. Study of Promoted Cu based catalysts for hydrogenolysis of methyl formate to methanol. J. Xiamen Univ. Nat. Sci. 1997, 36, 381–387. [Google Scholar]
- Jitao, L.; Qiangu, Y.; Weide, Z. Synthesis methanol under low temperature and low pressure by two step process. Sci. Technol. Chem. Ind. 2000, 8, 17–19. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Chung, B.Z.; Hsieh, C.R. An effective catalyst, Cu-B2O3/SiO2, for hydrogenolysis of methyl formate and one-step synthesis of methanol in slurry-phase with potassium methoxide. Catal. Lett. 1996, 41, 213–220. [Google Scholar] [CrossRef]
- Parry, E.P. An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity. J. Catal. 1963, 2, 371–379. [Google Scholar] [CrossRef]
- Huang, F. Research for control the acidity of RFCC catalyst. Chem. Eng. Oil Gas 2001, 6, 287–289. [Google Scholar]
- Lu, S.; Fu, H.; Wang, L. Infrared spectroscopic study on the surface Bronsted and Lewis acidity of fluoridated hydrotreating catalysts F·N·W/Al2O3·SiO2. J. Mol. Catal. 1993, 7, 471–474. [Google Scholar] [CrossRef]
- Wang, J.; Shan, J.; Tian, Y. Catalytic cracking of n-heptane over Fe modified HZSM-5 nanosheet to produce light olefins. Fuel 2021, 306, 121725. [Google Scholar] [CrossRef]
- Kamiguchi, S.; Seki, Y.; Satake, A. Catalytic cracking of methyl tert-butyl ether to isobutene over Brønsted and Lewis acid sites on solid-state molybdenum sulfide clusters with an octahedral metal framework. J. Clust. Sci. 2015, 26, 653–660. [Google Scholar] [CrossRef]
- Yang, J.; Wu, X.; Cheng, Y. Novel solid acid with both Brønsted and Lewis acid sites for biodiesel synthesis. Kinet. Catal. 2013, 54, 703–708. [Google Scholar] [CrossRef]
- Jiang, W.; Gao, X.; Dong, L. Aerobic oxidative desulfurization via magnetic mesoporous silica-supported tungsten oxide catalysts. Pet. Sci. 2020, 17, 1422–1431. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Wang, Y. High performance of supported Cu-based catalysts modulated via phosphamide coordination in acetylene hydrochlorination. Appl. Catal. A Gen. 2020, 591, 117408. [Google Scholar] [CrossRef]
- Elgayyar, T.; Atwi, R.; Tuel, A. Contributions and limitations of IR spectroscopy of CO adsorption to the characterization of bimetallic and nanoalloy catalysts. Catal. Today 2021, 373, 59–68. [Google Scholar] [CrossRef]
- Niu, Y.; Li, F.; Yang, K. Preparation and characterization of sulfated TiO2 with rhodium modification used in esterification reaction and decomposition of methyl orange. Chin. J. Chem. Eng. 2016, 24, 767–774. [Google Scholar] [CrossRef]
- Moayedi, H.; Kazemian, S. Zeta potentials of suspended humus in multivalent cationic saline solution and its effect on electro-osomosis behavior. J. Dispers. Sci. Technol. 2013, 34, 283–294. [Google Scholar] [CrossRef]
- Song, M.; Zhang, B.; Zhai, Z.; Liu, S.; Wang, L.; Liu, G. Highly dispersed Pt stabilized by ZnOx-Si on self-pillared zeolite nanosheets for propane dehydrogenation. Ind. Eng. Chem. Res. 2023, 62, 3853–3861. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, X.; Wei, S.; Yang, H.; Zhang, F.; Wang, P.; Xie, M.; Ma, J. Cu (I) immobilized on functionalized SBA-15: A recyclable catalyst for the synthesis of 1,3-diynes using terminal alkynes without base. Catal. Commun. 2013, 39, 24–29. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Zhou, H.; Chu, S.; Liu, L.; Feng, Z.; Qin, X.; Qi, J.; Hou, J.; Li, H.; et al. Rivet of cobalt in siliceous zeolite for catalytic ethane dehydrogenation. Chem 2023, 9, 637–649. [Google Scholar] [CrossRef]
- Wang, L.; Cui, Y.; Meng, X.; Gates, B.C.; Xiao., F.; Guan, E.; Wang, Y. Silica accelerates the selective hydrogenation of CO2 to methanol on cobalt catalysts. Nat. Commun. 2020, 11, 1033. [Google Scholar] [CrossRef] [Green Version]
- Tehrani, S.; Irani, M.; Tavasoli, A. Studies on accelerated deactivation of ruthenium-promoted alumina-supported alkalized cobalt Fischer-Tropsch synthesis catalyst. J. Nat. Gas Chem. 2011, 20, 65–71. [Google Scholar] [CrossRef]
- Xie, Y.; Li, B.; Weng, W.Z. Mechanistic aspects of formation of sintering-resistant palladium nanoparticles over SiO2 prepared using Pd(acac)2 as precursor. Appl. Catal. A Gen. 2015, 504, 179–186. [Google Scholar] [CrossRef]
- Li, D.; Xu, F.; Tang, X.; Dai, S.; Pu, T.; Liu, X.; Tian, P.; Xuan, F.; Xu, Z.; Wachs, I.E.; et al. Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol. Nat. Catal. 2022, 5, 99–108. [Google Scholar] [CrossRef]
- Li, H.; Fang, W.; Wang, L.; Liu, Y.; Liu, L.; Sun, T.; Liao, C.; Zhu, Y.; Wang, L.; Xiao, F. Physical regulation of copper catalyst with a hydrophobic promoter for enhancing CO2 hydrogenation to methanol. Innovation 2023, 4, 100445. [Google Scholar] [CrossRef]
- Shi, W.; Gao, T.; Zhang, L. Tailoring the surface structures of iron oxide nanorods to support Au nanoparticles for CO oxidation. Chin. J. Catal. 2019, 40, 1884–1894. [Google Scholar] [CrossRef]
- Xiao, P.; Zhao, Y.; Wang, T. Polymeric carbon nitride/mesoporous silica composites as catalyst support for Au and Pt nanoparticles. Chem. Eur. J. 2014, 20, 2872–2878. [Google Scholar] [CrossRef]
- Ewing, C.S.; Veser, G.; McCarthy, J.J. Effect of support preparation and nanoparticle size on catalyst-support interactions between Pt and amorphous silica. J. Phys. Chem. C 2015, 119, 19934–19940. [Google Scholar] [CrossRef]
- Lueking, A.D.; Yang, R.T. Hydrogen spillover to enhance hydrogen storage-study of the effect of carbon physicochemical properties. Appl. Catal. A Gen. 2004, 265, 259–268. [Google Scholar] [CrossRef]
- Kong, X.; Xiao, J.; Chen, A. Enhanced catalytic denitrification performance of ruthenium-based catalysts by hydrogen spillover from a palladium promoter. J. Colloid Interface Sci. 2022, 608, 2973–2984. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, H.; Ryoo, R. Hydrogen spillover in nonreducible oxides: Mechanism and catalytic utilization. Nano Res. 2022, 15, 10357–10365. [Google Scholar] [CrossRef]
- Lennon, D.; Kennedy, D.R.; Webb, G. Deactivation and selectivity: The effect of hydrogen concentration in propyne hydrogenation over a silica-supported palladium catalyst//studies in surface science and catalysis. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1999; Volume 126, pp. 341–348. [Google Scholar] [CrossRef]
- Behrens, M. Chemical hydrogen storage by methanol: Challenges for the catalytic methanol synthesis from CO2. Recycl. Catal. 2015, 2, 78–86. [Google Scholar] [CrossRef]









| Samples | a SBET (m2/g) | a V (cm3/g) | a d (nm) | b D (%) | b SCu (m2/g) | b dCu (nm) |
|---|---|---|---|---|---|---|
| Cu-SiO2-CP | 208.3 | 0.8 | 18.4 | 50.0 | 339.0 | 2.0 |
| Cu-SiO2-AE | 350.8 | 0.7 | 18.4 | 109.6 | 742.9 | 0.9 |
| Catalyst | MF Conversion/% | Product Selectivity/% | ||
|---|---|---|---|---|
| MeOH | DME a | Others b | ||
| Cu-SiO2-CP | 83.9 | 92.1 | 5.7 | 2.2 |
| Cu-SiO2-AE | 95.3 | 99.8 | 0.1 | 0.1 |
| Catalyst | Cu Loading (wt%) | H2/MF (v/v) | Temp. (°C) | P (MPa) | LHSV (h−1) | MF Conv. (%) | MeOH Sel. (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cu-Cr | 23 | 4/1 | 140 | 0.1 | 1800 | 73.1 | 98.0 | [32] |
| Cu-Cr-Mn | 23 | 4/1 | 140 | 0.1 | 1800 | 76.15 | 99.55 | [32] |
| Cu-Mn-SiO2 | 19.2 | 4/1 | 180 | 0.5 | 10 | 84.4 | 94.4 | [33] |
| Cu-SiO2 | 19.2 | 4/1 | 180 | 0.5 | 10 | 51.5 | 90.4 | [33] |
| Cu-B2O3/SiO2 | - | 3/1 | 150 | 2.8 | - | 39 | 99.8 | [34] |
| Cu-SiO2-AE | 15 | 4/1 | 140 | 1.5 | 2.4 | 95.3 | 99.8 | This study |
| Cu-SiO2-CP | 15 | 4/1 | 140 | 1.5 | 2.4 | 83.9 | 92.1 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Liu, G.; Liu, Q.; Zhang, Y.; Ding, F.; Wang, K. A Cu-SiO2 Catalyst for Highly Efficient Hydrogenation of Methyl Formate to Methanol. Catalysts 2023, 13, 1038. https://doi.org/10.3390/catal13071038
Wu J, Liu G, Liu Q, Zhang Y, Ding F, Wang K. A Cu-SiO2 Catalyst for Highly Efficient Hydrogenation of Methyl Formate to Methanol. Catalysts. 2023; 13(7):1038. https://doi.org/10.3390/catal13071038
Chicago/Turabian StyleWu, Jincheng, Guoguo Liu, Qin Liu, Yajing Zhang, Fu Ding, and Kangjun Wang. 2023. "A Cu-SiO2 Catalyst for Highly Efficient Hydrogenation of Methyl Formate to Methanol" Catalysts 13, no. 7: 1038. https://doi.org/10.3390/catal13071038






