Recent Progress of Metal-Oxide-Based Catalysts for Non-Oxidative Coupling of Methane to Ethane and Hydrogen
Abstract
:1. Introduction
2. Thermal Catalytic NOCM
2.1. Fundamentals of Thermal Catalysis
2.2. Precious Metal Catalysts
2.3. Non-Precious Metal Catalysts
3. Photocatalytic NOCM
3.1. Fundamentals of Photocatalysis
3.2. Metal-Oxide-Based Catalysts
3.3. Metal-Modified Catalysts
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kerr, R.A. Natural Gas From Shale Bursts Onto the Scene. Science 2010, 328, 1624–1626. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zholobenko, V.L.; Moldovan, S.; Hu, D.; Wu, D.; Ordomsky, V.V.; Khodakov, A.Y. Stoichiometric methane conversion to ethane using photochemical looping at ambient temperature. Nat. Energy 2020, 5, 511–519. [Google Scholar] [CrossRef]
- Montzka, S.A.; Dlugokencky, E.J.; Butler, J.H. Non-CO2 greenhouse gases and climate change. Nature 2011, 476, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, S.; Chen, G. High-temperature steam reforming of methanol over ZnO–Al2O3 catalysts. Appl. Catal. B 2011, 101, 409–416. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Najari, S.; Saeidi, S.; Concepcion, P.; Dionysiou, D.D.; Bhargava, S.K.; Lee, A.F.; Wilson, K. Oxidative dehydrogenation of ethane: Catalytic and mechanistic aspects and future trends. Chem. Soc. Rev. 2021, 50, 4564–4605. [Google Scholar] [CrossRef]
- Xiao, Y.; Varma, A. Highly Selective Nonoxidative Coupling of Methane over Pt-Bi Bimetallic Catalysts. ACS Catal. 2018, 8, 2735–2740. [Google Scholar] [CrossRef]
- Julian, I.; Ramirez, H.; Hueso, J.L.; Mallada, R.; Santamaria, J. Non-oxidative methane conversion in microwave-assisted structured reactors. Chem. Eng. J. 2019, 377, 119764. [Google Scholar] [CrossRef]
- Wang, G.; Mu, X.; Li, J.; Zhan, Q.; Qian, Y.; Mu, X.; Li, L. Light-induced Nonoxidative Coupling of Methane using Stable Solid-Solutions. Angew. Chem. Int. Ed. Engl. 2021, 13, 20760–20764. [Google Scholar] [CrossRef]
- Meng, L.; Chen, Z.; Ma, Z.; He, S.; Hou, Y.; Li, H.-H.; Yuan, R.; Huang, X.-H.; Wang, X.; Wang, X.; et al. Gold plasmon-induced photocatalytic dehydrogenative coupling of methane to ethane on polar oxide surfaces. Energy Environ. Sci. 2018, 11, 294–298. [Google Scholar] [CrossRef]
- Huang, K.; Miller, J.B.; Huber, G.W.; Dumesic, J.A.; Maravelias, C.T. A General Framework for the Evaluation of Direct Nonoxidative Methane Conversion Strategies. Joule 2018, 2, 349–365. [Google Scholar] [CrossRef] [Green Version]
- Bajec, D.; Kostyniuk, A.; Pohar, A.; Likozar, B. Nonoxidative methane activation, coupling, and conversion to ethane, ethylene, and hydrogen over Fe/HZSM-5, Mo/HZSM-5, and Fe–Mo/HZSM-5 catalysts in packed bed reactor. Int. J. Energy Res. 2019, 43, 6852–6868. [Google Scholar] [CrossRef]
- Schwach, P.; Pan, X.L.; Bao, X.H. Direct Conversion of Methane to Value-Added Chemicals over Heterogeneous Catalysts: Challenges and Prospects. Chem. Rev. 2017, 117, 8497–8520. [Google Scholar] [CrossRef] [PubMed]
- Amos, R.D. An accurateab initiostudy of the multipole moments and polarizabilities of methane. Mol. Phys. 1979, 38, 33–45. [Google Scholar] [CrossRef]
- Reddy, P.V.L.; Kim, K.-H.; Song, H. Emerging green chemical technologies for the conversion of CH4 to value added products. Renew. Sustain. Energy Rev. 2013, 24, 578–585. [Google Scholar] [CrossRef]
- Belgued, M.; Pareja, P.; Amariglio, A.; Amariglio, H. Conversion of Methane into Higher Hydrocarbons on Platinum. Nature 1991, 352, 789–790. [Google Scholar] [CrossRef]
- Bajec, D.; Kostyniuk, A.; Pohar, A.; Likozar, B. Micro-kinetics of non-oxidative methane coupling to ethylene over Pt/CeO2 catalyst. Chem. Eng. J. 2020, 396, 125182. [Google Scholar] [CrossRef]
- Moya, S.F.; Martins, R.L.; Ota, A.; Kunkes, E.L.; Behrens, M.; Schmal, M. Nanostructured supported palladium catalysts—Non-oxidative methane coupling. Appl. Catal. A 2012, 411–412, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Xiao, Y.; Chowdhury, P.R.; Wu, Z.; Ma, T.; Chen, J.Z.; Wan, G.; Kim, T.-H.; Jing, D.; He, P.; et al. Direct methane activation by atomically thin platinum nanolayers on two-dimensional metal carbides. Nat. Catal. 2021, 4, 882–891. [Google Scholar] [CrossRef]
- Guo, X.; Fang, G.; Li, G.; Ma, H.; Fan, H.; Yu, L.; Ma, C.; Wu, X.; Deng, D.; Wei, M.; et al. Direct, Nonoxidative Conversion of Methane to Ethylene, Aromatics, and Hydrogen. Science 2014, 344, 616–619. [Google Scholar] [CrossRef]
- Xie, P.; Pu, T.; Nie, A.; Hwang, S.; Purdy, S.C.; Yu, W.; Su, D.; Miller, J.T.; Wang, C. Nanoceria-Supported Single-Atom Platinum Catalysts for Direct Methane Conversion. ACS Catal. 2018, 8, 4044–4048. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, H.W.; Han, S.J.; Lee, S.W.; Shin, J.; Kim, Y.T. Mechanistic and microkinetic study of non-oxidative methane coupling on a single-atom iron catalyst. Commun. Chem. 2020, 3, 58. [Google Scholar] [CrossRef] [PubMed]
- Dipu, A.L.; Ohbuchi, S.; Nishikawa, Y.; Iguchi, S.; Ogihara, H.; Yamanaka, I. Direct Nonoxidative Conversion of Methane to Higher Hydrocarbons over Silica-Supported Nickel Phosphide Catalyst. ACS Catal. 2019, 10, 375–379. [Google Scholar] [CrossRef]
- Han, S.J.; Lee, S.W.; Kim, H.W.; Kim, S.K.; Kim, Y.T. Nonoxidative Direct Conversion of Methane on Silica-Based Iron Catalysts: Effect of Catalytic Surface. ACS Catal. 2019, 9, 7984–7997. [Google Scholar] [CrossRef]
- Soulivong, D.; Norsic, S.; Taoufik, M.; Cope’ret, C.; Thivolle-Cazat, J.; Chakka, S.; Basset, J.-M. Non-Oxidative Coupling Reaction of Methane to Ethane and Hydrogen Catalyzed by the Silica-Supported Tantalum Hydride: (tSiO)2Ta-H. J. Am. Chem. Soc. 2008, 130, 5044–5045. [Google Scholar] [CrossRef]
- Moya, S.F.; Martins, R.L.; Schmal, M. Monodispersed and nanostructrured Ni/SiO2 catalyst and its activity for non oxidative methane activation. Appl. Catal. A 2011, 396, 159–169. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, J.; Pupucevski, M.; Impeng, S.; Yang, B.; Chen, G.; Kuboon, S.; Zhong, Q.; Faungnawakij, K.; Zheng, L.; et al. High-Performance Binary Mo–Ni Catalysts for Efficient Carbon Removal during Carbon Dioxide Reforming of Methane. ACS Catal. 2021, 11, 12087–12095. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.C.; Li, T.H.; Duan, Z.H.; Zhang, T.Y.; Yan, M.; Li, W.L.; Xiao, H.; Wang, Y.G.; Chang, C.R.; et al. Unravelling the Enigma of Nonoxidative Conversion of Methane on Iron Single-Atom Catalysts. Angew. Chem. Int. Ed. Engl. 2020, 59, 18586–18590. [Google Scholar] [CrossRef]
- Kashaboina, U.; Nishikawa, Y.; Wakisaka, Y.; Sirisit, N.; Nagamatsu, S.-I.; Bao, D.; Ariga-Miwa, H.; Takakusagi, S.; Inami, Y.; Kuriyama, F.; et al. Metamorphosis-like Transformation during Activation of In/SiO2 Catalyst for Non-oxidative Coupling of Methane: In Situ X-ray Absorption Fine Structure Analysis. Chem. Lett. 2019, 48, 1145–1147. [Google Scholar] [CrossRef]
- Al Rashid, M.H.; Dipu, A.; Nishikawa, Y.; Ogihara, H.; Inami, Y.; Obuchi, S.; Yamanaka, I.; Nagamatsu, S.; Kido, D.; Asakura, K. Active Phase Structure of the SiO2-supported Nickel Phosphide Catalysts for Non-oxidative Coupling of Methane (NOCM) Reactions. E-J. Surf. Sci. Nanotechnol. 2020, 18, 24–27. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Xi, Y.; Zhang, G.; Phillips, D.L.; Guo, W. A Reaction Mechanism of Methane Coupling on a Silica-Supported Single-Site Tantalum Catalyst. Organometallics 2014, 33, 2172–2181. [Google Scholar] [CrossRef]
- Singh, S.P.; Anzai, A.; Kawaharasaki, S.; Yamamoto, A.; Yoshida, H. Non-oxidative coupling of methane over Pd-loaded gallium oxide photocatalysts in a flow reactor. Catal. Today 2020, 375, 264–272. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Jansen, I.; Schuttlefield Christus, J.D. Renewable energy based catalytic CH4 conversion to fuels. Catal. Sci. Technol. 2014, 4, 2397–2411. [Google Scholar] [CrossRef]
- Kato, Y.; Yoshida, H.; Hattorib, T. Photoinduced non-oxidative coupling of methane over silica-alumina and alumina around room temperature. Chem. Commun. 1998, 21, 2389–2390. [Google Scholar] [CrossRef]
- Yuliati, L.; Tsubota, M.; Satsuma, A.; Itoh, H.; Yoshida, H. Photoactive sites on pure silica materials for nonoxidative direct methane coupling. J. Catal. 2006, 238, 214–220. [Google Scholar] [CrossRef]
- Yoshida, H.; Chaskar, M.G.; Kato, Y.; Hattori, T. Active sites on silica-supported zirconium oxide for photoinduced direct methane conversion and photoluminescence. J. Photochem. Photobiol. A 2003, 160, 47–53. [Google Scholar] [CrossRef]
- Kato, Y.; Matsushita, N.; Yoshida, H.; Hattori, T. Highly active silica–alumina–titania catalyst for photoinduced non-oxidative methane coupling. Catal. Commun. 2002, 3, 99–103. [Google Scholar] [CrossRef]
- Yoshida, H.; Matsushita, N.; Kato, Y.; Hattori, T. Synergistic Active Sites on SiO2-Al2O3-TiO2 Photocatalysts for Direct Methane Coupling. J. Phys. Chem. B 2003, 107, 8355–8362. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, S.; Ma, J.; Mine, S.; Toyao, T.; Matsuoka, M.; Wang, L.; Zhang, J. Non-oxidative Coupling of Methane: N-type Doping of Niobium Single Atoms in TiO(2)-SiO(2) Induces Electron Localization. Angew. Chem. Int. Ed. Engl. 2021, 60, 11901–11909. [Google Scholar] [CrossRef]
- Yoshida, H.; Hamajima, T.; Kato, Y.; Shibata, J.; Satsuma, A.; Hattori, T. Active Ag species in MFI zeolite for direct methane conversion in the light and dark. Res. Chem. Intermed. 2003, 29, 7–9. [Google Scholar] [CrossRef]
- Li, L.; Cai, Y.Y.; Li, G.D.; Mu, X.Y.; Wang, K.X.; Chen, J.S. Synergistic effect on the photoactivation of the methane C-H bond over Ga(3+)-modified ETS-10. Angew. Chem. Int. Ed. Engl. 2012, 51, 4702–4706. [Google Scholar] [CrossRef] [PubMed]
- Yuliati, L.; Hamajima, T.; Hattori, T.; Yoshida, H. Highly dispersed Ce(III) species on silica and alumina as new photocatalysts for non-oxidative direct methane coupling. Chem. Commun. (Camb) 2005, 38, 4824–4826. [Google Scholar] [CrossRef] [PubMed]
- Yuliati, L.; Hamajima, T.; Hattori, T.; Yoshida, H. Nonoxidative Coupling of Methane over Supported Ceria Photocatalysts. J. Phys. Chem. C 2008, 112, 7223–7232. [Google Scholar] [CrossRef]
- Li, L.; Li, G.D.; Yan, C.; Mu, X.Y.; Pan, X.L.; Zou, X.X.; Wang, K.X.; Chen, J.S. Efficient sunlight-driven dehydrogenative coupling of methane to ethane over a Zn(+)-modified zeolite. Angew. Chem. Int. Ed. Engl. 2011, 50, 8299–8303. [Google Scholar] [CrossRef]
- Yuliati, L.; Hattori, T.; Itoh, H.; Yoshida, H. Photocatalytic nonoxidative coupling of methane on gallium oxide and silica-supported gallium oxide. J. Catal. 2008, 257, 396–402. [Google Scholar] [CrossRef]
- Ma, J.; Tan, X.; Zhang, Q.; Wang, Y.; Zhang, J.; Wang, L. Exploring the Size Effect of Pt Nanoparticles on the Photocatalytic Nonoxidative Coupling of Methane. ACS Catal. 2021, 11, 3352–3360. [Google Scholar] [CrossRef]
- Singh, S.P.; Yamamoto, A.; Fudo, E.; Tanaka, A.; Kominami, H.; Yoshida, H. A Pd-Bi Dual-Cocatalyst-Loaded Gallium Oxide Photocatalyst for Selective and Stable Nonoxidative Coupling of Methane. ACS Catal. 2021, 11, 13768–13781. [Google Scholar] [CrossRef]
- Wu, S.; Tan, X.; Lei, J.; Chen, H.; Wang, L.; Zhang, J. Ga-Doped and Pt-Loaded Porous TiO2-SiO2 for Photocatalytic Nonoxidative Coupling of Methane. J. Am. Chem. Soc. 2019, 141, 6592–6600. [Google Scholar] [CrossRef]
- Yoshida, H.; Matsushita, N.; Kato, Y.; Hattori, T. Active sites in sol–gel prepared silica-alumina for photoinduced non-oxidative methane coupling. Phys. Chem. Chem. Phys. 2002, 4, 2459–2465. [Google Scholar] [CrossRef]
- Talebi, P.; Kistanov, A.A.; Rani, E.; Singh, H.; Pankratov, V.; Pankratova, V.; King, G.; Huttula, M.; Cao, W. Unveiling the role of carbonate in nickel-based plasmonic core@shell hybrid nanostructure for photocatalytic water splitting. Appl. Energy 2022, 322, 119461. [Google Scholar] [CrossRef]
- Song, H.; Meng, X.; Wang, Z.-J.; Liu, H.; Ye, J. Solar-Energy-Mediated Methane Conversion. Joule 2019, 3, 1606–1636. [Google Scholar] [CrossRef]
- Schwarz, H.; Shaik, S.; Li, J. Electronic Effects on Room-Temperature, Gas-Phase C-H Bond Activations by Cluster Oxides and Metal Carbides: The Methane Challenge. J. Am. Chem. Soc. 2017, 139, 17201–17212. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Ma, Y.; Wu, X.; Jiang, Y.; Hu, Y.H. Highly efficient light-driven methane coupling under ambient conditions based on an integrated design of a photocatalytic system. Green Chem. 2020, 22, 4669–4675. [Google Scholar] [CrossRef]
- Zhang, W.; Fu, C.; Low, J.; Duan, D.; Ma, J.; Jiang, W.; Chen, Y.; Liu, H.; Qi, Z.; Long, R.; et al. High-performance photocatalytic nonoxidative conversion of methane to ethane and hydrogen by heteroatoms-engineered TiO2. Nat. Commun. 2022, 13, 2806. [Google Scholar] [CrossRef]


| Entry | Catalysts | Temperature (K) | Reaction Conditions | C2H6 Selectivity (%) | CH4 Conversion (%) | Reference |
|---|---|---|---|---|---|---|
| 1 | Pt | 523 | 100 mg, 400 mL min−1 CH4 | - | 19.3 | [16] |
| 2 | Pt/CeO2 | 1053 | 0.36 g, 0.094 h−1 CH4 | 84.8 | 7.92 | [17] |
| 3 | Pd/α-Al2O3 | 550 | 300 mg, 300 mL min−1 CH4 | 4 | - | [18] |
| 4 | Pt/Mo2TiC2 | 1023 | 50 mg, 12.9 GHSV(h−1) | 85 | 6.5 | [19] |
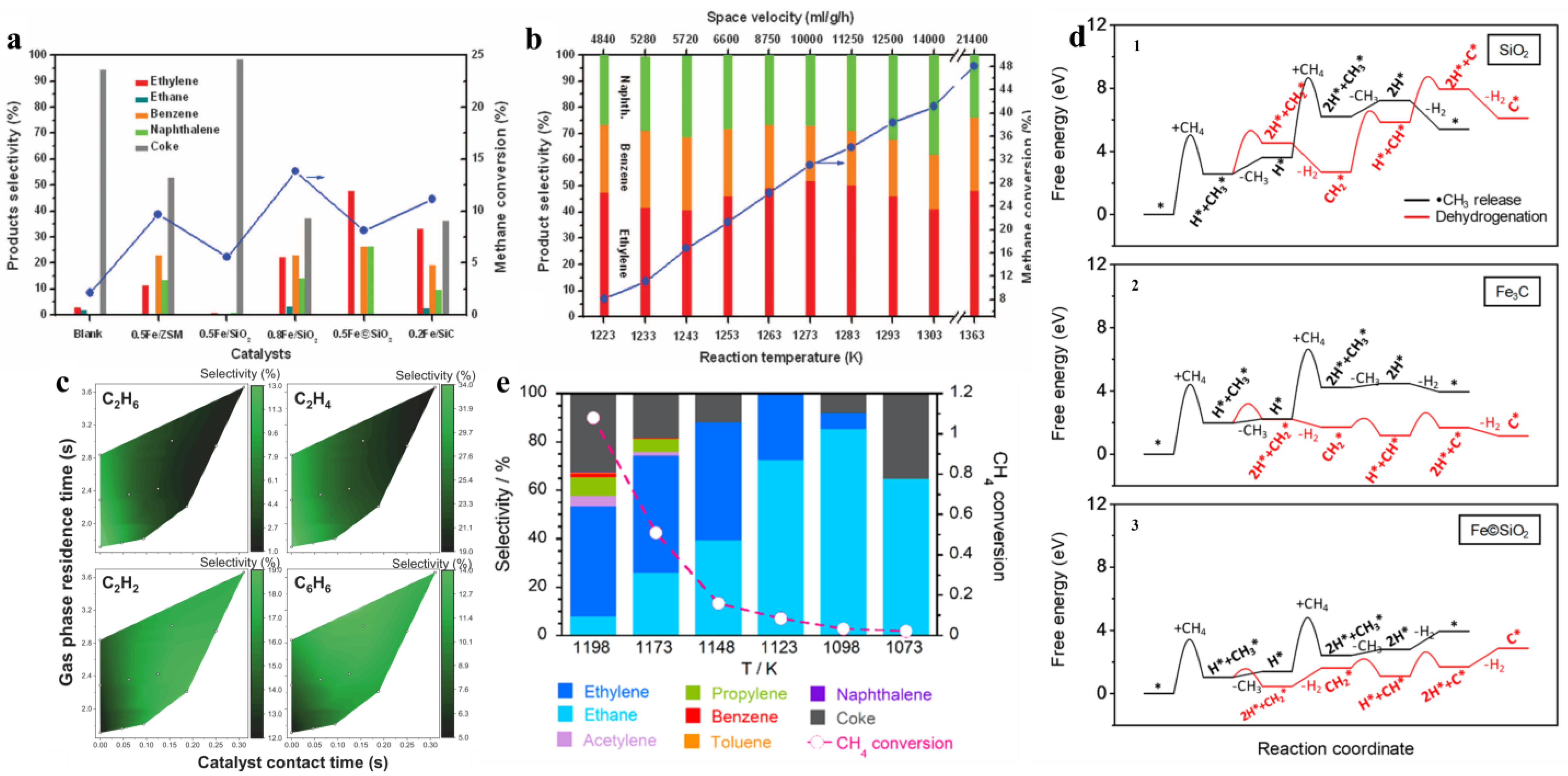
| 5 | Fe©SiO2 | 1223 | 4.84 L gcat−1 h−1 CH4 | 2 | 15 | [20] |
| 6 | Pt1@CeO2 | 1173 | 0.2 g, 20 mL min−1 CH4 | 43.6 | ~6 | [21] |
| 5 | Fe/SiO2 | 1293 | 1.2 g, 2.4 mL gcat−1 s−1 | 13 | 12.2 | [22] |
| 6 | Fe/HZSM-5 | 973 | 0.5 g, 6L gcat−1 h−1 CH4 | 23 | 3 | [12] |
| 7 | Fe©SiO2 | 1098 | 100 mg, 20 mL min−1 CH4 | 80 | 15 | [23] |
| 8 | Fe©SiO2 | 1353 | 0.6 g, 8000 mL gcat−1 h−1 CH4 | 7.9 | 6 | [24] |
| 9 | Ta/SiO2 | 573 | 300 mg, 30 mL min−1 CH4 | 50 | 0.1 | [25] |
| 10 | NiOx/SiO2 | 700 | 300 mg, 300 mL min−1 CH4 | 16 | - | [26] |
| Catalysts | Reaction Conditions | C2H6 Selectivity (% or μmol g−1 h−1) | CH4 Conversion (% or μmol g−1 h−1) | Reference | |
|---|---|---|---|---|---|
| 1 | SiO2-Al2O3 | 250 W Xe lamp, 1.0 g, 100 μmol CH4 | 0.01 | 5.9 | [34] |
| 2 | FSM-16 | 300 W Xe lamp, 0.2 g, 200 μmol CH4 | 1.63 | 5.7 | [35] |
| 3 | ZrO2/SiO2 | 250 W Xe lamp, 0.5 g, 200 μmol CH4 | 0.11 | 0.1 | [36] |
| 4 | SiO2-Al2O3-TiO2 | 250 W Xe lamp, 1.0 g, 200 μmol CH4 | 2.07 | 2.5 | [37] |
| 5 | SiO2-Al2O3-TiO2 | 250 W Xe lamp, 1.0 g, 200 μmol CH4 | 2.07 | 3.7 | [38] |
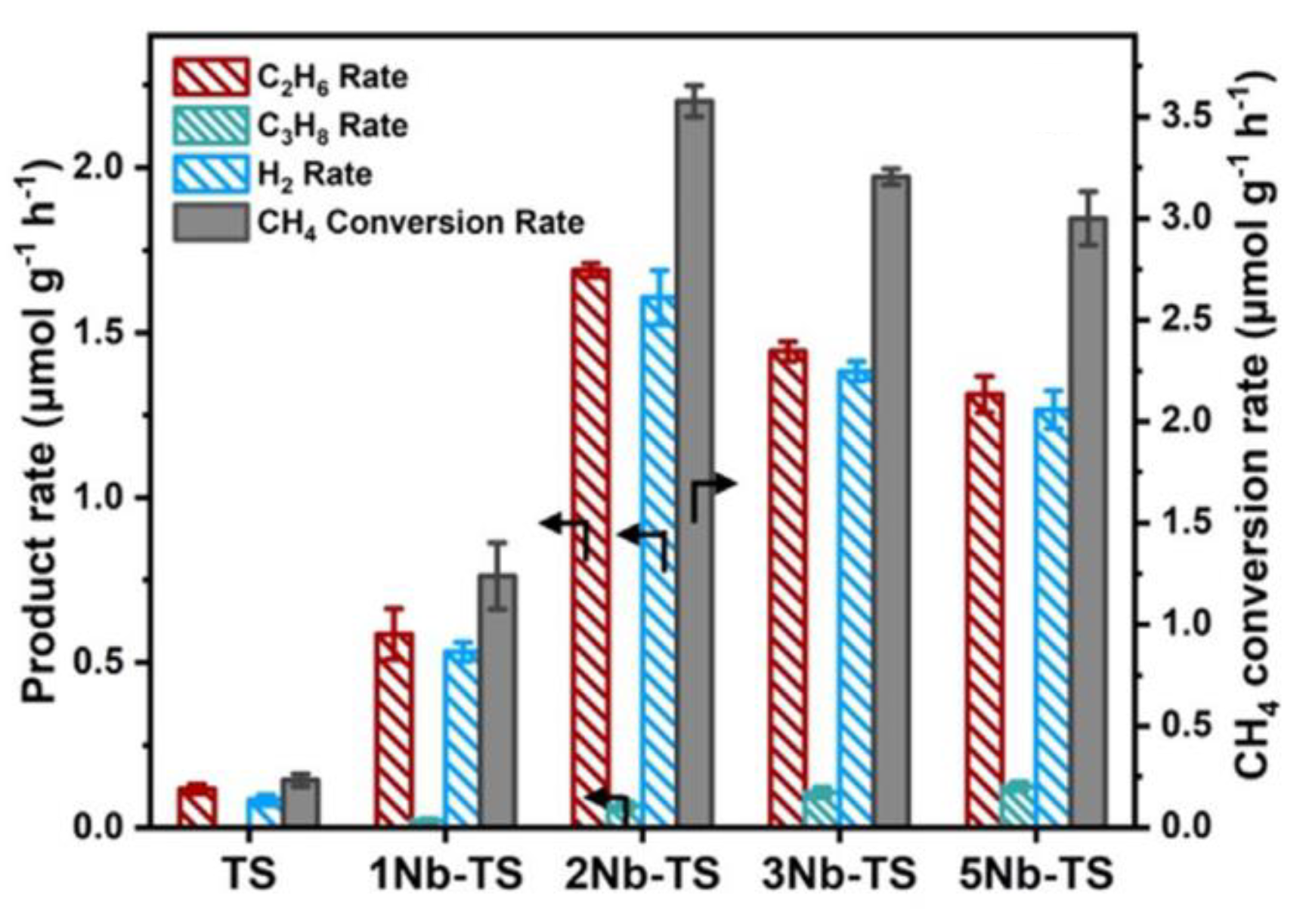
| 6 | Nb-TiO2-SiO2 | 300 W Xe lamp, 0.1 g, 1 mL CH4 | 1.7 μmol g−1 h−1 | 3.5 μmol g−1 h−1 | [39] |
| 7 | Ag/MFI | 250 W Xe lamp, 0.2 g, 200 μmol CH4 | 4.40 | 4.0 | [40] |
| 8 | Ga-ETS-10 | high-pressure Hg lamp, 0.2 g, 200 μmol CH4 | 70 | 15 | [41] |
| 9 | Ag-HPW/TiO2 | 400 W Xe lamp, 0.1 g, 0.3 MPa CH4 | 150 μmol g−1 h−1 | 4.96 | [2] |
| 10 | Ce/SiO2 | 300 W Xe lamp, 0.2 g, 200 μmol CH4 | 94 | 13 | [42] |
| 11 | Ce/Al2O3 | 300 W Xe lamp, 0.2 g, 200 μmol CH4 | 20 | 15 | [43] |
| 12 | Zn2+-ZSM-5− | high-pressure Hg lamp, 1.0 g, 200 μmol CH4 | 99 | 6 μmol g−1 h−1 | [44] |
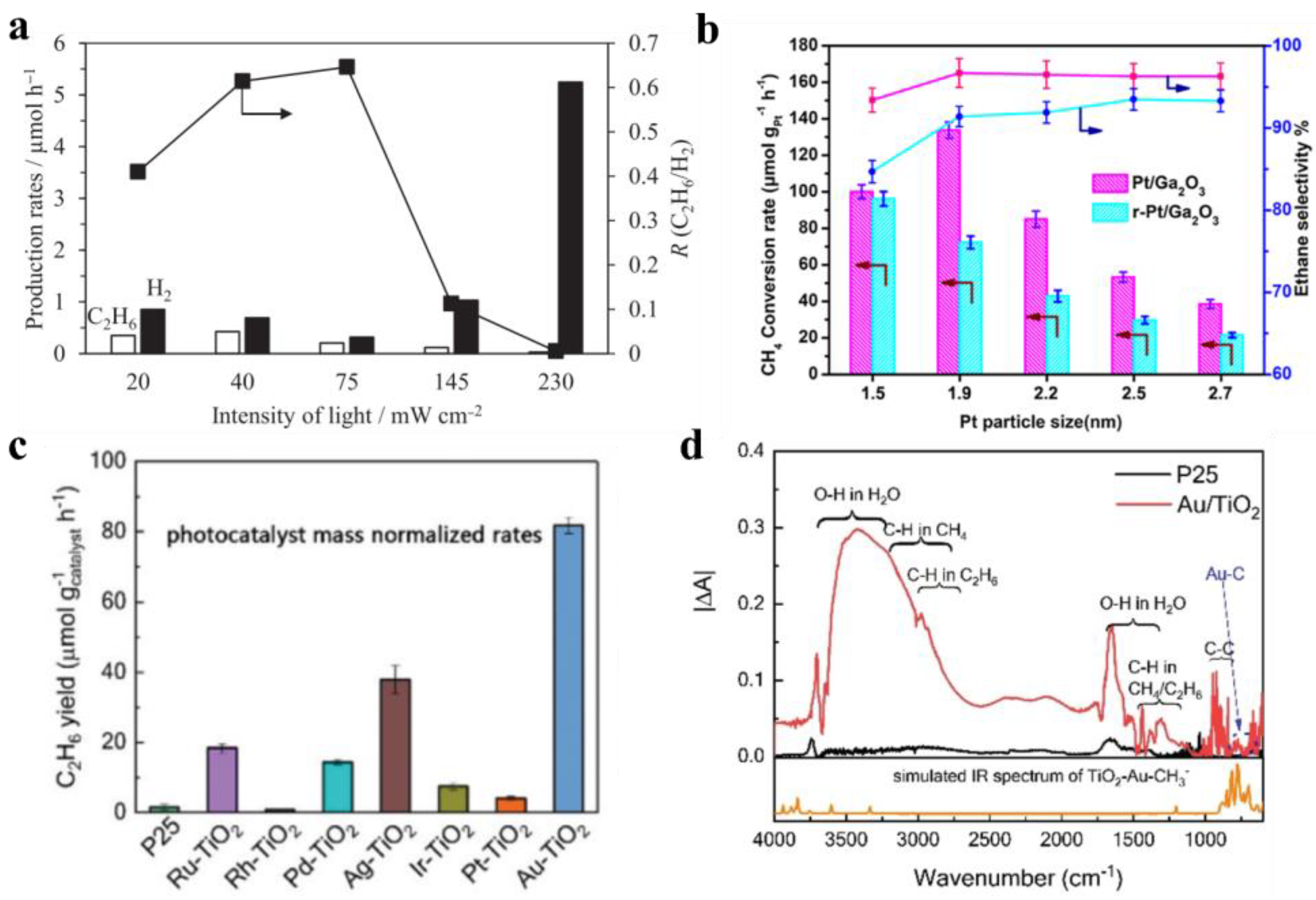
| 13 | Ga2O3 | 300 W Xe lamp, 0.2 g, 200 μmol CH4 | 86 | 17 | [45] |
| 14 | Pt/Ga2O3 | 300 W Xe lamp, 0.2 g, 1 mL CH4 | 90 | 140 μmol g−1 h−1 | [46] |
| 15 | Pd/Ga2O3 | 300 W Xe lamp, 0.8 g, 30 mL min−1 CH4 | 0.125 μmol g−1 h−1 | - | [32] |
| 16 | Pd-Bi/Ga2O3 | 300 W Xe lamp, 0.8 g, 450 h−1 CH4 | 97 | 0.03 | [47] |
| 17 | Pt/HGTS | 300 W Xe lamp, 0.2 g, 44.6 μmol CH4 | 3.48 μmol g−1 h−1 | 28 | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Rao, Z.; Huang, Z.; Chen, Y.; Wang, F.; Zhou, Y. Recent Progress of Metal-Oxide-Based Catalysts for Non-Oxidative Coupling of Methane to Ethane and Hydrogen. Catalysts 2023, 13, 719. https://doi.org/10.3390/catal13040719
Wang J, Rao Z, Huang Z, Chen Y, Wang F, Zhou Y. Recent Progress of Metal-Oxide-Based Catalysts for Non-Oxidative Coupling of Methane to Ethane and Hydrogen. Catalysts. 2023; 13(4):719. https://doi.org/10.3390/catal13040719
Chicago/Turabian StyleWang, Junbu, Zhiqiang Rao, Zeai Huang, Yaolin Chen, Fang Wang, and Ying Zhou. 2023. "Recent Progress of Metal-Oxide-Based Catalysts for Non-Oxidative Coupling of Methane to Ethane and Hydrogen" Catalysts 13, no. 4: 719. https://doi.org/10.3390/catal13040719






