Visible-Light-Driven BiOBr-TiO2-Attapulgite Photocatalyst with Excellent Photocatalytic Activity for Multiple Xanthates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Materials
2.1.1. Phase Analysis
2.1.2. Morphology Analysis
2.1.3. Surface Chemical State Analysis
2.1.4. TG-DSC Analysis
2.2. Photocatalytic Activity
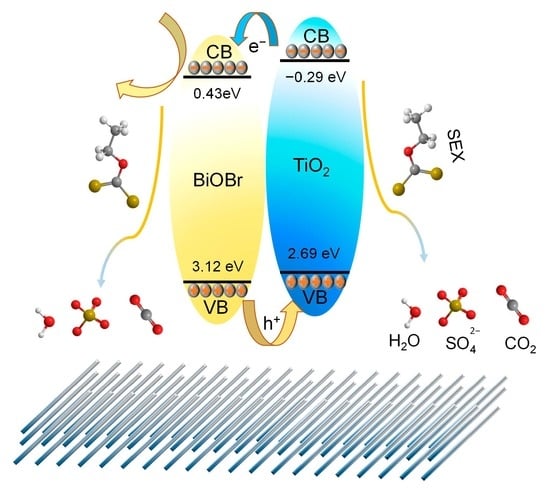
2.3. Possible Degradation Mechanism
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Microstructure Characterization
3.3. Preparation of BiOBr-TiO2-Attapulgite Composites
3.4. Measurement of Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shu, K.; Chuaicham, C.; Noguchi, Y.; Xu, L.; Sasaki, K. In-situ hydrothermal synthesis of Fe-doped hydroxyapatite photocatalyst derived from converter slag toward xanthate photodegradation and Cr(VI) reduction under visible-light irradiation. Chem. Eng. J. 2023, 459, 141474. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, G.; Liu, J.; Liu, Y.; Zhai, J.; Zhang, H.; Yu, H. Visible-light-driven photodegradation of xanthate in a continuous fixed-bed photoreactor: Experimental study and modeling. Chem. Eng. J. 2023, 461, 141833. [Google Scholar] [CrossRef]
- Zhou, P.; Shen, Y.; Zhao, S.; Li, G.; Cui, B.; Wei, D.; Shen, Y. Synthesis of clinoptilolite-supported BiOCl/TiO2 heterojunction nanocomposites with highly-enhanced photocatalytic activity for the complete degradation of xanthates under visible light. Chem. Eng. J. 2021, 407, 126697. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, T.; Zheng, S.; Sun, Z.; Li, C. Adsorptive and photocatalytic behaviour of PANI/TiO2/metakaolin composites for the removal of xanthate from aqueous solution. Miner. Eng. 2021, 171, 107129. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, Y.; Zhang, X.; Cheng, J.; Xie, Y.; Zhang, Y.; Yin, X.; Song, F.; Cui, H. Novel CdS/PANI/MWCNTs photocatalysts for photocatalytic degradation of xanthate in wastewater. Sep. Purif. Technol. 2023, 309, 123022. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, P.; Zhao, S.; Li, A.; Chen, Y.; Bai, J.; Han, C.; Wei, D.; Ao, Y. Synthesis of high-efficient TiO2/clinoptilolite photocatalyst for complete degradation of xanthate. Miner. Eng. 2020, 159, 106640. [Google Scholar] [CrossRef]
- Zou, M.; Tan, C.; Yang, H.; Kuang, D.; Nie, Z.; Zhou, H. Facile preparation of recyclable and flexible BiOBr@ TiO2/PU-SF composite porous membrane for efficient photocatalytic degradation of mineral flotation wastewater. J. Water Process Eng. 2022, 50, 103127. [Google Scholar] [CrossRef]
- Yuan, F.; Zheng, Y.; Gao, D.; Wang, L.; Hu, X. Facile assembly and enhanced visible-light-driven photocatalytic activity of S-scheme BiOBr/g-C3N4 heterojunction for degrading xanthate in wastewater. J. Mol. Liq. 2022, 366, 120279. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Shen, X.; Zhang, Y.; Lou, Y.; Pan, C.; Zhu, Y.; Xu, J. A plasmonic Z-scheme Ag@AgCl/PDI photocatalyst for the efficient elimination of organic pollutants, antibiotic resistant bacteria and antibiotic resistance genes. Appl. Catal. B Environ. 2023, 324, 122220. [Google Scholar] [CrossRef]
- Wei, S.; Kamali, A.R. Trifunctional mesoporous magnetic adsorbent-photocatalyst nanocomposite for efficient removal of potassium ethyl xanthate from mining wastewater. J. Water Process Eng. 2022, 49, 103067. [Google Scholar] [CrossRef]
- Zhou, P.; Shen, Y.; Zhao, S.; Bai, J.; Han, C.; Liu, W.; Wei, D. Facile synthesis of clinoptilolite-supported Ag/TiO2 nanocomposites for visible-light degradation of xanthates. J. Taiwan Inst. Chem. Eng. 2021, 122, 231–240. [Google Scholar] [CrossRef]
- Shu, K.; Chuaicham, C.; Noguchi, Y.; Xu, L.; Sasaki, K. Charge transfer mechanism through S-scheme heterojunction in in-situ synthesized TiO2/Fe-doped hydroxyapatite for improved photodegradation of xanthate. J. Hazard. Mater. 2023, 460, 132337. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Teng, Q.; Sun, B.; Yang, Z.; Liu, S.; Zhu, X. Synthesis scaly Ag-TiO2 loaded fly ash magnetic bead particles for treatment of xanthate wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126795. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Tran, N.M.; Tri, N.N.; Sreedhar, A.; Noh, J.S. Highly surface-active Si-doped TiO2/Ti3C2Tx heterostructure for gas sensing and photodegradation of toxic matters. Chem. Eng. J. 2021, 425, 131437. [Google Scholar] [CrossRef]
- Bott-Neto, J.L.; Martins, T.S.; Oliveira Jr, O.N.; Marken, F. Controlled electrodeposition of brookite TiO2 for photoelectroanalysis at printed carbon electrodes. Appl. Surf. Sci. 2023, 640, 158316. [Google Scholar] [CrossRef]
- Wang, S.-q.; Li, X.-x.; Wu, J.-j. Preparation of TiO2/graphene oxide and their photocatalytic properties at room temperature. J. Fuel Chem. Technol. 2022, 50, 1307–1316. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, Y.; Liu, H.; Yan, Y.; Yan, F. Reinforced UHMWPE composites by grafting TiO2 on ATP nanofibers for improving thermal and tribological properties. Tribol. Int. 2022, 172, 107585. [Google Scholar] [CrossRef]
- Zhou, L.; Yan, Y.; Mao, H.; Zhou, S.; Hui, J.; Li, H.; Li, M.; Zhao, Y.; Zhang, Q.; Xia, S. Development of attapulgite based catalytic membrane for activation of peroxymonosulfate: A singlet oxygen-dominated catalytic oxidation process for sulfamethoxazole degradation. Sep. Purif. Technol. 2023, 312, 123382. [Google Scholar] [CrossRef]
- Yuan, S.; Peng, J.; Zhang, X.; Lin, D.; Geng, H.; Han, B.; Zhang, M.; Wang, H. A mechanically robust slippery surface with ‘corn-like’ structures fabricated by in-situ growth of TiO2 on attapulgite. Chem. Eng. J. 2021, 415, 128953. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Zhang, W.; Liu, J.; Zhong, H.; Zhao, Y. Photocatalytic activity of attapulgite–BiOCl–TiO2 toward degradation of methyl orange under UV and visible light irradiation. Mater. Res. Bull. 2015, 66, 109–114. [Google Scholar] [CrossRef]
- Tan, Z.; Zhang, S.; Yue, X.; Zhao, F.; Xi, F.; Yan, D.; Ling, H.; Zhang, R.; Tang, F.; You, K.; et al. Attapulgite as a cost-effective catalyst for low-energy consumption amine-based CO2 capture. Sep. Purif. Technol. 2022, 298, 121577. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Zhou, S.; Chen, H.; Zhong, H.; Zhao, Y.; Wang, X. Magnetically separable attapulgite−TiO2−Fe O composites with superior activity towards photodegradation of methyl orange under visible light radiation. J. Ind. Eng. Chem. 2014, 20, 3884–3889. [Google Scholar] [CrossRef]
- Cao, W.; Wang, W.; Yang, Z.; Wang, W.; Chen, W.; Wu, K. Enhancing photocathodic protection performance by controlled synthesis of Bi/BiOBr/TiO2 NTAs Z-scheme heterojunction films. J. Alloys Compd. 2023, 960, 170675. [Google Scholar] [CrossRef]
- Yin, Y.; Kang, X.; Han, B. Two-dimensional materials: Synthesis and applications in the electro-reduction of carbon dioxide. Chem. Synth. 2022, 2, 19. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, C.; Wu, Z.; Liu, Y.; Sun, S. A review on the progress of the photocatalytic removal of refractory pollutants from water by BiOBr-based nanocomposites. Chemosphere 2022, 308, 136107. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wang, P.; Zhan, S. Shedding light on the role of interfacial chemical bond in heterojunction photocatalysis. Nano Res. 2022, 15, 10158–10170. [Google Scholar] [CrossRef]
- Zhao, S.; Lu, Y.; Lu, R.; Hu, Y.; Rodriguez, R.D.; Chen, J. Constructing BiOBr/TiO2 heterostructure nanotubes for enhanced adsorption/photocatalytic performance. J. Water Process Eng. 2023, 54, 103972. [Google Scholar] [CrossRef]
- Eskandari, P.; Amarloo, E.; Zangeneh, H.; Rezakazemi, M.; Zamani, M.R.; Aminabhavi, T.M. Photocatalytic activity of visible-light-driven L-Proline-TiO2/BiOBr nanostructured materials for dyes degradation: The role of generated reactive species. J. Environ. Manag. 2023, 326, 116691. [Google Scholar] [CrossRef]
- Huang, X.; Sun, M.; Humayun, M.; Li, S.; Zhao, J.; Chen, H.; Li, Z. In-situ synthesis of efficient N-graphyne/Bi/BiOBr photocatalysts for contaminants removal and nitrogen fixation. J. Alloys Compd. 2023, 976, 173025. [Google Scholar] [CrossRef]
- Swathi, K.S.; Gopalakrishna Naik, K. Structural, morphological, and optical studies of sol–gel spin coated TiO2 thin films. Mater. Today Proc. 2023, 960, 170335. [Google Scholar] [CrossRef]
- Zhao, M.; Qin, J.; Wang, N.; Zhang, Y.; Cui, H. Reconstruction of surface oxygen vacancy for boosting CO2 photoreduction mediated by BiOBr/CdS heterojunction. Sep. Purif. Technol. 2024, 329, 125179. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Zhang, Q.; Weng, W.; Wan, H. Attapulgite as natural catalyst for glucose isomerization to fructose in water. Catal. Commun. 2017, 99, 20–24. [Google Scholar] [CrossRef]
- Barkouch, H.; Bessbousse, H.; Amar, M.; Bouzzine, S.M.; Hamidi, M.; El Mhammedi, M.A.; Alaoui, O.T. Bismuth-doped TiO2 enable solar photocatalytic water treatment. Opt. Mater. 2023, 146, 114507. [Google Scholar] [CrossRef]
- Deng, J.; Xu, D.; Zhang, J.; Xu, Q.; Yang, Y.; Wei, Z.; Su, Z. Cs3Bi2Br9/BiOBr S-scheme heterojunction for selective oxidation of benzylic C−H bonds. J. Mater. Sci. Technol. 2023, 924, 166608. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, B.; Wang, H.; Chen, Y.; Fan, M.; Dong, L.; Li, B.; Chen, G. Exposed 110 facets of BiOBr anchored to marigold-like MnCo2O4 with abundant interfacial electron transfer bridges and efficient activation of peroxymonosulfate. J. Colloid Interface Sci. 2023, 653, 867–878. [Google Scholar] [CrossRef]
- Yue, C.; Lin, Y.; Sang, J.; Li, Z.; Zhang, P.; Fan, M.; Leng, Y.; Jiang, P.; Haryono, A. BiOBr/Bi24O31Br10 heterojunctions in situ prepared by microwave-assisted hydrothermal method for aerobic oxidation of glycerol to formic acid. Mater. Today Commun. 2022, 31, 103270. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zha, X.; Luo, Y.; Hu, Y.; Chen, G.; He, X. Interfacial chemical bond and oxygen vacancies modulated Mo2S3/BiOBr high-low junctions for enhanced photocatalysis gatifloxacin degradation. Appl. Surf. Sci. 2023, 641, 158548. [Google Scholar] [CrossRef]
- Tan, Y.; Yin, C.; Zheng, S.; Di, Y.; Sun, Z.; Li, C. Design and controllable preparation of Bi2MoO6/attapulgite photocatalyst for the removal of tetracycline and formaldehyde. Appl. Clay Sci. 2021, 215, 106319. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.; Liu, W.; Hu, X.; Zhang, C.; Zhou, Z.; Lang, J.; Wu, G.; Zhang, Y.; Yang, J.; et al. Regulating mechanical performance of poly (l-lactide acid) stent by the combined effects of heat and aqueous media. Int. J. Biol. Macromol. 2023, 242, 124987. [Google Scholar] [CrossRef]
- Wang, L.; Wang, T.; Zhang, Y.; Peng, X.; Song, W.; Yang, J.; Yuan, C. Oxidation behaviors of Hongqian heavy crude oil characterized by TG-DSC-FTIR-MS within full temperature regions. Fuel 2023, 353, 129242. [Google Scholar] [CrossRef]
- Zhu, S.-R.; Wu, M.-K.; Zhao, W.-N.; Yi, F.-Y.; Tao, K.; Han, L. Fabrication of heterostructured BiOBr/Bi24O31Br10/TiO2 photocatalyst by pyrolysis of MOF composite for dye degradation. J. Solid State Chem. 2017, 255, 17–26. [Google Scholar] [CrossRef]
- Qi, Y.; Shen, Y.; Zhao, S.; Jiang, X.; Ma, R.; Cui, B.; Zhao, Q.; Wei, D. Degradation of multiple xanthates using highly efficient visible light-responsive BiOBr-TiO2 composite photocatalysts. J. Ind. Eng. Chem. 2023, in press. [CrossRef]
- Shi, Z.; Zhang, Y.; Shen, X.; Duoerkun, G.; Zhu, B.; Zhang, L.; Li, M.; Chen, Z. Fabrication of g-C3N4/BiOBr heterojunctions on carbon fibers as weaveable photocatalyst for degrading tetracycline hydrochloride under visible light. Chem. Eng. J. 2020, 386, 124010. [Google Scholar] [CrossRef]













| Photocatalyst | Light Sources | Pollutants | Dosage (mg/L) | Degradation Rate | Irradiation Time (min) | Ref. |
|---|---|---|---|---|---|---|
| TiO2/clinoptilolite | Mercury lamp | SIPX | 20 | Over 90% | 30 | [6] |
| BiOBr/g-C3N4 | Xe lamp | SEX | 30 | 96.1% | 120 | [8] |
| Ag/TiO2/clinoptilolite | Xe lamp | SIPX | 20 | Approx. 60% | 180 | [11] |
| BiOCl/TiO2/clinoptilolite | Xe lamp | SIPX | 20 | 91.2% | 180 | [3] |
| BTA | Xe lamp | SEX | 20 | 94.8% | 40 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Zhao, S.; Jiang, X.; Kang, Z.; Gao, S.; Liu, W.; Shen, Y. Visible-Light-Driven BiOBr-TiO2-Attapulgite Photocatalyst with Excellent Photocatalytic Activity for Multiple Xanthates. Catalysts 2023, 13, 1504. https://doi.org/10.3390/catal13121504
Qi Y, Zhao S, Jiang X, Kang Z, Gao S, Liu W, Shen Y. Visible-Light-Driven BiOBr-TiO2-Attapulgite Photocatalyst with Excellent Photocatalytic Activity for Multiple Xanthates. Catalysts. 2023; 13(12):1504. https://doi.org/10.3390/catal13121504
Chicago/Turabian StyleQi, Yaozhong, Sikai Zhao, Xiaoyu Jiang, Zhangke Kang, Shuling Gao, Wengang Liu, and Yanbai Shen. 2023. "Visible-Light-Driven BiOBr-TiO2-Attapulgite Photocatalyst with Excellent Photocatalytic Activity for Multiple Xanthates" Catalysts 13, no. 12: 1504. https://doi.org/10.3390/catal13121504