Synergistic Catalytic Effects of Alloys of Noble Metal Nanoparticles Supported on Two Different Supports: Crystalline Zeolite Sn-Beta and Carbon Nanotubes for Glycerol Conversion to Methyl Lactate
Abstract
:1. Introduction
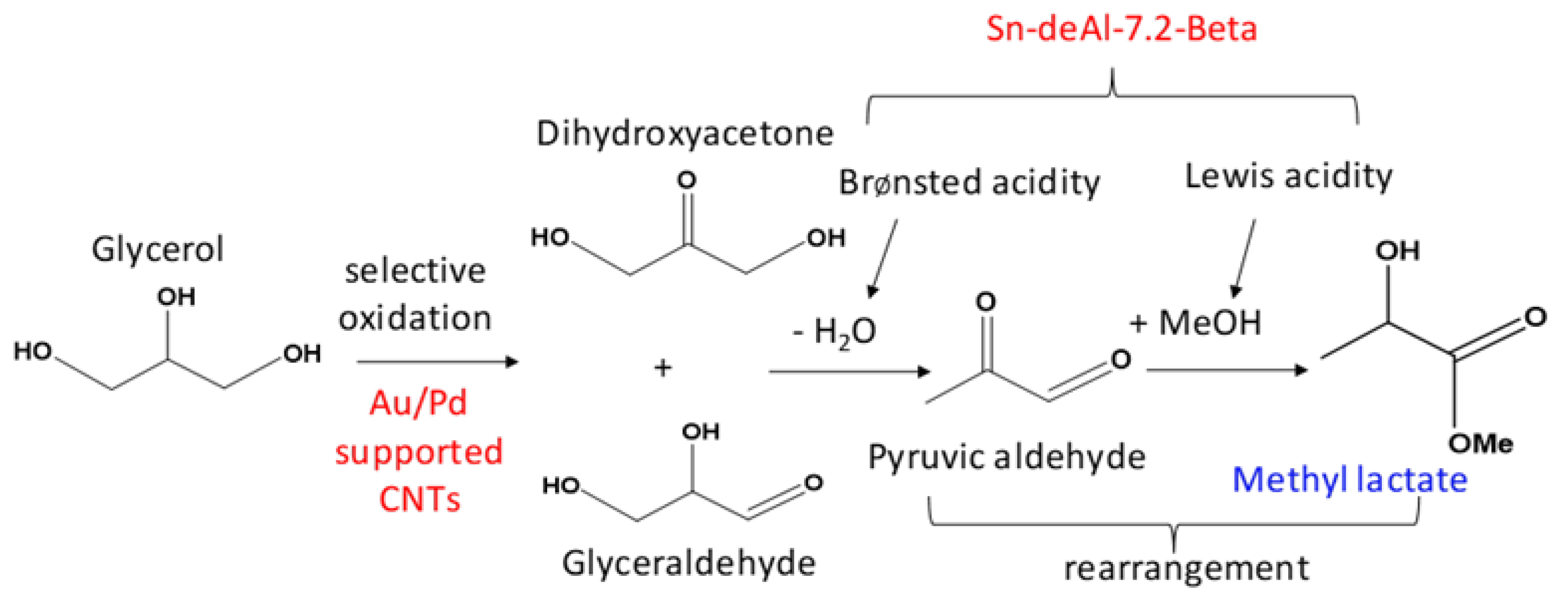
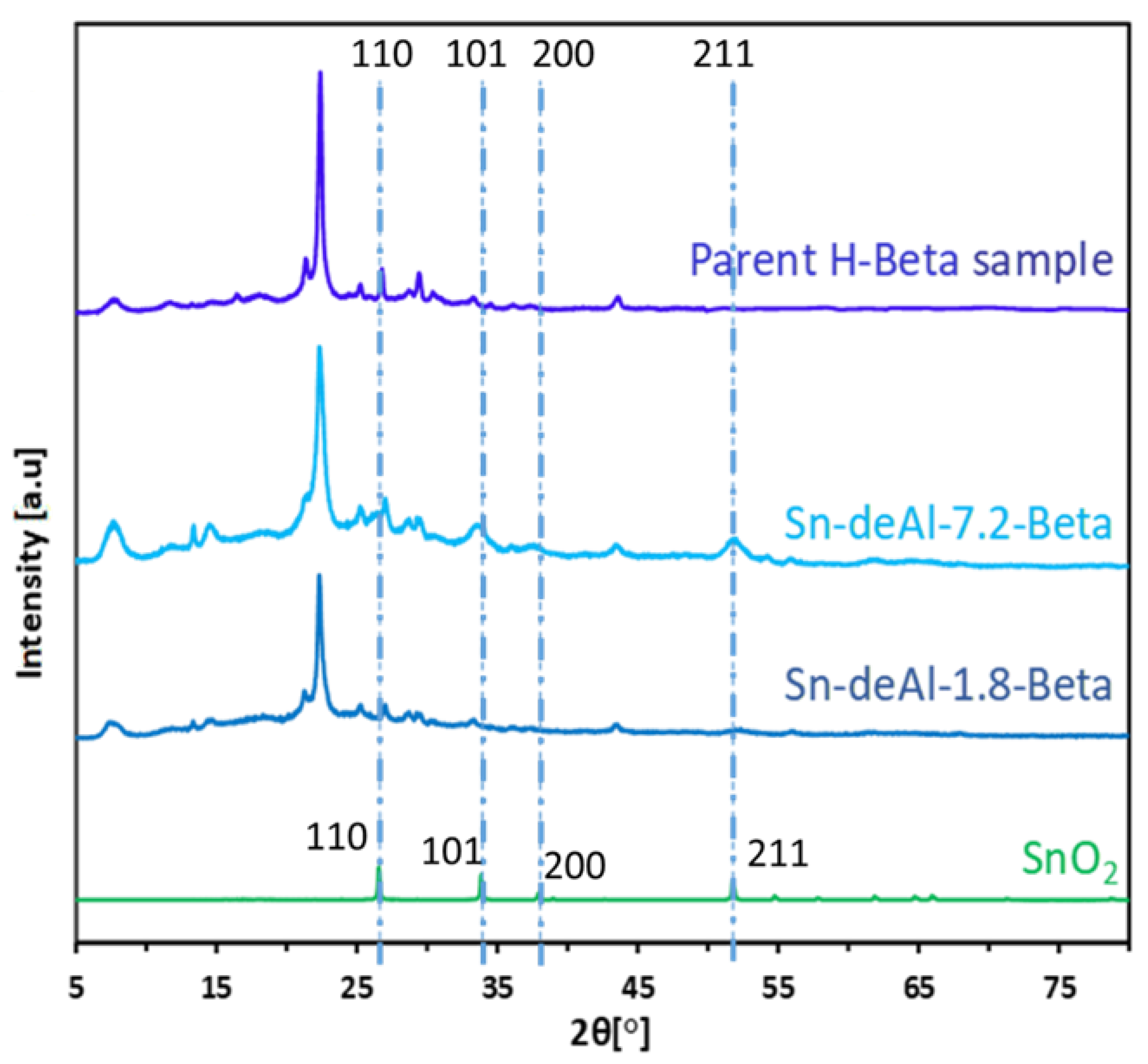
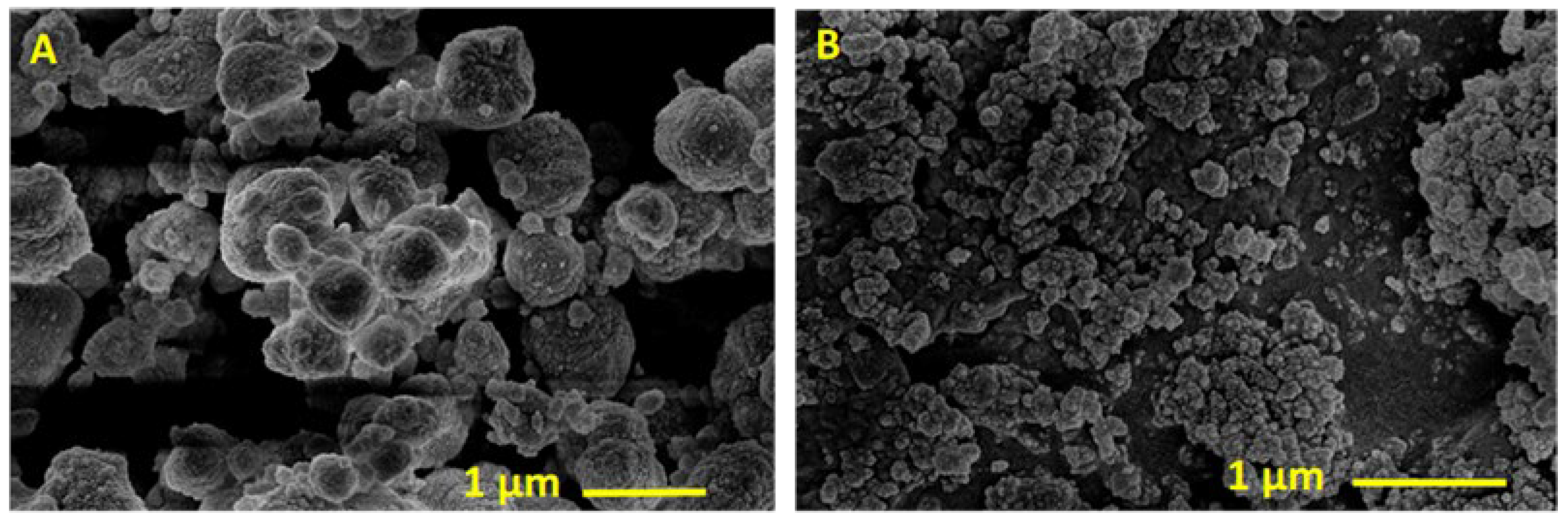
2. Results and Discussion
A Comparison between the Activities of Two Catalytic Systems for the Synthesis of ML from Glycerol
3. Experimental Section
3.1. Materials
3.2. Catalyst Synthesis
3.3. Catalytic Tests
3.4. Catalyst Characterization Methods
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mardiana, S.; Azhari, N.J.; Ilmi, T.; Kadja, G.T. Hierarchical zeolite for biomass conversion to biofuel: A review. Fuel 2022, 309, 122119. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Dapsens, P.Y.; Mondelli, C.; Pérez-Ramírez, J. Biobased chemicals from conception toward industrial reality: Lessons learned and to be learned. ACS Catal. 2012, 2, 1487–1499. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Gustavsson, L.; Börjesson, P.; Johansson, B.; Svenningsson, P. Reducing CO2 emissions by substituting biomass for fossil fuels. Energy 1995, 20, 1097–1113. [Google Scholar] [CrossRef]
- Azar, C.; Lindgren, K.; Larson, E.; Möllersten, K. Carbon capture and storage from fossil fuels and biomass–Costs and potential role in stabilizing the atmosphere. Clim. Chang. 2006, 74, 47–79. [Google Scholar] [CrossRef]
- Okoye, P.U.; Hameed, B.H. Review on recent progress in catalytic carboxylation and acetylation of glycerol as a byproduct of biodiesel production. Renew. Sustain. Energy Rev. 2016, 53, 558–574. [Google Scholar] [CrossRef]
- Sun, P.; Ling, W.; Wang, H.; Huang, J.; Liao, Y.; Wang, C. Hierarchical zeolite with open tin sites stabilized by metal ions catalyze the conversion of polysaccharides to alkyl lactates. Mol. Catal. 2023, 545, 113226. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef]
- Dusselier, M.; Van Wouwe, P.; Dewaele, A.; Makshina, E.; Sels, B.F. Lactic acid as a platform chemical in the biobased economy: The role of chemocatalysis. Energy Environ. Sci. 2013, 6, 1415–1442. [Google Scholar] [CrossRef]
- Tang, B.; Wang, D.; Li, A.; Tang, H.M.; Yang, E.C.; Dai, W. Constructing core-shell structured Au/Snβ@ mesosilica composite for one-pot base-free conversion of glycerol to methyl lactate. Microporous Mesoporous Mater. 2023, 347, 112348. [Google Scholar] [CrossRef]
- Dapsens, P.Y.; Mondelli, C.; Pérez-Ramírez, J. Design of Lewis-acid centres in zeolitic matrices for the conversion of renewables. Chem. Soc. Rev. 2015, 44, 7025–7043. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.H.; Beltramini, J.N.; Fan, X.Y.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of biomass into chemicals over metal catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef] [PubMed]
- Román-Leshkov, Y.; Moliner, M.; Labinger, J.A.; Davis, M.E. Mechanism of glucose isomerization using a solid lewis acid catalyst in water. Angew. Chem. Int. Ed. 2010, 49, 8954–8957. [Google Scholar] [CrossRef] [PubMed]
- Gounder, R.; Davis, M.E. Monosaccharide and disaccharide isomerization over Lewis acid sites in hydrophobic and hydrophilic molecular sieves. J. Catal. 2013, 308, 176–188. [Google Scholar] [CrossRef]
- Asgar Pour, Z.; Boer, D.G.; Fang, S.; Tang, Z.; Pescarmona, P.P. Bimetallic Zeolite Beta Beads with Hierarchical Porosity as Brønsted-Lewis Solid Acid Catalysts for the Synthesis of Methyl Lactate. Catalysts 2021, 11, 1346. [Google Scholar] [CrossRef]
- Mal, N.K.; Ramaswamy, A.V. Synthesis and catalytic properties of large-pore Sn-β and Al-free Sn-β molecular sieves. Chem. Commun. 1997, 8, 425–426. [Google Scholar] [CrossRef]
- Camblor, M.A.; Corma, A.; Valencia, S. Spontaneous nucleation and growth of pure silica zeolite-β free of connectivity defects. Chem. Commun. 1996, 20, 2365–2366. [Google Scholar] [CrossRef]
- Van Grieken, R.; Martos, C.; Sánchez-Sánchez, M.; Serrano, D.P.; Melero, J.A.; Iglesias, J.; Cubero, A.G. Synthesis of Sn-silicalite from hydrothermal conversion of SiO2-SnO2 xerogels. Microporous Mesoporous Mater. 2009, 119, 176–185. [Google Scholar] [CrossRef]
- Dijkmans, J.; Gabriëls, D.; Dusselier, M.; De Clippel, F.; Vanelderen, P.; Houthoofd, K.; Malfliet, A.; Pontikes, Y.; Sels, B.F. Productive sugar isomerization with highly active Sn in dealuminated β zeolites. Green Chem. 2013, 15, 2777–2785. [Google Scholar] [CrossRef]
- Valtchev, V.; Majano, G.; Mintova, S.; Pérez-Ramírez, J. Tailored crystalline microporous materials by post-synthesis modification. Chem. Soc. Rev. 2013, 42, 263–290. [Google Scholar] [CrossRef] [PubMed]
- Dijkmans, J.; Dusselier, M.; Gabriëls, D.; Houthoofd, K.; Magusin, P.C.M.M.; Huang, S.; Pontikes, Y.; Trekels, M.; Vantomme, A.; Giebeler, L.; et al. Cooperative catalysis for multistep biomass conversion with Sn/Al beta zeolite. ACS Catal. 2015, 5, 928–940. [Google Scholar] [CrossRef]
- Tang, B.; Dai, W.; Wu, G.; Guan, N.; Li, L.; Hunger, M. Improved post synthesis strategy to Sn-beta zeolites as lewis acid catalysts for the ring-opening hydration of epoxides. ACS Catal. 2014, 4, 2801–2810. [Google Scholar] [CrossRef]
- Verboekend, D.; Vilé, G.; Pérez-Ramírez, J. Hierarchical Y and USY zeolites designed by post-synthetic strategies. Adv. Funct. Mater. 2012, 22, 916–928. [Google Scholar] [CrossRef]
- Sun, H.; Peng, P.; Wang, Y.; Li, C.; Subhan, F.; Bai, P.; Xing, W.; Zhang, Z.; Liu, Z.; Yan, Z. Preparation, scale-up and application of meso-ZSM-5 zeolite by sequential desilication–dealumination. J. Porous Mater. 2017, 24, 1513–1525. [Google Scholar] [CrossRef]
- Liang, D.; Gao, J.; Sun, H.; Chen, P.; Hou, Z.; Zheng, X. Selective oxidation of glycerol with oxygen in a base-free aqueous solution over MWNTs supported Pt catalysts. Appl. Catal. B Environ. 2011, 106, 423–432. [Google Scholar] [CrossRef]
- Rodrigues, E.G.; Carabineiro, S.A.; Delgado, J.J.; Chen, X.; Pereira, M.F.; Órfão, J.J. Gold supported on carbon nanotubes for the selective oxidation of glycerol. J. Catal. 2012, 285, 83–91. [Google Scholar] [CrossRef]
- Asgar Pour, Z.; Abduljawad, M.M.; Alassmy, Y.A.; Cardon, L.; Van Steenberge, P.H.M.; Sebakhy, K.O. A Comparative Review of Binder-Containing Extrusion and Alternative Shaping Techniques for Structuring of Zeolites into Different Geometrical Bodies. Catalysts 2023, 13, 656. [Google Scholar] [CrossRef]
- Asgar Pour, Z.; Alassmy, Y.A.; Sebakhy, K.O. A Survey on Zeolite Synthesis and the Crystallization Process: Mechanism of Nucleation and Growth Steps. Crystals 2023, 13, 959. [Google Scholar] [CrossRef]
- Asgar Pour, Z.; Sebakhy, K.O. A Review on the Effects of Organic Structure-Directing Agents on the Hydrothermal Synthesis and Physicochemical Properties of Zeolites. Chemistry 2022, 4, 431–446. [Google Scholar] [CrossRef]
- Ma, Z.; Dai, S. Development of novel supported gold catalysts: A materials perspective. Nano Res. 2011, 4, 3–32. [Google Scholar] [CrossRef]
- Tang, Z.; Boer, D.G.; Syariati, A.; Enache, M.; Rudolf, P.; Heeres, H.J.; Pescarmona, P.P. Base-free conversion of glycerol to methyl lactate using a multifunctional catalytic system consisting of Au–Pd nanoparticles on carbon nanotubes and Sn-MCM-41-XS. Green Chem. 2019, 21, 4115–4126. [Google Scholar] [CrossRef]
- Wang, J.; Okumura, K.; Jaenicke, S.; Chuah, G.K. Post-synthesized zirconium-containing Beta zeolite in Meerwein-Ponndorf-Verley reduction: Pros and cons. Appl. Catal. A Gen. 2015, 493, 112–120. [Google Scholar] [CrossRef]
- Van Der Graaff, W.N.; Li, G.; Mezari, B.; Pidko, E.A.; Hensen, E.J. Synthesis of Sn-Beta with Exclusive and High Framework Sn Content. ChemCatChem 2015, 7, 1152–1160. [Google Scholar] [CrossRef]
- Emeis, C.A. ChemInform Abstract: Determination of Integrated Molar Extinction Coefficients for IR Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Luo, H.Y.; Lewis, J.D.; Román-Leshkov, Y. Lewis acid zeolites for biomass conversion: Perspectives and challenges on reactivity, synthesis, and stability. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 663–692. [Google Scholar] [CrossRef]
- Saikia, K.; Rajkumari, K.; Moyon, N.S.; Basumatary, S.; Halder, G.; Rashid, U.; Rokhum, S.L. Sulphonated biomass-based catalyst for solketal synthesis by acetalization of glycerol–A byproduct of biodiesel production. Fuel Process. Technol. 2022, 238, 107482. [Google Scholar] [CrossRef]
- Wadlinger, R.L.; Kerr, G.T.; Rosinski, E.J. Catalytic Composition of a Crystalline Zeolite, Modil Oil Corporation. U.S. Patent 3,308,069, 7 March 1967. [Google Scholar]
- Sebakhy, K.O.; Vitale, G.; Pereira-Almao, P. Production of Highly Dispersed Ni within Nickel Silicate Materials with the MFI Structure for the Selective Hydrogenation of Olefins. Ind. Eng. Chem. Res. 2019, 58, 8597–8611. [Google Scholar] [CrossRef]
- Sebakhy, K.O.; Vitale, G.; Pereira-Almao, P. Dispersed Ni-doped Aegirine Nanocatalysts for the Selective Hydro-genation of Olefinic Molecules. ACS. Appl. Nano Mater. 2018, 1, 6269–6280. [Google Scholar] [CrossRef]
- El Hariri El Nokab, M. Formation and Structural Analysis of Ultra Low-Density Silica Based Aerogels. Master’s Thesis, University of Siegen, Siegen, Germany, 2018. [Google Scholar] [CrossRef]
- Asgar Pour, Z.; Koelewijn, R.; El Hariri El Nokab, M.; van der Wel, P.C.; Sebakhy, K.O.; Pescarmona, P.P. Binder-free Zeolite Beta Beads with Hierarchical Porosity: Synthesis and Application as Heterogeneous Catalysts for Anisole Acylation. ChemCatChem 2022, 14, e202200518. [Google Scholar] [CrossRef]
- El Hariri El Nokab, M.; Sebakhy, K.O. Solid State NMR Spectroscopy a Valuable Technique for Structural Insights of Advanced Thin Film Materials: A Review. Nanomater. 2022, 11, 1494. [Google Scholar] [CrossRef]
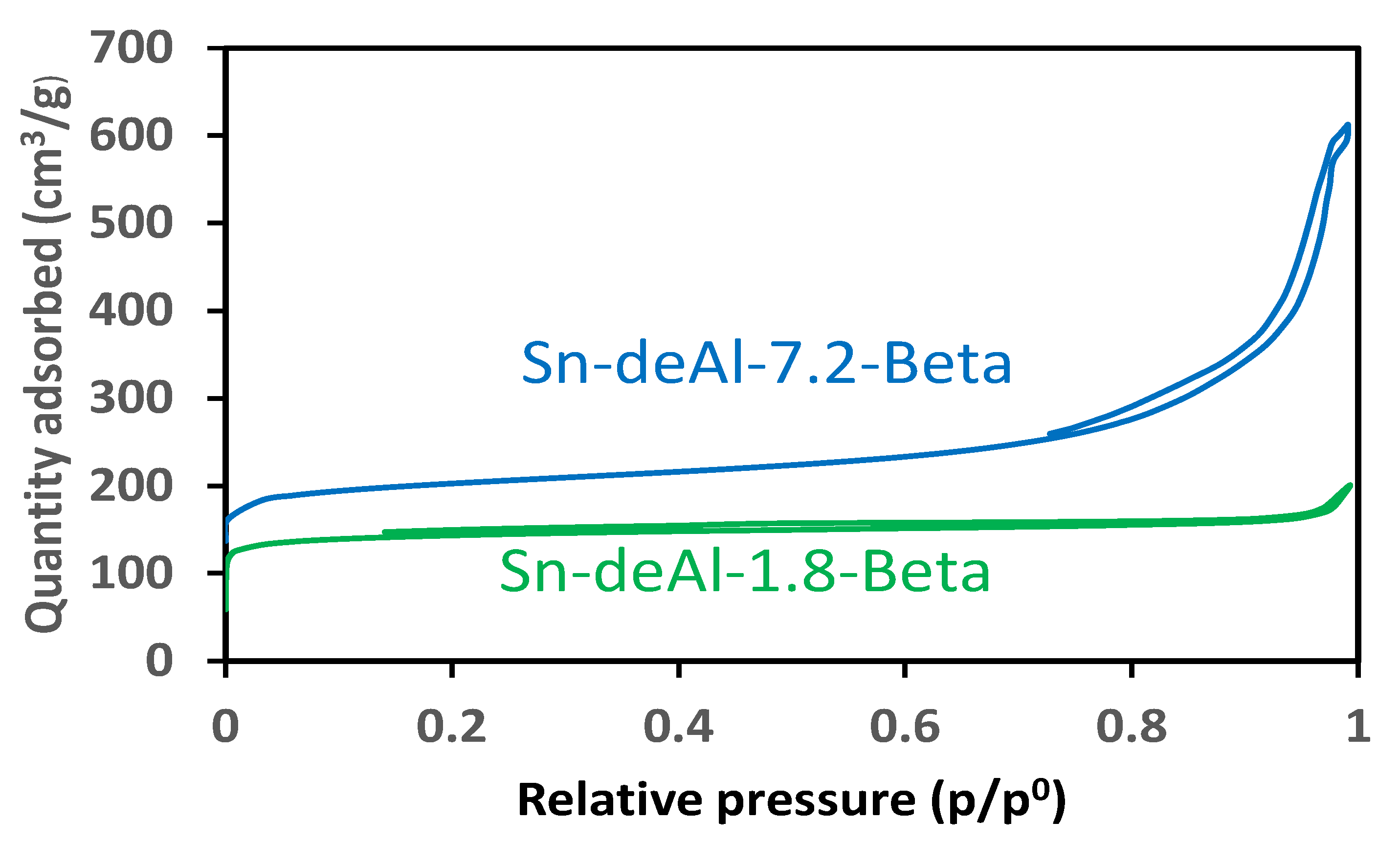
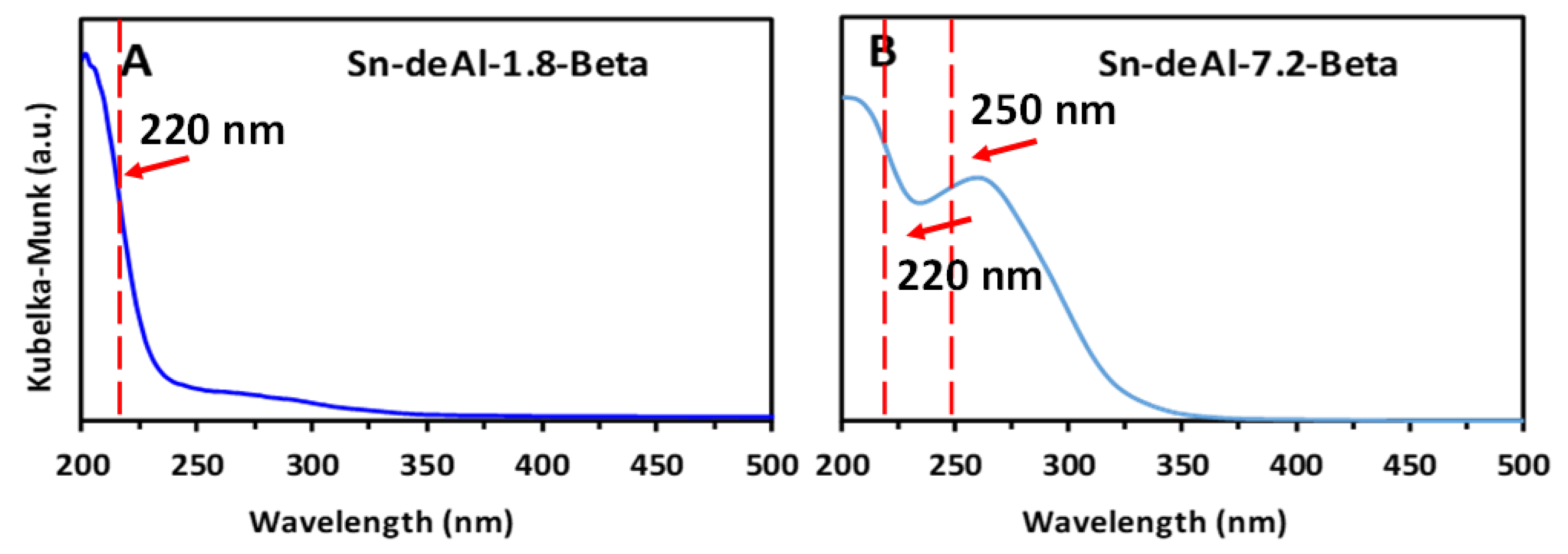
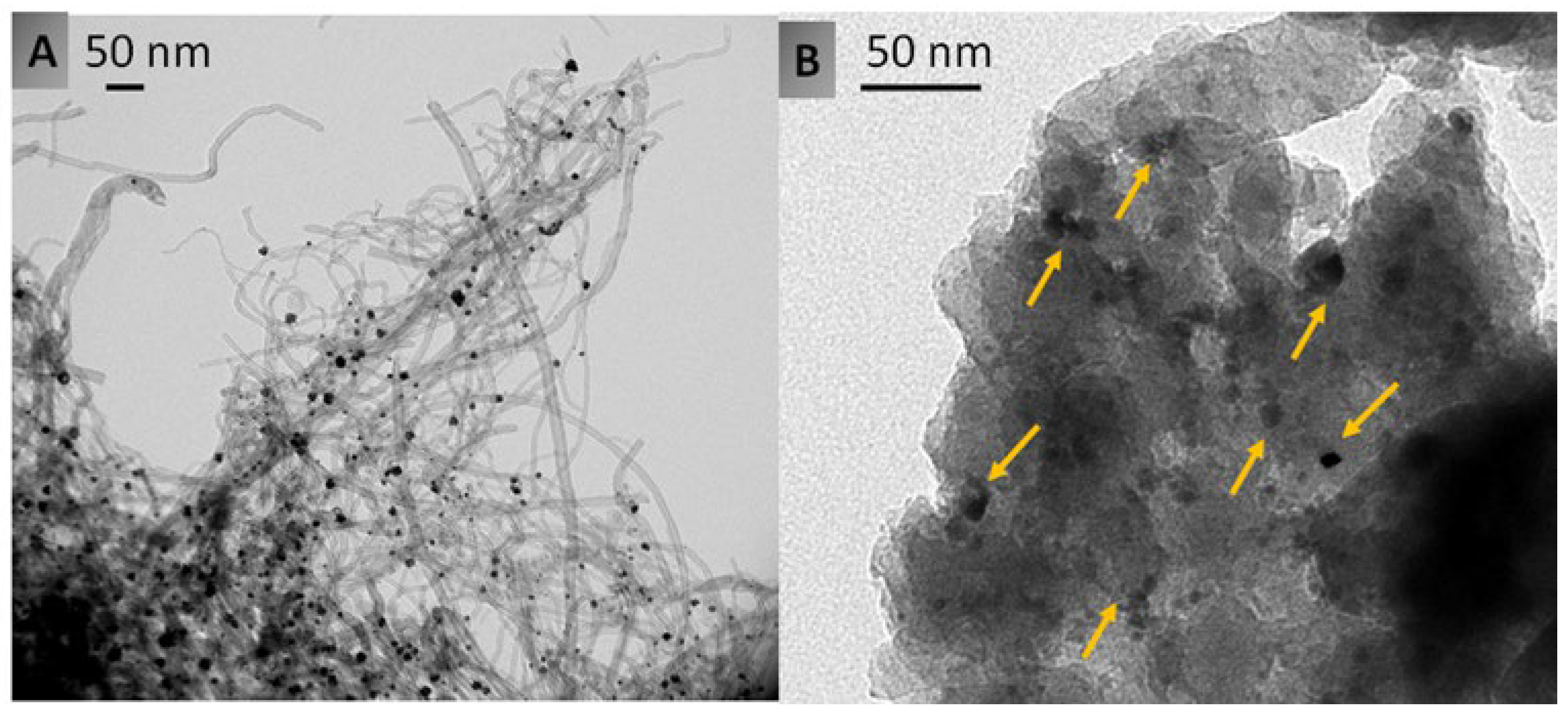
| Entry | Catalyst | Specific Surface Area (m2/g) a | Micropore Volume (cm3/g) a | Total Pore Volume (cm3/g) a | Acid Conc. (M) | Si/Al Molar Ratio b | Si/Sn Molar Ratio c |
|---|---|---|---|---|---|---|---|
| 1 | Sn-deAl-1.8-Beta | 465 | 0.18 | 0.28 | 1.8 | 87 | 48 |
| 2 | Sn-deAl-7.2-Beta | 507 | 0.15 | 0.83 | 7.2 | 131 | 108 |
| Entry | Catalyst | Lewis Acidity (µmol/g) | Brønsted Acidity (µmol/g) | Lewis/Brønsted Ratio |
|---|---|---|---|---|
| 150 °C | 150 °C | 150 °C | ||
| 1 | Sn-deAl-1.8-Beta | 15 | 39 | 3 |
| 2 | Sn-deAl-7.2-Beta | 90 | 9 | 10 |
| Entry | Catalyst | Conv. (%) | Yield of ML (%) | Selectivity (%) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ML | MP | MGo | DMO | 1,2 GF | DMT | MGe | |||||
| 1 | Sn-deAl-7.2-Beta + Au-Pd-F-CNTs | 58 | 45 | 77 | n.d. | 8.6 | 2.7 | 2.4 | n.d. | 4.8 | This work |
| 2 | Sn-deAl-7.2-Beta + Au-Pd-F-CNTs a | 70 | 49 | 69 | n.d. | 12.6 | 0 | 6.1 | 7.1 | 4.5 | This work |
| 3 | Sn-deAl-1.8-Beta + Au-Pd-F-CNTs | 29 | 17 | 70 | n.d. | 8.2 | 0 | 16.9 | n.d | 4.9 | This work |
| 4 | Au-Pd-Sn-deAl-7.2-Beta-DP b | 34 | 26 | 76 | n.d. | 12.5 | 0 | 2.1 | 0.5 | 6.3 | This work |
| 5 | Sn-deAl-1.8-Beta-B + Au-Pd-F-CNTs c | 29 | 20 | 67 | n.d. | 5.8 | 4.8 | 5.8 | 7.1 | 3.9 | [17] |
| 6 | Sn-deAl-7.2-Beta + Au-Pd-F-CNTs d | 69 | 57 | 82 | n.d. | 0.1 | 0 | 11.1 | 3.5 | 3 | This work |
| 7 | Au-Pd-Sn-deAl-7.2-Beta-DP d | 65 | 50.7 | 78 | n.d. | 9 | 0 | 2 | 6 | 5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asgar Pour, Z.; Abduljawad, M.M.; Alassmy, Y.A.; Alnafisah, M.S.; El Hariri El Nokab, M.; Van Steenberge, P.H.M.; Sebakhy, K.O. Synergistic Catalytic Effects of Alloys of Noble Metal Nanoparticles Supported on Two Different Supports: Crystalline Zeolite Sn-Beta and Carbon Nanotubes for Glycerol Conversion to Methyl Lactate. Catalysts 2023, 13, 1486. https://doi.org/10.3390/catal13121486
Asgar Pour Z, Abduljawad MM, Alassmy YA, Alnafisah MS, El Hariri El Nokab M, Van Steenberge PHM, Sebakhy KO. Synergistic Catalytic Effects of Alloys of Noble Metal Nanoparticles Supported on Two Different Supports: Crystalline Zeolite Sn-Beta and Carbon Nanotubes for Glycerol Conversion to Methyl Lactate. Catalysts. 2023; 13(12):1486. https://doi.org/10.3390/catal13121486
Chicago/Turabian StyleAsgar Pour, Zahra, Marwan M. Abduljawad, Yasser A. Alassmy, Mohammed S. Alnafisah, Mustapha El Hariri El Nokab, Paul H. M. Van Steenberge, and Khaled O. Sebakhy. 2023. "Synergistic Catalytic Effects of Alloys of Noble Metal Nanoparticles Supported on Two Different Supports: Crystalline Zeolite Sn-Beta and Carbon Nanotubes for Glycerol Conversion to Methyl Lactate" Catalysts 13, no. 12: 1486. https://doi.org/10.3390/catal13121486








