Recent Advances of Doping and Surface Modifying Carbon Nitride with Characterization Techniques
Abstract
:1. Introduction
2. Doped CN Systems
2.1. Metal Doping
2.2. Non-Metal Doping
2.3. Co-Doping
3. Surface-Functionalized Modification
4. Characterization of Modified CN
4.1. X-ray Photoelectron Spectroscopy
4.2. Solid-State NMR
4.3. Fourier Transform Infrared Spectra
4.4. Elemental Mapping
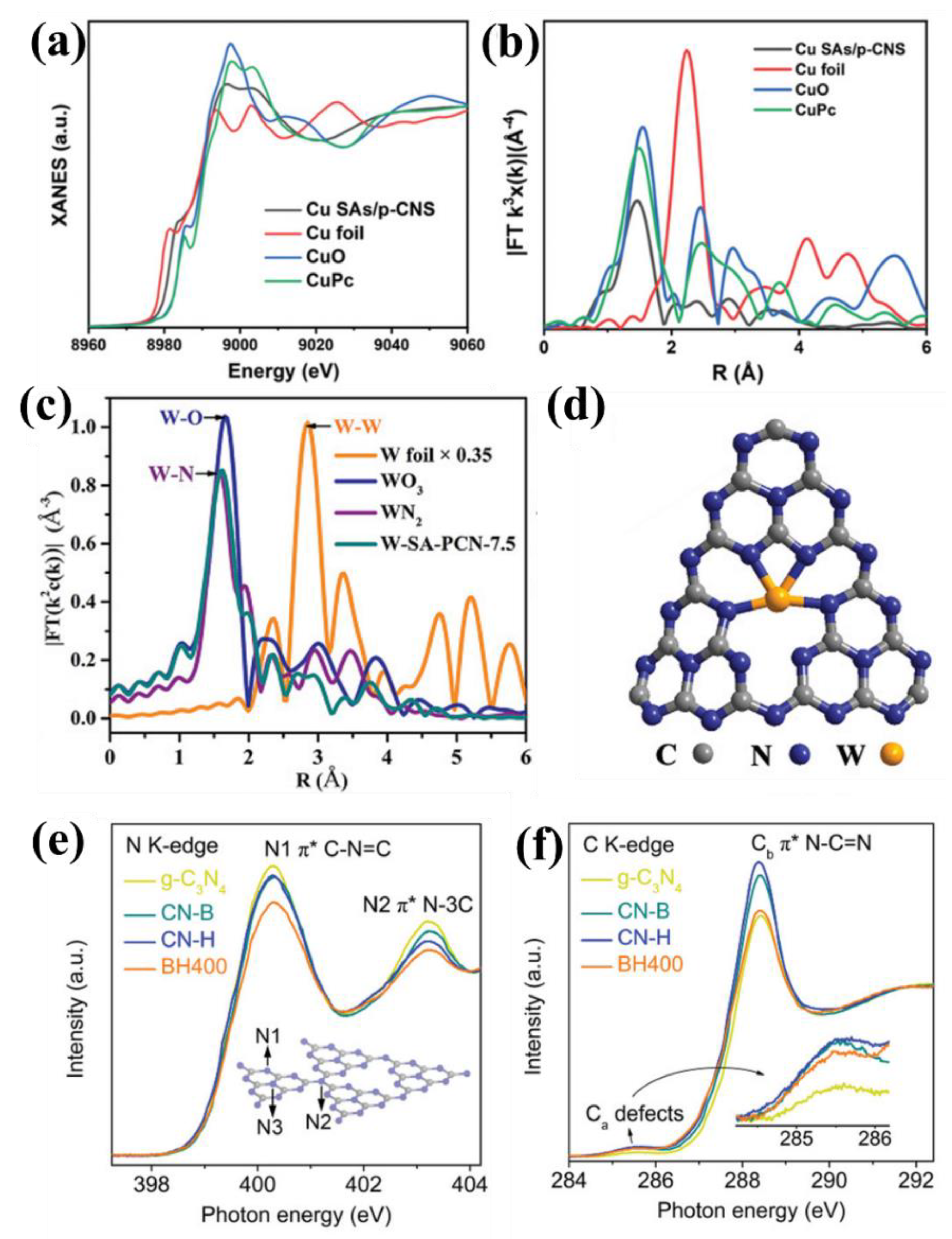
4.5. X-ray Absorption Spectroscopy
5. Summary and Outlook
- (1)
- The typically used characterization methods are non-real-time processes. Therefore, the results cannot reflect the real state of the photocatalyst during photoreaction. To account for the effects of doping and surface modification on the photocatalytic performance of CN, more in situ characterization techniques besides in situ DRIFTs are needed.
- (2)
- Some have reported the decomposition of pristine CN during the photocatalytic reaction. The question of how to improve the photostability of doped/surface-modified CN should be carefully considered, as it is of great importance to its practical applications.
- (3)
- As for co-doping methods, it is difficult to control the location of the dopants. Much work should be done to adjust the doping structure to better exploit the synergistic effect.
- (1)
- Doping carbon nitride to form a heterojunction with other semiconductor materials is a worthwhile research direction, particularly the new S-scheme heterojunction scheme, which can not only promote the separation of electron-hole pairs, but also maintain the strong redox ability of the catalyst.
- (2)
- The doping of single-atom noble metals can maximize the atom utilization and significantly enhance the photoreactivity. Recently, the synergistic effect of single-atom noble metals and metal particles has become a popular research direction.
- (3)
- Recently, the fabrication of crystalline CN (CCN) has attracted much attention due to its good crystallization, which provides an opportunity to further improve the photocatalytic activity of CCN photocatalysts by using doping and surface modification approaches.
- (4)
- Guiding practice with theory can also be used in photocatalyst design. Before preparation of the CN photocatalyst, DFT calculations can be used in advance for photocatalyst screening by predicting the effects of doping and surface modification on the electronic structure, interfacial charge transfer and energy band structure of CN.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CN | Carbon nitride |
| BCN | Bulk graphitic carbon nitride |
| HAADF-STEM | High-angle annular dark-field scanning transmission electron microscopy |
| CCN | Crystalline carbon nitride |
| HCCP | Hexachlorocyclotriphosphazene |
| D-A | Donor-acceptor |
| XAS | X-ray absorption spectroscopy |
| XANES | X-ray absorption near-edge structure |
| EXAFS | Extended X-ray absorption fine structure |
References
- Zeng, Z.; Su, Y.; Quan, X.; Choi, W.; Zhang, G.; Liu, N.; Kim, B.; Chen, S.; Yu, H.; Zhang, S. Single-atom platinum confined by the interlayer nanospace of carbon nitride for efficient photocatalytic hydrogen evolution. Nano Energy 2020, 69, 104409. [Google Scholar] [CrossRef]
- Ding, Z.; Sun, M.; Liu, W.; Sun, W.; Meng, X.; Zheng, Y. Ultrasonically synthesized N-TiO2/Ti3C2 composites: Enhancing sonophotocatalytic activity for pollutant degradation and nitrogen fixation. Sep. Purif. Technol. 2021, 276, 119287. [Google Scholar] [CrossRef]
- Zhao, G.; Ma, W.; Wang, X.; Xing, Y.; Hao, S.; Xu, X. Self-water-absorption-type two-dimensional composite photocatalyst with high-efficiency water absorption and overall water-splitting performance. Adv. Powder Mater. 2022, 1, 100008. [Google Scholar] [CrossRef]
- Lin, Z.; Shao, C.; Jiang, S.; Sun, C.; Song, S. Hollow graphene with apparent potential difference to boost charge directional transfer for photocatalytic H2 evolution. Appl. Catal. B Environ. 2020, 268, 118742. [Google Scholar] [CrossRef]
- Luo, J.; He, J.; Jiang, S.; Sun, C.; Song, S. In-plane coupling electric field driving charge directional transfer for highly efficient H2 bubble evolution. Chem. Eng. J. 2020, 396, 125365. [Google Scholar] [CrossRef]
- Peng, J.; Shen, J.; Yu, X.; Tang, H.; Zulfiqar; Liu, Q. Construction of LSPR-enhanced 0D/2D CdS/MoO3− S-scheme heterojunctions for visible-light-driven photocatalytic H2 evolution. Chin. J. Catal. 2021, 42, 87–96. [Google Scholar] [CrossRef]
- Yang, C.; Tan, Q.; Li, Q.; Zhou, J.; Fan, J.; Li, B.; Sun, J.; Lv, K. 2D/2D Ti3C2 MXene/g-C3N4 nanosheets heterojunction for high efficient CO2 reduction photocatalyst: Dual effects of urea. Appl. Catal. B Environ. 2020, 268, 118738. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.; He, H. Enhanced photocatalytic oxidation of NO over g-C3N4-TiO2 under UV and visible light. Appl. Catal. B Environ. 2016, 184, 28–34. [Google Scholar] [CrossRef]
- Yu, H.; Yuan, R.; Gao, D.; Xu, Y.; Yu, J. Ethyl acetate-induced formation of amorphous MoSx nanoclusters for improved H2-evolution activity of TiO2 photocatalyst. Chem. Eng. J. 2019, 375, 121934. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, J.; Zhang, G.; Huang, J.; Liu, P.; Antonietti, M.; Wang, X. Synthesis of bulk and nanoporous carbon nitride polymers from ammonium thiocyanate for photocatalytic hydrogen evolution. J. Mater. Chem. 2011, 21, 13032–13039. [Google Scholar] [CrossRef]
- Sun, L.-J.; Su, H.-W.; Xu, D.-F.; Wang, L.-L.; Tang, H.; Liu, Q.-Q. Carbon hollow spheres as cocatalyst of Cu-doped TiO2 nanoparticles for improved photocatalytic H2 generation. Rare Met. 2022, 41, 2063–2073. [Google Scholar] [CrossRef]
- Low, J.; Zhang, L.; Tong, T.; Shen, B.; Yu, J. TiO2/MXene Ti3C2 composite with excellent photocatalytic CO2 reduction activity. J. Catal. 2018, 361, 255–266. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Wang, W.; Tao, Y.; Fan, J.; Yan, Z.; Shang, H.; Phillips, D.L.; Chen, M.; Li, G. Fullerene–Graphene Acceptor Drives Ultrafast Carrier Dynamics for Sustainable CdS Photocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2022, 32, 2201357. [Google Scholar] [CrossRef]
- Ou, H.; Ning, S.; Zhu, P.; Chen, S.; Han, A.; Kang, Q.; Hu, Z.; Ye, J.; Wang, D.; Li, Y. Carbon Nitride Photocatalysts with Integrated Oxidation and Reduction Atomic Active Centers for Improved CO2 Conversion. Angew. Chem. Int. Ed. Engl. 2022, 134, e202206579. [Google Scholar] [CrossRef]
- Ma, J.; Jin, D.; Li, Y.; Xiao, D.; Jiao, G.; Liu, Q.; Guo, Y.; Xiao, L.; Chen, X.; Li, X.; et al. Photocatalytic conversion of biomass-based monosaccharides to lactic acid by ultrathin porous oxygen doped carbon nitride. Appl. Catal. B Environ. 2021, 283, 119520. [Google Scholar] [CrossRef]
- Du, A.; Sanvito, S.; Li, Z.; Wang, D.; Jiao, Y.; Liao, T.; Sun, Q.; Ng, Y.H.; Zhu, Z.; Amal, R.; et al. Hybrid graphene and graphitic carbon nitride nanocomposite: Gap opening, electron-hole puddle, interfacial charge transfer, and enhanced visible light response. J. Am. Chem. Soc. 2012, 134, 4393–4397. [Google Scholar] [CrossRef]
- Chen, J.; Kang, N.; Fan, J.; Lu, C.; Lv, K. Carbon nitride for photocatalytic water splitting to produce hydrogen and hydrogen peroxide. Mater. Today Chem. 2022, 26, 101028. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Zeng, H.; Li, Z.; Li, G.; Cui, X.; Jin, M.; Xie, T.; Liu, L.; Jiang, M.; Zhong, X.; Zhang, Y.; et al. Interfacial Engineering of TiO2/Ti3C2 MXene/Carbon Nitride Hybrids Boosting Charge Transfer for Efficient Photocatalytic Hydrogen Evolution. Adv. Energy Mater. 2021, 12, 2102765. [Google Scholar] [CrossRef]
- An, S.; Zhang, G.; Li, K.; Huang, Z.; Wang, X.; Guo, Y.; Hou, J.; Song, C.; Guo, X. Self-Supporting 3D Carbon Nitride with Tunable n → п* Electronic Transition for Enhanced Solar Hydrogen Production. Adv. Mater. 2021, 33, e2104361. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Yang, S.; He, J.; Jiang, S.; Sun, C.; Song, S. In situ growing graphene on g-C3N4 with barrier-free interface and polarization electric field for strongly boosting solar energy conversion into H2 energy. Appl. Catal. B Environ. 2021, 287, 119986. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wu, G.; Chen, W. Porous graphitic carbon nitride synthesized via direct polymerization of urea for efficient sunlight-driven photocatalytic hydrogen production. Nanoscale 2012, 4, 5300–5303. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bai, X.; Ci, Q.; Du, L.; Ren, X.; Phillips, D.L. Near-Field Drives Long-Lived Shallow Trapping of Polymeric C3N4 for Efficient Photocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2021, 31, 2103978. [Google Scholar] [CrossRef]
- Shi, H.; Long, S.; Hu, S.; Hou, J.; Ni, W.; Song, C.; Li, K.; Gurzadyan, G.G.; Guo, X. Interfacial charge transfer in 0D/2D defect-rich heterostructures for efficient solar-driven CO2 reduction. Appl. Catal. B Environ. 2019, 245, 760–769. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, L.-J.; Pan, W.-G.; Wei, Z.-Z.; Liang, X.-Y.; Guo, R.-T. Granular Polymeric Carbon Nitride with Carbon Vacancies for Enhanced Photocatalytic Hydrogen Evolution. Sol. RRL 2021, 5, 2000796. [Google Scholar] [CrossRef]
- Xie, P.; Ding, J.; Yao, Z.; Pu, T.; Zhang, P.; Huang, Z.; Wang, C.; Zhang, J.; Zecher-Freeman, N.; Zong, H.; et al. Oxo dicopper anchored on carbon nitride for selective oxidation of methane. Nat. Commun. 2022, 13, 1375. [Google Scholar] [CrossRef]
- Goswami, T.; Bhatt, H.; Yadav, D.K.; Ghosh, H.N. Interfacing g-C3N4 Nanosheets with CdS Nanorods for Enhanced Photocatalytic Hydrogen Evolution: An Ultrafast Investigation. J. Phys. Chem. B 2022, 126, 572–580. [Google Scholar] [CrossRef]
- Zhou, A.-Q.; Yang, J.-M.; Zhu, X.-W.; Zhu, X.-L.; Liu, J.-Y.; Zhong, K.; Chen, H.-X.; Chu, J.-Y.; Du, Y.-S.; Song, Y.-H.; et al. Self-assembly construction of NiCo LDH/ultrathin g-C3N4 nanosheets photocatalyst for enhanced CO2 reduction and charge separation mechanism study. Rare Met. 2022, 41, 2118–2128. [Google Scholar] [CrossRef]
- Fang, S.; Xia, Y.; Lv, K.; Li, Q.; Sun, J.; Li, M. Effect of carbon-dots modification on the structure and photocatalytic activity of g-C3N4. Appl. Catal. B Environ. 2016, 185, 225–232. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, Z.; Li, Q.; Li, X.; Fang, S.; Wu, X.; Li, M.; Ding, Y.; Liu, B.; Yang, C.; et al. Fabrication of high photoreactive carbon nitride nanosheets by polymerization of amidinourea for hydrogen production. Appl. Catal. B Environ. 2019, 245, 197–206. [Google Scholar] [CrossRef]
- Guan, K.; Li, J.; Lei, W.; Wang, H.; Tong, Z.; Jia, Q.; Zhang, H.; Zhang, S. Synthesis of sulfur doped g-C3N4 with enhanced photocatalytic activity in molten salt. J. Mater. 2021, 7, 1131–1142. [Google Scholar] [CrossRef]
- Xiong, T.; Cen, W.; Zhang, Y.; Dong, F. Bridging the g-C3N4 Interlayers for Enhanced Photocatalysis. ACS Catal. 2016, 6, 2462–2472. [Google Scholar] [CrossRef]
- Li, Y.; Gu, M.; Zhang, X.; Fan, J.; Lv, K.; Carabineiro, S.A.C.; Dong, F. 2D g-C3N4 for advancement of photo-generated carrier dynamics: Status and challenges. Mater. Today 2020, 41, 270–303. [Google Scholar] [CrossRef]
- Škuta, R.; Matějka, V.; Foniok, K.; Smýkalová, A.; Cvejn, D.; Gabor, R.; Kormunda, M.; Smetana, B.; Novák, V.; Praus, P. On P-doping of graphitic carbon nitride with hexachlorotriphosphazene as a source of phosphorus. Appl. Surf. Sci. 2021, 552, 149490. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Z.; Fang, Y.; Fu, X.; Wang, X. In Situ Synthesis of Phosphorus-Doped Polymeric Carbon Nitride Sheets for Photoelectrochemical Water Oxidation. Sol. RRL 2020, 4, 2000168. [Google Scholar] [CrossRef]
- Patnaik, S.; Sahoo, D.P.; Parida, K. Recent advances in anion doped g-C3N4 photocatalysts: A review. Carbon 2021, 172, 682–711. [Google Scholar] [CrossRef]
- Bai, Y.; Zheng, Y.; Wang, Z.; Hong, Q.; Liu, S.; Shen, Y.; Zhang, Y. Metal-doped carbon nitrides: Synthesis, structure and applications. New J. Chem. 2021, 45, 11876–11892. [Google Scholar] [CrossRef]
- Li, X.; Bi, W.; Zhang, L.; Tao, S.; Chu, W.; Zhang, Q.; Luo, Y.; Wu, C.; Xie, Y. Single-Atom Pt as Co-Catalyst for Enhanced Photocatalytic H2 Evolution. Adv. Mater. 2016, 28, 2427–2431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Long, R.; Zhang, Y.; Duan, D.; Xiong, Y.; Zhang, Y.; Bi, Y. Direct Observation of Dynamic Bond Evolution in Single-Atom Pt/C3N4 Catalysts. Angew. Chem. Int. Ed. Engl. 2020, 59, 6224–6229. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Huang, Z.; Gao, X.; Hong, X.; Zhu, J.; Wang, G.; Wu, Y.; Zeng, J.; Zheng, X. Synergy between Palladium Single Atoms and Nanoparticles via Hydrogen Spillover for Enhancing CO2 Photoreduction to CH4. Adv. Mater. 2022, 34, e2200057. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; He, X.; Zhong, H.; Singh, D.J.; Zhang, L.; Wang, R. Solid salt confinement effect: An effective strategy to fabricate high crystalline polymer carbon nitride for enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 246, 349–355. [Google Scholar] [CrossRef]
- Guo, F.; Hu, B.; Yang, C.; Zhang, J.; Hou, Y.; Wang, X. On-Surface Polymerization of In-Plane Highly Ordered Carbon Nitride Nanosheets toward Photocatalytic Mineralization of Mercaptan Gas. Adv. Mater. 2021, 33, e2101466. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.; Cheng, B.; Ho, W.; Yu, J. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B Environ. 2015, 176–177, 44–52. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Liu, J.; Fan, X.; Wang, B.; Wang, M.; Ren, W.; Wang, J.; Li, M.; Shi, J. Brand new P-doped g-C3N4: Enhanced photocatalytic activity for H2 evolution and Rhodamine B degradation under visible light. J. Mater. Chem. A 2015, 3, 3862–3867. [Google Scholar] [CrossRef]
- Wang, H.; Huang, G.; Chen, Z.; Li, W. Carbon Self-Doped Carbon Nitride Nanosheets with Enhanced Visible-Light Photocatalytic Hydrogen Production. Catalysts 2018, 8, 366. [Google Scholar] [CrossRef]
- Fang, J.; Fan, H.; Li, M.; Long, C. Nitrogen self-doped graphitic carbon nitride as efficient visible light photocatalyst for hydrogen evolution. J. Mater. Chem. A 2015, 3, 13819–13826. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, M.; Yang, W.; Xiao, Y.; Zeng, L.; Wu, X.; Xu, Q.; Su, C.; He, Q. Homogeneous Carbon/Potassium-Incorporation Strategy for Synthesizing Red Polymeric Carbon Nitride Capable of Near-Infrared Photocatalytic H2 Production. Adv. Mater. 2021, 33, e2101455. [Google Scholar] [CrossRef]
- Dong, H.; Zuo, Y.; Song, N.; Hong, S.; Xiao, M.; Zhu, D.; Sun, J.; Chen, G.; Li, C. Bimetallic synergetic regulating effect on electronic structure in cobalt/vanadium co-doped carbon nitride for boosting photocatalytic performance. Appl. Catal. B Environ. 2021, 287, 119954. [Google Scholar] [CrossRef]
- Cheng, L.; Yue, X.; Wang, L.; Zhang, D.; Zhang, P.; Fan, J.; Xiang, Q. Dual-Single-Atom Tailoring with Bifunctional Integration for High-Performance CO2 Photoreduction. Adv. Mater. 2021, 33, 2105135. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, C.; Yang, Z.; Li, S.; Chu, C.; Chen, B. Simultaneously Tuning Band Structure and Oxygen Reduction Pathway toward High-Efficient Photocatalytic Hydrogen Peroxide Production Using Cyano-Rich Graphitic Carbon Nitride. Adv. Funct. Mater. 2021, 31, 2105731. [Google Scholar] [CrossRef]
- Wu, S.; Yu, H.; Chen, S.; Quan, X. Enhanced Photocatalytic H2O2 Production over Carbon Nitride by Doping and Defect Engineering. ACS Catal. 2020, 10, 14380–14389. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Xu, W.; Sun, Y.; Wang, T.; Zhang, Y.; Dong, J.; Hou, W.; Wu, N.; Wu, L.; et al. Zn-doped tri-s-triazine crystalline carbon nitrides for efficient hydrogen evolution photocatalysis. Appl. Catal. A Gen. 2019, 582, 117118. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Wang, H.; Xiao, B.; Zhang, W.; Zhao, X.; Lv, T.; Thangamuthu, M.; Zhang, J.; Guo, Y.; et al. Single-atom Cu anchored catalysts for photocatalytic renewable H2 production with a quantum efficiency of 56. Nat. Commun. 2022, 13, 58. [Google Scholar] [CrossRef]
- Hu, X.; Song, J.; Luo, J.; Zhang, H.; Sun, Z.; Li, C.; Zheng, S.; Liu, Q. Single-atomic Pt sites anchored on defective TiO2 nanosheets as a superior photocatalyst for hydrogen evolution. J. Energy Chem. 2021, 62, 1–10. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, C.; Lv, K.; Li, X.; Li, Q.; Fan, J. Single atomic Au induced dramatic promotion of the photocatalytic activity of TiO2 hollow microspheres. Chem. Commun. 2020, 56, 1745–1748. [Google Scholar] [CrossRef]
- Li, X.; Hu, Z.; Li, Q.; Lei, M.; Fan, J.; Carabineiro, S.A.C.; Liu, Y.; Lv, K. Three in one: Atomically dispersed Na boosting the photoreactivity of carbon nitride towards NO oxidation. Chem. Commun. 2020, 56, 14195–14198. [Google Scholar] [CrossRef]
- Vazquez-Gonzalez, M.; Liao, W.C.; Cazelles, R.; Wang, S.; Yu, X.; Gutkin, V.; Willner, I. Mimicking Horseradish Peroxidase Functions Using Cu2+-Modified Carbon Nitride Nanoparticles or Cu2+-Modified Carbon Dots as Heterogeneous Catalysts. ACS Nano 2017, 11, 3247–3253. [Google Scholar] [CrossRef]
- Wang, S.; Zhan, J.; Chen, K.; Ali, A.; Zeng, L.; Zhao, H.; Hu, W.; Zhu, L.; Xu, X. Potassium-Doped g-C3N4 Achieving Efficient Visible-Light-Driven CO2 Reduction. ACS Sustain. Chem. Eng. 2020, 8, 8214–8222. [Google Scholar] [CrossRef]
- Liao, Z.; Li, C.; Shu, Z.; Zhou, J.; Li, T.; Wang, W.; Zhao, Z.; Xu, L.; Shi, L.; Feng, L. K–Na co-doping in crystalline polymeric carbon nitride for highly improved photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 26318–26328. [Google Scholar] [CrossRef]
- Liu, J.; Fu, W.; Liao, Y.; Fan, J.; Xiang, Q. Recent advances in crystalline carbon nitride for photocatalysis. J. Mater. Sci. Technol. 2021, 91, 224–240. [Google Scholar] [CrossRef]
- Lin, L.; Ou, H.; Zhang, Y.; Wang, X. Tri-s-triazine-Based Crystalline Graphitic Carbon Nitrides for Highly Efficient Hydrogen Evolution Photocatalysis. ACS Catal. 2016, 6, 3921–3931. [Google Scholar] [CrossRef]
- Du, J.; Fan, Y.; Gan, X.; Dang, X.; Zhao, H. Three-dimension branched crystalline carbon nitride: A high efficiency photoelectrochemical sensor of trace Cu2+ detection. Electrochim. Acta 2020, 330, 135336. [Google Scholar] [CrossRef]
- Wang, W.; Cui, J.; Sun, Z.; Xie, L.; Mu, X.; Huang, L.; He, J. Direct Atomic-Scale Structure and Electric Field Imaging of Triazine-Based Crystalline Carbon Nitride. Adv. Mater. 2021, 33, e2106359. [Google Scholar] [CrossRef]
- Liu, W.; Peng, R.; Ye, X.; Guo, J.; Luo, L. Sulfur doping and structure defect functionalized carbon nitride nanosheets with enhanced photocatalytic degradation activity. Appl. Surf. Sci. 2021, 560, 150013. [Google Scholar] [CrossRef]
- Yan, B.; Yang, G. Enhancing electron density of bulk g-C3N4 through phosphorus doping for promoting photocatalytic hydrogen evolution reaction. Appl. Surf. Sci. 2021, 570, 151186. [Google Scholar] [CrossRef]
- Cui, M.; Cui, K.; Liu, X.; Chen, X.; Guo, Z.; Chen, Y.; Li, C.X. Insights into the photocatalytic peroxymonosulfate activation over defective boron-doped carbon nitride for efficient pollutants degradation. J. Hazard. Mater. 2021, 418, 126338. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Hou, L.; Guo, X.; Fu, R.; Sun, J. Construction of covalent bonding oxygen-doped carbon nitride/graphitic carbon nitride Z-scheme heterojunction for enhanced visible-light-driven H2 evolution. Chem. Eng. J. 2020, 383, 123132. [Google Scholar] [CrossRef]
- Aleksandrzak, M.; Kijaczko, M.; Kukulka, W.; Baranowska, D.; Baca, M.; Zielinska, B.; Mijowska, E. Boosting of photocatalytic hydrogen evolution via chlorine doping of polymeric carbon nitride. Beilstein J. Nanotechnol. 2021, 12, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Gogoi, D.; Devi, M.; Dhar, S.S.; Peela, N.R. Synergistic Effect of Metal Complex and Dual Doped Graphitic Carbon Nitride for Superior Photocatalytic Hydrogen Evolution. Energy Fuels 2021, 35, 15223–15233. [Google Scholar] [CrossRef]
- Wang, Y.; Godin, R.; Durrant, J.R.; Tang, J. Efficient Hole Trapping in Carbon Dot/Oxygen-Modified Carbon Nitride Heterojunction Photocatalysts for Enhanced Methanol Production from CO2 under Neutral Conditions. Angew. Chem. Int. Ed. Engl. 2021, 60, 20811–20816. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A reveiw. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Guo, H.; Shu, Z.; Chen, D.; Tan, Y.; Zhou, J.; Meng, F.; Li, T. One-step synthesis of S-doped g-C3N4 nanosheets for improved visible-light photocatalytic hydrogen evolution. Chem. Phys. 2020, 533, 110714. [Google Scholar] [CrossRef]
- Wang, X.; Meng, J.; Zhang, X.; Liu, Y.; Ren, M.; Yang, Y.; Guo, Y. Controllable Approach to Carbon-Deficient and Oxygen-Doped Graphitic Carbon Nitride: Robust Photocatalyst Against Recalcitrant Organic Pollutants and the Mechanism Insight. Adv. Funct. Mater. 2021, 31, 2010763. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Ma, Y.; Fang, S.; Kong, F.; Pang, X. Photocatalytic degradation of dinotefuran by layered phosphorus-doped carbon nitride and its mechanism. J. Photochem. Photobiol. A Chem. 2021, 414, 113287. [Google Scholar] [CrossRef]
- Liu, B.; Ye, L.; Wang, R.; Yang, J.; Zhang, Y.; Guan, R.; Tian, L.; Chen, X. Phosphorus-Doped Graphitic Carbon Nitride Nanotubes with Amino-rich Surface for Efficient CO2 Capture, Enhanced Photocatalytic Activity, and Product Selectivity. ACS Appl. Mater. Interfaces 2018, 10, 4001–4009. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Guan, J.; Jiang, Y.; Hou, T.; Mu, X. Facile synthesis of phosphorus doped graphitic carbon nitride polymers with enhanced visible-light photocatalytic activity. Mater. Res. Bull. 2013, 48, 3485–3491. [Google Scholar] [CrossRef]
- Zhang, Y.; Antonietti, M. Photocurrent generation by polymeric carbon nitride solids: An initial step towards a novel photovoltaic system. Chem. Asian J. 2010, 5, 1307–1311. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Wu, Z.; Yu, H.; Mo, D.; Wang, H.; Xiao, Z.; Zhou, C. Nitrogen self-doped g-C3N4 nanosheets with tunable band structures for enhanced photocatalytic tetracycline degradation. J. Colloid Interface Sci. 2019, 536, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Xu, G.; Yu, Q.; Yang, K.; Li, H. Nitrogen self-doped high specific surface area graphite carbon nitride for photocatalytic degradating of methylene blue. J. Nanopart. Res. 2019, 21, 224. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Zhang, B.; Yuan, M.; Ma, Y.; Kong, F. A facile strategy for photocatalytic degradation of seven neonicotinoids over sulfur and oxygen co-doped carbon nitride. Chemosphere 2020, 253, 126672. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lei, J.; Wang, F.; Wang, L.; Hoffmann, M.R.; Liu, Y.; In, S.-I.; Zhang, J. Carbon nitride nanotubes with in situ grafted hydroxyl groups for highly efficient spontaneous H2O2 production. Appl. Catal. B Environ. 2021, 288, 119993. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Lin, S.; Fu, X.; Wang, X. Molecular doping of carbon nitride photocatalysts with tunable bandgap and enhanced activity. J. Catal. 2014, 310, 24–30. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, X.; Chen, P.; Li, D.; Zhang, Q.; Liu, H.; Zhong, J.; Lv, W.; Liu, G. Synchronous construction of a porous intramolecular D-A conjugated polymer via electron donors for superior photocatalytic decontamination. J. Hazard. Mater. 2022, 424, 127379. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Y.; Qi, R.; Cao, C.; Liu, X.; Zheng, Y.; Song, W. Enhanced electron separation on in-plane benzene-ring doped g-C3N4 nanosheets for visible light photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 244, 459–464. [Google Scholar] [CrossRef]
- Hwang, J.; Park, J.; Kim, Y.J.; Ha, Y.H.; Park, C.E.; Chung, D.S.; Kwon, S.-K.; Kim, Y.-H. Indolo[3,2-b]indole-Containing Donor–Acceptor Copolymers for High-Efficiency Organic Solar Cells. Chem. Mater. 2017, 29, 2135–2140. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Z.; Zhang, S.; Ye, H.; Kong, K.; Gong, X.; Hua, J.; Tian, H. Molecular Engineering of Donor-Acceptor Conjugated Polymer/g-C3N4 Heterostructures for Significantly Enhanced Hydrogen Evolution Under Visible-Light Irradiation. Adv. Funct. Mater. 2018, 28, 1804512. [Google Scholar] [CrossRef]
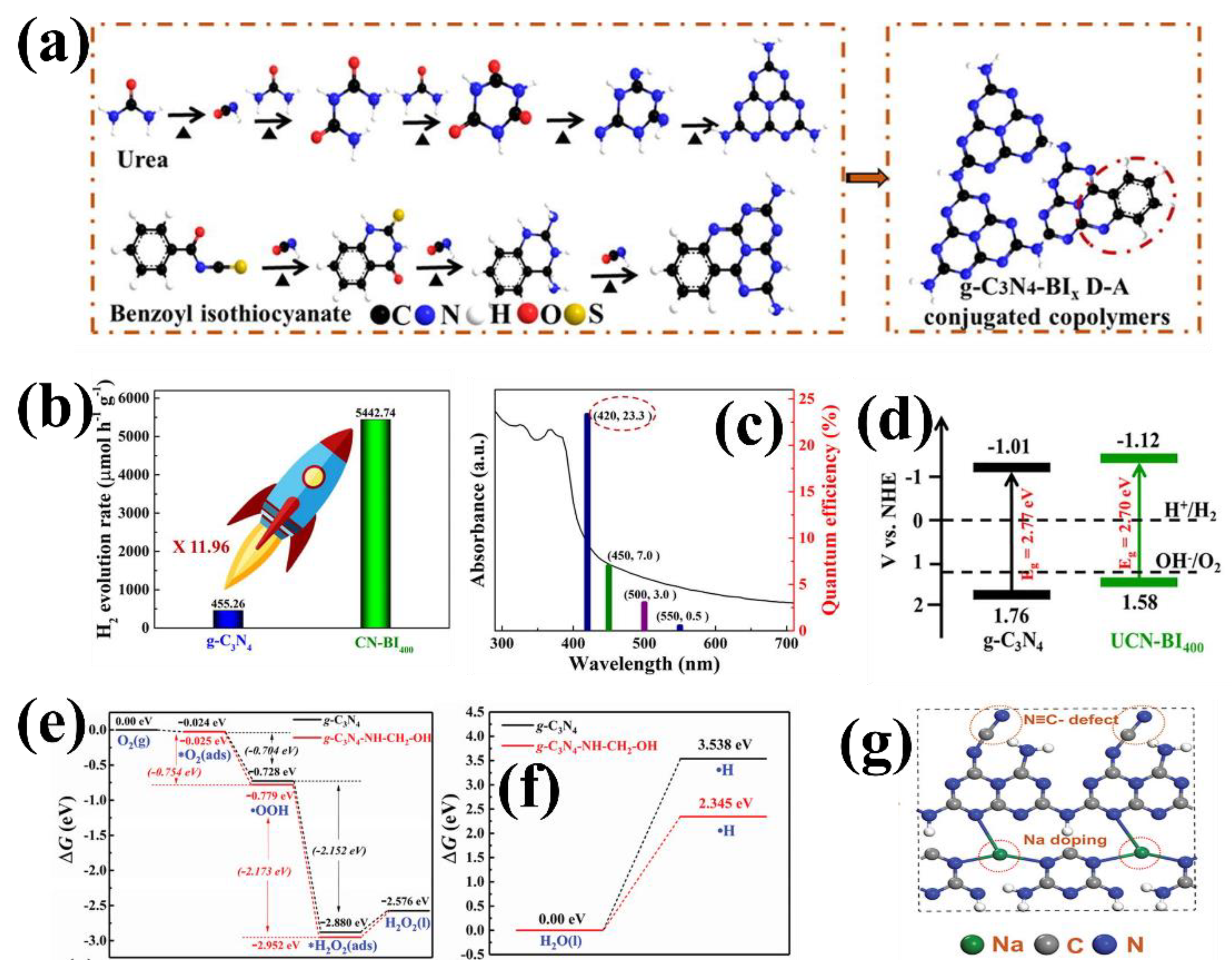
- Che, H.; Li, C.; Li, C.; Liu, C.; Dong, H.; Song, X. Benzoyl isothiocyanate as a precursor to design of ultrathin and high-crystalline g-C3N4-based donor–acceptor conjugated copolymers for superior photocatalytic H2 production. Chem. Eng. J. 2021, 410, 127791. [Google Scholar] [CrossRef]
- Liu, B.; Du, J.; Ke, G.; Jia, B.; Huang, Y.; He, H.; Zhou, Y.; Zou, Z. Boosting O2 Reduction and H2O Dehydrogenation Kinetics: Surface N-Hydroxymethylation of g-C3N4 Photocatalysts for the Efficient Production of H2O2. Adv. Funct. Mater. 2021, 32, 2111125. [Google Scholar] [CrossRef]
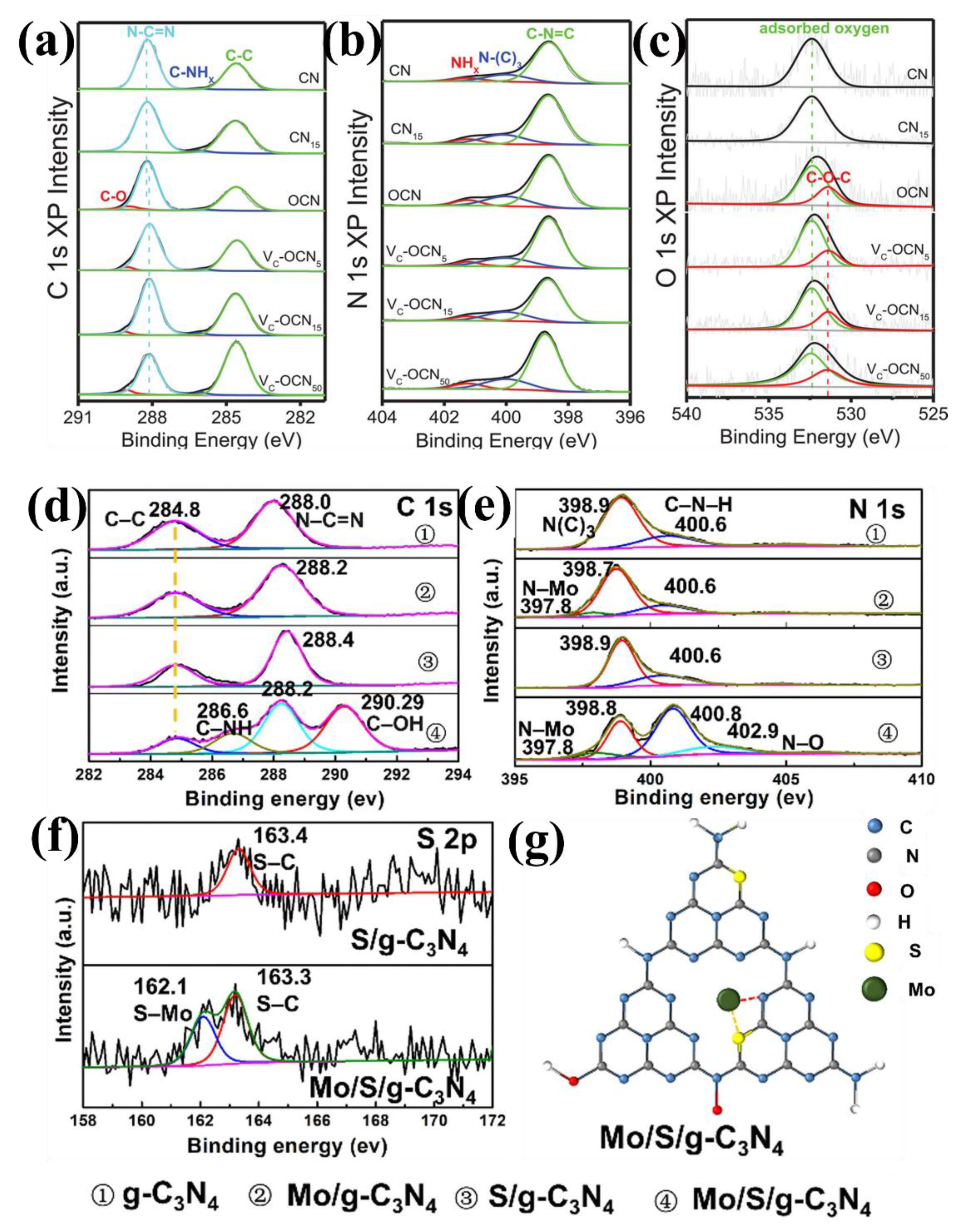
- Li, Y.; Zhu, S.; Liang, Y.; Li, Z.; Wu, S.; Chang, C.; Luo, S.; Cui, Z. One-step synthesis of Mo and S co-doped porous g-C3N4 nanosheets for efficient visible-light photocatalytic hydrogen evolution. Appl. Surf. Sci. 2021, 536, 147743. [Google Scholar] [CrossRef]
- Luo, L.; Gong, Z.; Ma, J.; Wang, K.; Zhu, H.; Li, K.; Xiong, L.; Guo, X.; Tang, J. Ultrathin sulfur-doped holey carbon nitride nanosheets with superior photocatalytic hydrogen production from water. Appl. Catal. B Environ. 2021, 284, 119742. [Google Scholar] [CrossRef]
- Wang, S.; He, F.; Zhao, X.; Zhang, J.; Ao, Z.; Wu, H.; Yin, Y.; Shi, L.; Xu, X.; Zhao, C.; et al. Phosphorous doped carbon nitride nanobelts for photodegradation of emerging contaminants and hydrogen evolution. Appl. Catal. B Environ. 2019, 257, 117931. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, S.; Chen, S.; Li, D.; Zhang, X.; Shao, W.; Sun, X.; Xie, J.; Zhao, Z.; Zhang, Q.; et al. Enhanced Singlet Oxygen Generation in Oxidized Graphitic Carbon Nitride for Organic Synthesis. Adv. Mater. 2016, 28, 6940–6945. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Y.; Cao, Y.; Du, R.; Bao, Z.; Zhang, S.; Shao, F.; Ji, W.; Yang, J.; Zhuang, G.; et al. Trace water triggers high-efficiency photocatalytic hydrogen peroxide production. J. Energy Chem. 2022, 64, 47–54. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Y.; Lyu, L.; Zeng, Q.; Xing, X.; Hu, C. Electronic Structure Modulation of Graphitic Carbon Nitride by Oxygen Doping for Enhanced Catalytic Degradation of Organic Pollutants through Peroxymonosulfate Activation. Environ. Sci. Technol. 2018, 52, 14371–14380. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, Y.; Zong, X.; Nie, H.; Niu, L.; An, L.; Qu, D.; Wang, X.; Kang, Z.; Sun, Z. Photocatalyst for High-Performance H2 Production: Ga-Doped Polymeric Carbon Nitride. Angew. Chem. Int. Ed. Engl. 2021, 60, 6124–6129. [Google Scholar] [CrossRef]
- Wang, G.; Huang, R.; Zhang, J.; Mao, J.; Wang, D.; Li, Y. Synergistic Modulation of the Separation of Photo-Generated Carriers via Engineering of Dual Atomic Sites for Promoting Photocatalytic Performance. Adv. Mater. 2021, 33, e2105904. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Shi, W.X.; Zhuang, G.L.; Zhao, Q.P.; Ren, J.; Zhang, P.; Yin, H.Q.; Lu, T.B.; Zhang, Z.M. W Single-Atom Catalyst for CH4 Photooxidation in Water Vapor. Adv. Mater. 2022, 34, e2204448. [Google Scholar] [CrossRef]
- Zhao, D.; Dong, C.L.; Wang, B.; Chen, C.; Huang, Y.C.; Diao, Z.; Li, S.; Guo, L.; Shen, S. Synergy of Dopants and Defects in Graphitic Carbon Nitride with Exceptionally Modulated Band Structures for Efficient Photocatalytic Oxygen Evolution. Adv. Mater. 2019, 31, e1903545. [Google Scholar] [CrossRef] [PubMed]

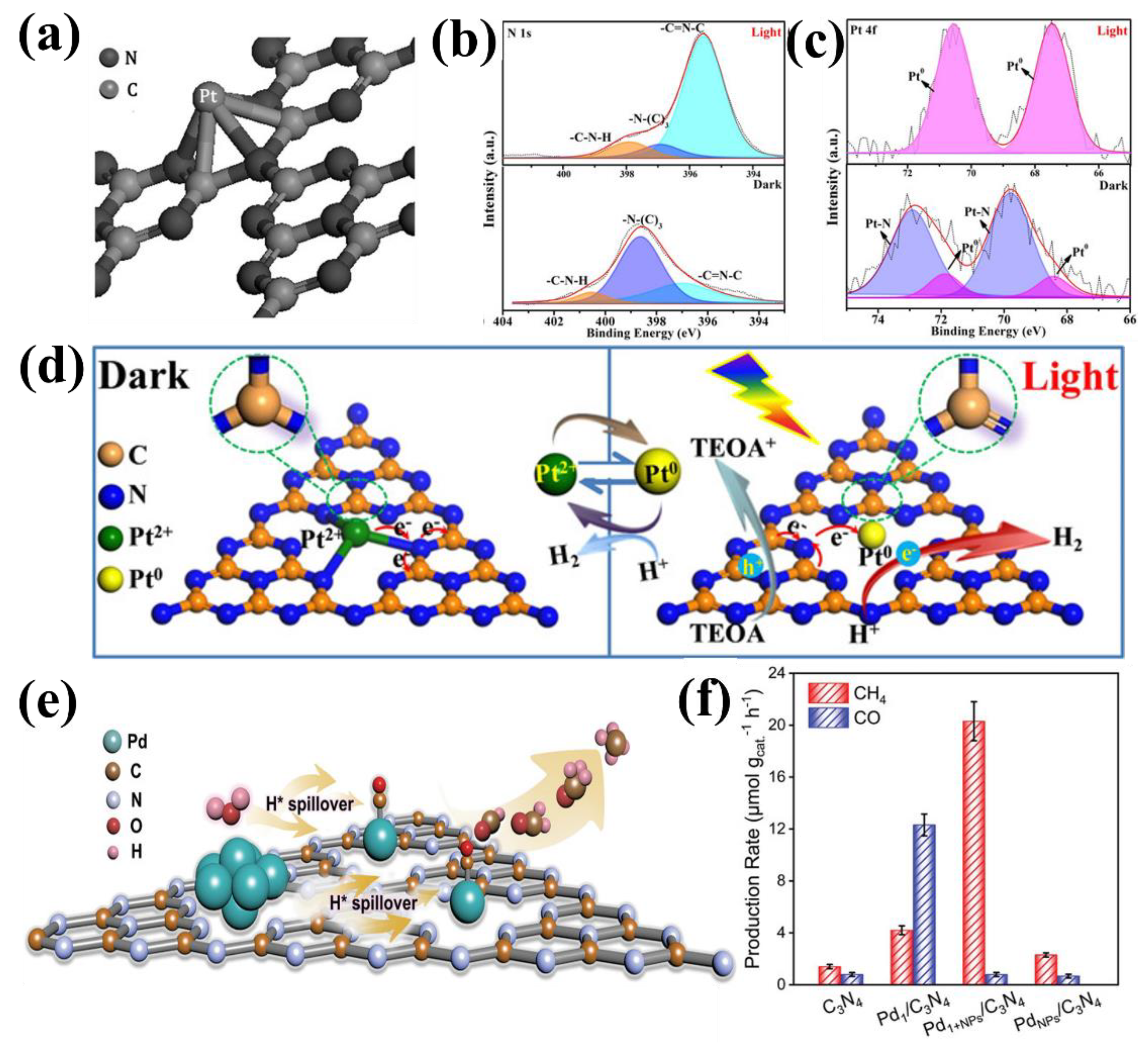
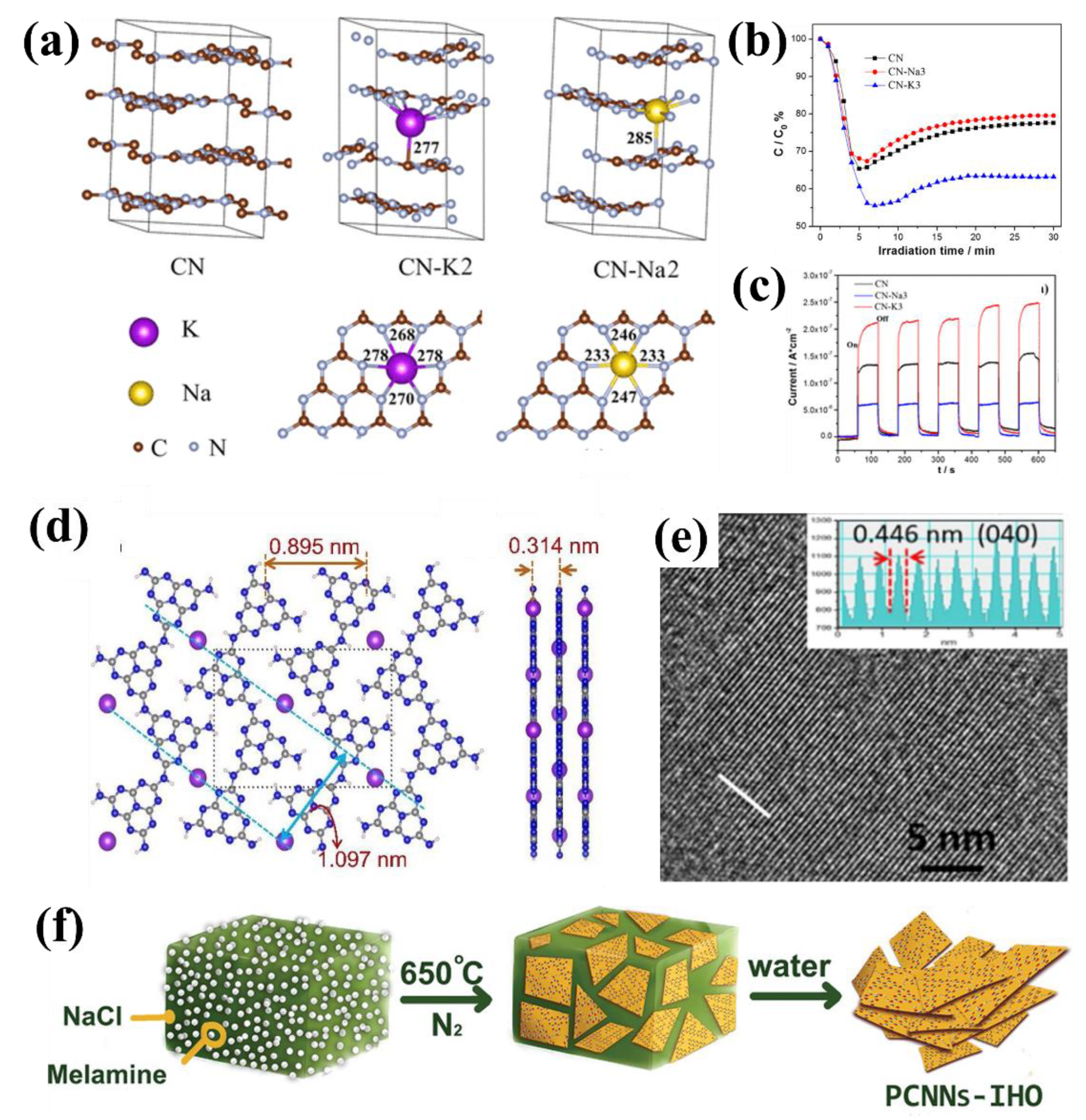
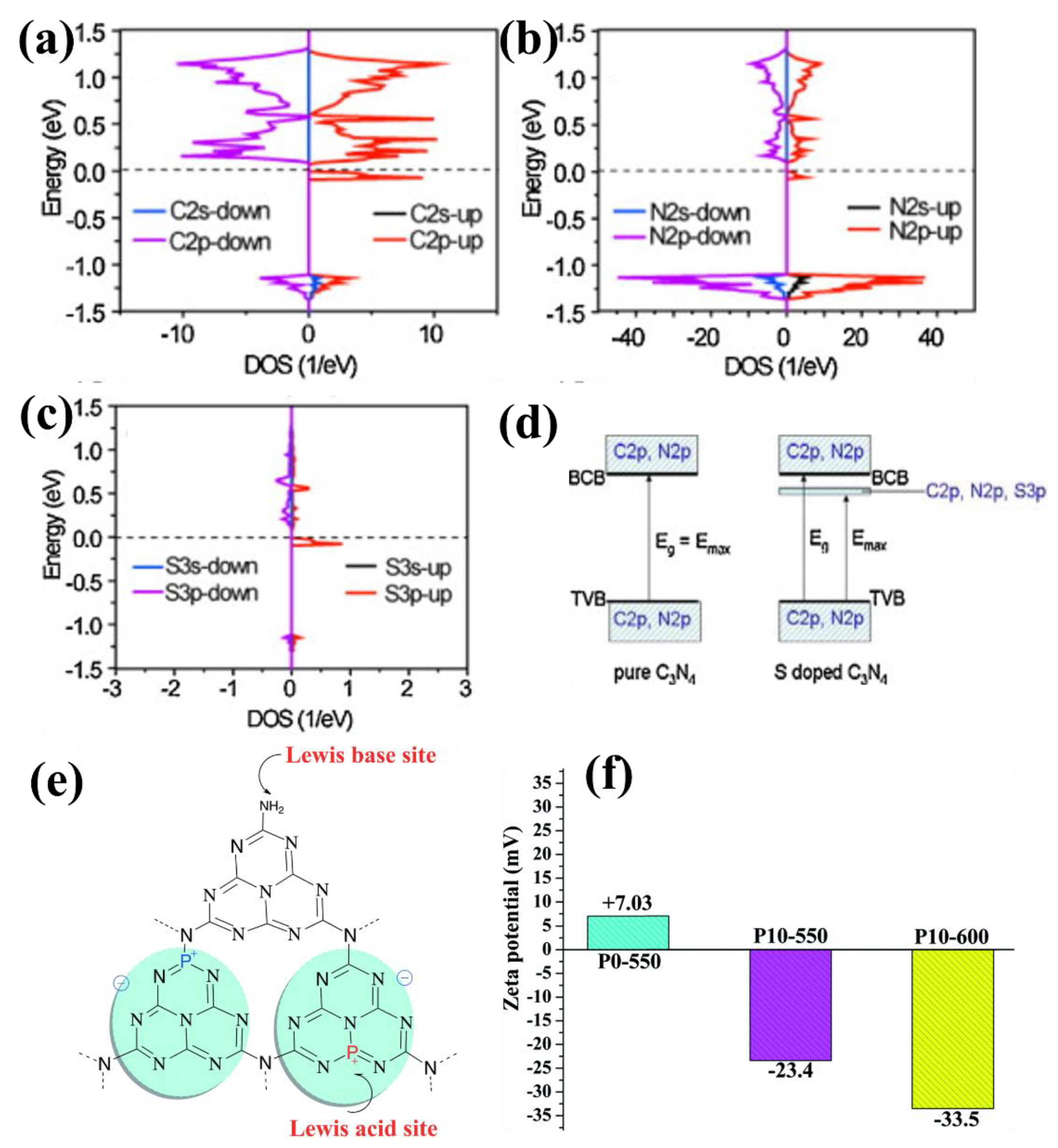
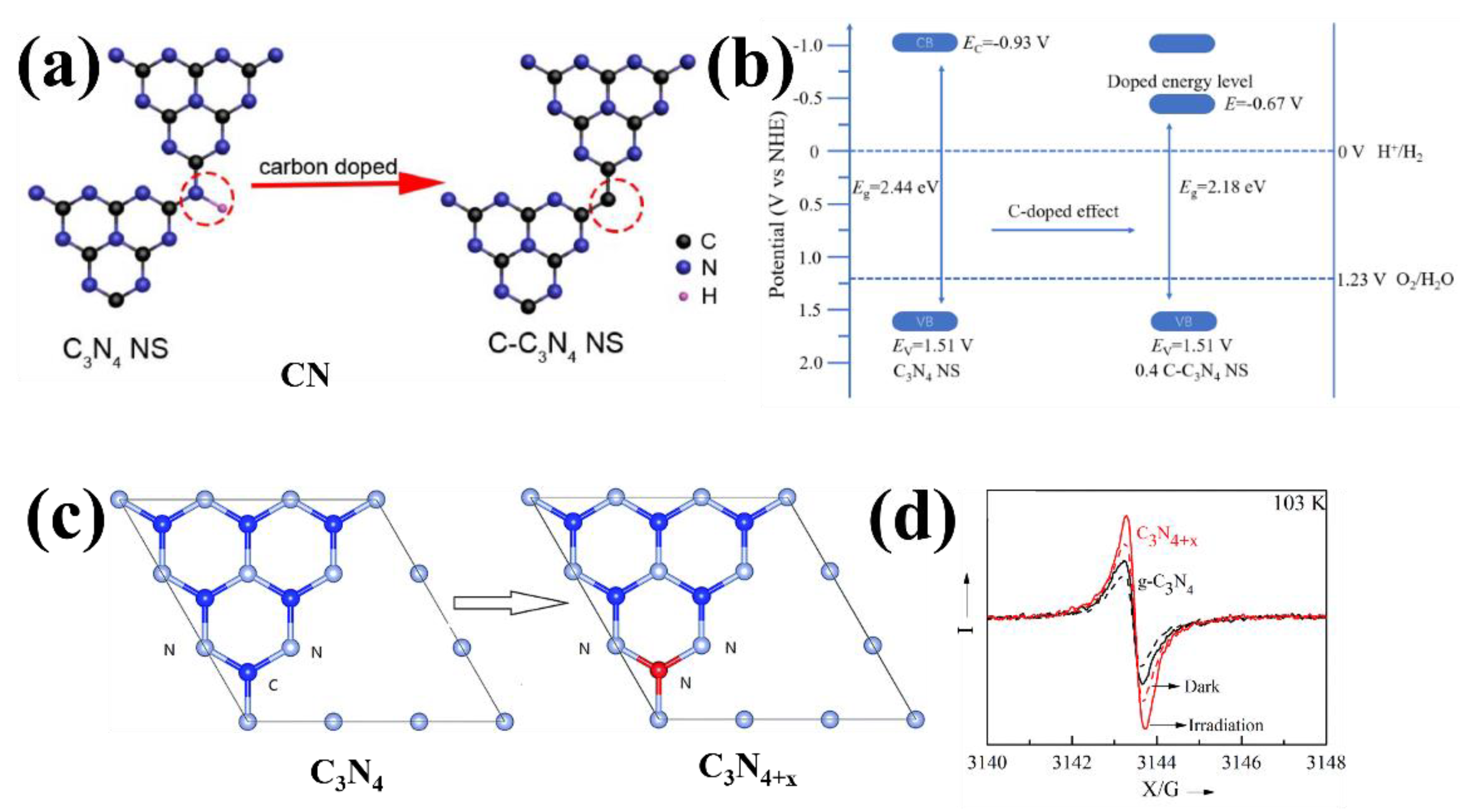


| Entry | Catalysts | Dopant Source | Application | Activity | Rate Enhanced | Ref. |
|---|---|---|---|---|---|---|
| 1 | Pt–CN | H2PtCl6 | H2 evolution | 318 µmol h−1 | 50 | [41] |
| 2 | Pt–CN | H2PtCl6 | H2 evolution | 14.7 mmol h−1 g−1 | 1340 | [42] |
| 3 | Pd1+NPs/C3N4 | Pd(acac)2 | CO2 reduction | 20.3 µmol CH4 g−1 h−1 | - | [43] |
| 4 | CN-K | KBr | NO removal | around 40% | - | [35] |
| 5 | CCN | KCl | H2 evolution | 1356 μmol h−1 g−1 | 22 | [44] |
| 6 | PCNNs-IHO | NaCl | CH3SH mineralization | − | - | [45] |
| 7 | S–CN | thiourea | CH3OH production | 1.12 μmol g−1 | 1.38 | [46] |
| 8 | P–CN | HCCP | H2 evolution | 50.6 mmol h−1 | 2.9 | [47] |
| 9 | C–C3N4 | glucose | H2 evolution | 6545.8 µmol g−1 | 3 | [48] |
| 10 | C3N4+x | hydrazine hydrate | H2 evolution | 44.28 mmol h−1 | 4.6 | [49] |
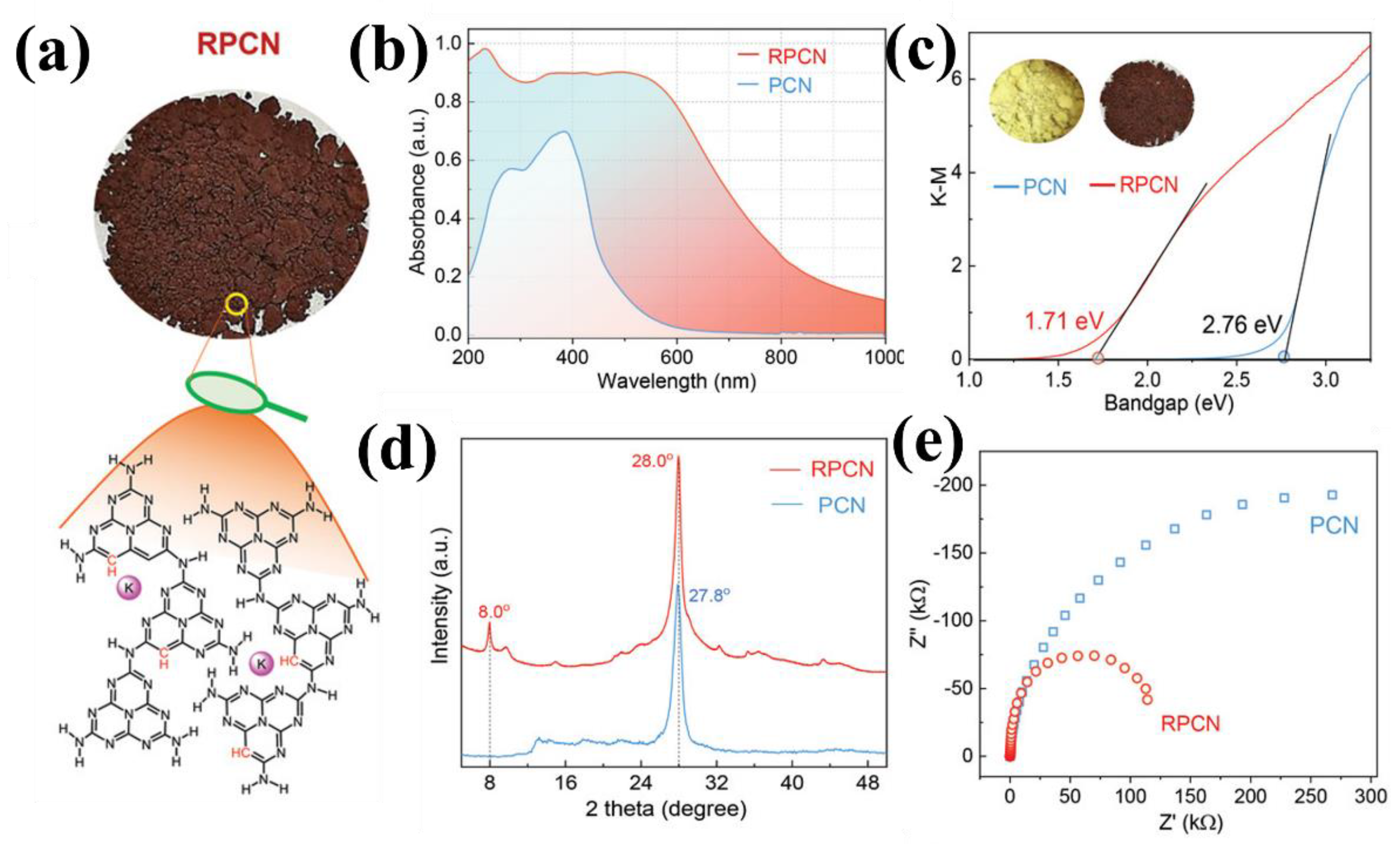
| 11 | RPCN | 2,4,6-triamine-pyrimidine + KCl | H2 evolution | 640 µmol h−1 g−1 500~780 nm | - | [50] |
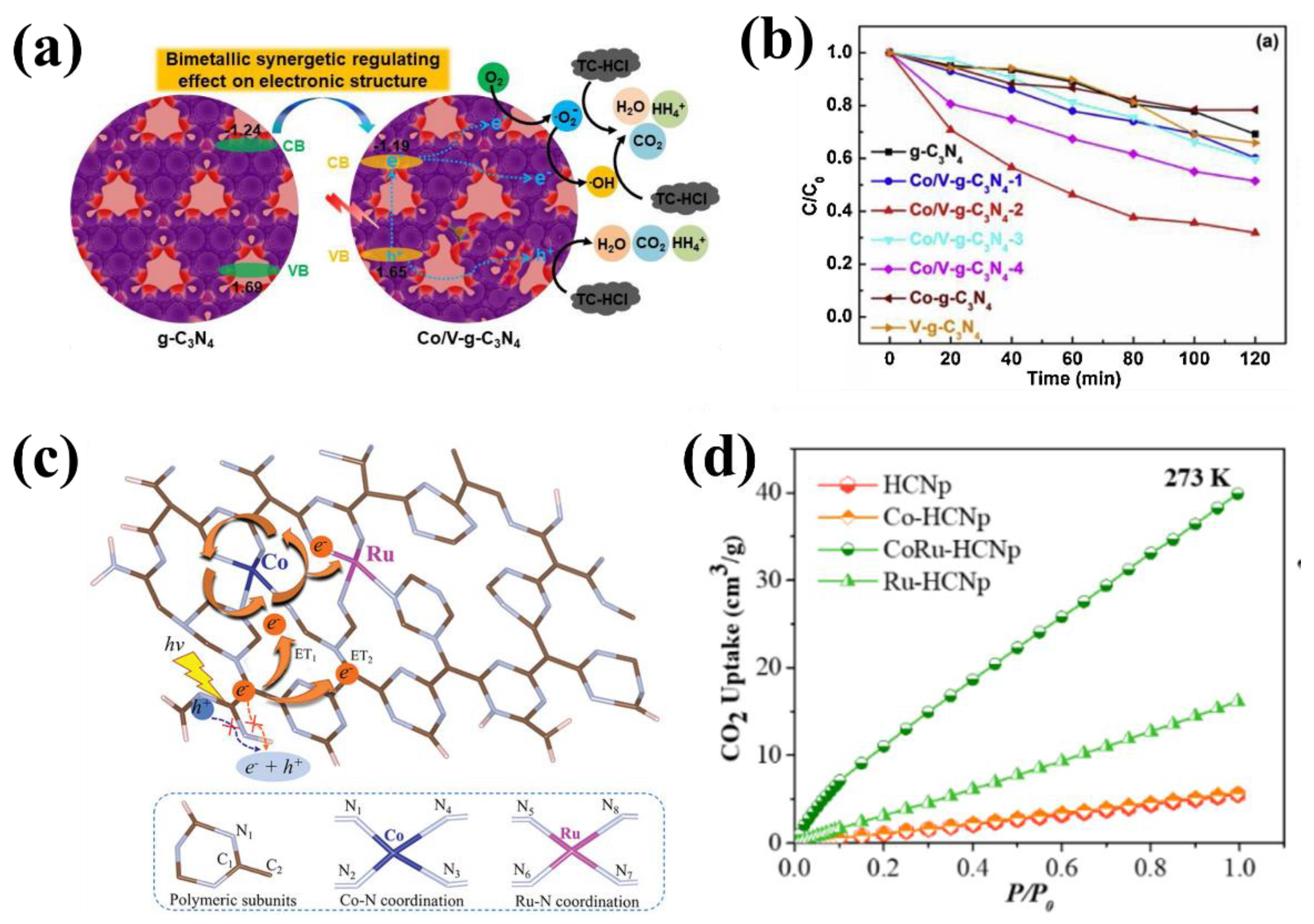
| 12 | Co/V–g–C3N4 | vanadyl acetylacetonate + cobalt acetate | TC–HCl degradation | 0.0108 min−1 | 4 | [51] |
| 13 | Co/Ru–CN | RuCl3 + Co(NO3)2·6H2O | CO2 reduction | 95.6 µmol g−1 | 3 | [52] |
| 14 | Na/g–C3N4 | NaCl | H2O2 production | 7.01 mM | 220 | [53] |
| Characterization Techniques | Information | Limitations |
|---|---|---|
| XPS | elemental composition, chemical structure, bonding environment | surface characterization techniques |
| NMR | chemical environment | limited sensitivity, difficult for quantitative analysis |
| FT-IR | functional groups triggering new vibrational modes | only sensitive to organic groups, not for detection of trace elements directly |
| Elemental mapping | intuitive display of the presence of foreign elements | only reflects the spatial distribution of the element, without considering the chemical environment |
| XAS | coordination environment and electronic state of single atomic CN | only reflects the average information of all species in the catalyst |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Fang, S.; Shen, Q.; Fan, J.; Li, Q.; Lv, K. Recent Advances of Doping and Surface Modifying Carbon Nitride with Characterization Techniques. Catalysts 2022, 12, 962. https://doi.org/10.3390/catal12090962
Chen J, Fang S, Shen Q, Fan J, Li Q, Lv K. Recent Advances of Doping and Surface Modifying Carbon Nitride with Characterization Techniques. Catalysts. 2022; 12(9):962. https://doi.org/10.3390/catal12090962
Chicago/Turabian StyleChen, Jinbao, Shun Fang, Qun Shen, Jiajie Fan, Qin Li, and Kangle Lv. 2022. "Recent Advances of Doping and Surface Modifying Carbon Nitride with Characterization Techniques" Catalysts 12, no. 9: 962. https://doi.org/10.3390/catal12090962






