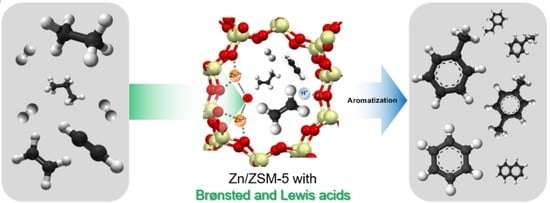
Efficient Utilization of Hydrocarbon Mixture to Produce Aromatics over Zn/ZSM-5 and Physically Mixed with ZSM-5
Abstract
:1. Introduction
2. Results
2.1. Catalyst Characterization
2.2. Catalytic Activity Tests
2.3. Remarks about Utilization of Methane as A Primary Component of Natural Gas, Including Shale Gas
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Borry, R.W.; Kim, Y.H.; Huffsmith, A.; Reimer, J.A.; Iglesia, E. Structure and Density of Mo and Acid Sites in Mo-Exchanged H−ZSM5 Catalysts for Nonoxidative Methane Conversion. J. Phys. Chem. B 1999, 103, 5787–5796. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Li, S.; Meitzner, G.D.; Iglesia, E. Methane Conversion to Aromatics on Mo/H-ZSM5: Structure of Molybdenum Species in Working Catalysts. J. Phys. Chem. B 2001, 105, 506–513. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Ohnishi, R.; Ichikawa, M. Bifunctional Catalysis of Mo/HZSM-5 in the Dehydroaromatization of Methane to Benzene and Naphthalene XAFS/TG/DTA/MASS/FTIR Characterization and Supporting Effects. J. Catal. 1999, 181, 175–188. [Google Scholar] [CrossRef]
- Tshabalala, T.E.; Coville, N.J.; Scurrell, M.S. Dehydroaromatization of methane over doped Pt/Mo/H-ZSM-5 zeolite catalysts: The promotional effect of tin. Appl. Catal. A Gen. 2014, 485, 238–244. [Google Scholar] [CrossRef]
- Farrell, B.L.; Igenegbai, V.O.; Linic, S. A Viewpoint on Direct Methane Conversion to Ethane and Ethylene Using Oxidative Coupling on Solid Catalysts. ACS Catal. 2016, 6, 4340–4346. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Fang, G.; Li, G.; Ma, H.; Fan, H.; Yu, L.; Ma, C.; Wu, X.; Deng, D.; Wei, M.; et al. Direct, Nonoxidative Conversion of Methane to Ethylene, Aromatics, and Hydrogen. Science 2014, 344, 616–619. [Google Scholar] [CrossRef]
- Lim, T.H.; Kim, D.H. Characteristics of Mn/H-ZSM-5 catalysts for methane dehydroaromatization. Appl. Catal. A Gen. 2019, 577, 10–19. [Google Scholar] [CrossRef]
- Gim, M.Y.; Han, S.J.; Kang, T.H.; Song, J.H.; Kim, T.H.; Kim, D.H.; Lee, K.-Y.; Song, I.K. Benzene, Toluene, and Xylene Production by Direct Dehydroaromatization of Methane Over WOy/HZSM-5 Catalysts. J. Nanosci. Nanotechnol. 2017, 17, 8226–8231. [Google Scholar] [CrossRef]
- Kim, J.; Abbott, M.S.; Go, D.B.; Hicks, J.C. Enhancing C–H Bond Activation of Methane via Temperature-Controlled, Catalyst—Plasma Interactions. ACS Energ. Lett. 2016, 1, 94–99. [Google Scholar] [CrossRef]
- Kameshima, S.; Tamura, K.; Ishibashi, Y.; Nozaki, T. Pulsed dry methane reforming in Plasma—Enhanced catalytic reaction. Catal. Today 2015, 256, 67–75. [Google Scholar] [CrossRef]
- Zheng, X.; Tan, S.; Dong, L.; Li, S.; Chen, H. Silica—Coated LaNiO3 nanoparticles for non-thermal plasma assisted dry reforming of methane: Experimental and kinetic studies. Chem. Eng. J. 2015, 265, 147–156. [Google Scholar] [CrossRef]
- Mahammadunnisa, S.; Reddy, P.M.K.; Ramaraju, B.; Subrahmanyam, C. Catalytic Nonthermal Plasma Reactor for Dry Reforming of Methane. Energy Fuels 2013, 27, 4441–4447. [Google Scholar] [CrossRef]
- Zheng, X.-G.; Tan, S.-Y.; Dong, L.-C.; Li, S.-B.; Chen, H.-M.; Wei, S.-A. Experimental and kinetic investigation of the plasma catalytic dry reforming of methane over perovskite LaNiO3 nanoparticles. Fuel Process. Technol. 2015, 137, 250–258. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, X.; Mei, D.; Ashford, B.; Tu, X. Plasma—Catalytic dry reforming of methane over γ-Al2O3 supported metal catalysts. Catal. Today 2015, 256, 80–87. [Google Scholar] [CrossRef]
- Kim, J.; Jeoung, J.; Jeon, J.; Kim, J.; Mok, Y.S.; Ha, K.-S. Effects of dielectric particles on non−oxidative coupling of methane in a dielectric barrier discharge plasma reactor. Chem. Eng. J. 2019, 377, 119896. [Google Scholar] [CrossRef]
- Ma, D.; Shu, Y.; Bao, X.; Xu, Y. Methane Dehydro—Aromatization under Nonoxidative Conditions over Mo/HZSM-5 Catalysts: EPR Study of the Mo Species on/in the HZSM-5 Zeolite. J. Catal. 2000, 189, 314–325. [Google Scholar] [CrossRef]
- Raad, M.; Hamieh, S.; Toufaily, J.; Hamieh, T.; Pinard, L. Propane aromatization on hierarchical Ga/HZSM-5 catalysts. J. Catal. 2018, 366, 223–236. [Google Scholar] [CrossRef]
- Tshabalala, T.E.; Scurrell, M.S. Aromatization of n-hexane over Ga, Mo and Zn modified H-ZSM-5 zeolite catalysts. Catal. Commun. 2015, 72, 49–52. [Google Scholar] [CrossRef]
- Ma, L.; Zou, X. Cooperative catalysis of metal and acid functions in Re-HZSM-5 catalysts for ethane dehydroaromatization. Appl. Catal. B Environ. 2019, 243, 703–710. [Google Scholar] [CrossRef]
- Bhan, A.; Delgass, W.N. Propane Aromatization over HZSM-5 and Ga/HZSM-5 Catalysts. Catal. Rev. 2008, 50, 19–151. [Google Scholar] [CrossRef]
- Nishi, K.; Komai, S.-i.; Inagaki, K.; Satsuma, A.; Hattori, T. Structure and catalytic properties of Ga-MFI in propane aromatization. Appl. Catal. A Gen. 2002, 223, 187–193. [Google Scholar] [CrossRef]
- Rodrigues, V.d.O.; Júnior, A.C.F. On catalyst activation and reaction mechanisms in propane aromatization on Ga/HZSM5 catalysts. Appl. Catal. A Gen. 2012, 435, 68–77. [Google Scholar] [CrossRef]
- Wang, J.; Kang, M.; Zhang, Z.; Wang, X. Propane Aromatization over Mo/HZSM-5 Catalysts. J. Nat. Gas Chem. 2002, 11, 43–50. [Google Scholar]
- Solymosi, F.; Széchenyi, A. Aromatization of n-butane and 1-butene over supported Mo2C catalyst. J. Catal. 2004, 223, 221–231. [Google Scholar] [CrossRef]
- Mehdad, A.; Lobo, R.F. Ethane and ethylene aromatization on Zinc—Containing zeolites. Catal. Sci. Technol. 2017, 7, 3562–3572. [Google Scholar] [CrossRef]
- Kazansky, V.B.; Subbotina, I.R.; Rane, N.; van Santen, R.A.; Hensen, E.J.M. On two alternative mechanisms of ethane activation over ZSM-5 zeolite modified by Zn2+ and Ga1+ cations. Phys. Chem. Chem. Phys. 2005, 7, 3088–3092. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, S.; Xu, X.; Jiang, H. Ethane aromatization and evolution of carbon deposits over nanosized and microsized Zn/ZSM−5 catalysts. Catal. Sci. Technol. 2020, 10, 835–843. [Google Scholar] [CrossRef]
- Gong, Q.; Fang, T.; Xie, Y.; Zhang, R.; Liu, M.; Barzagli, F.; Li, J.; Hu, Z.; Zhu, Z. High-Efficiency Conversion of Methanol to BTX Aromatics Over a Zn-Modified Nanosheet-HZSM-5 Zeolite. Ind. Eng. Chem. Res. 2021, 60, 1633–1641. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Bai, T.; Chen, T.F.; Fan, W.T.; Zhang, X. Conversion of Methanol to Light Aromatics on Zn-Modified Nano-HZSM-5 Zeolite Catalysts. Ind. Eng. Chem. Res. 2014, 53, 14932–14940. [Google Scholar] [CrossRef]
- Li, J.; Gong, Q.; Liu, Y.; Kang, R.; Yang, C.; Qiu, M.; Hu, Z.; Zhu, Z. Insight into the Formation of COX By-Products in Methanol-to-Aromatics Reaction over Zn/HZSM-5: Significantly Affected by the Chemical State of Surface Zn Species. Chem. Cat. Chem. 2019, 11, 4755–4764. [Google Scholar]
- Hsieh, C.-Y.; Chen, Y.-Y.; Lin, Y.-C. Ga-Substituted Nanoscale HZSM-5 in Methanol Aromatization: The Cooperative Action of the Brønsted Acid and the Extra-Framework Ga Species. Ind. Eng. Chem. Res. 2018, 57, 7742–7751. [Google Scholar] [CrossRef]
- Xin, Y.; Qi, P.; Duan, X.; Lin, H.; Yuan, Y. Enhanced Performance of Zn-Sn/HZSM-5 Catalyst for the Conversion of Methanol to Aromatics. Catal. Lett. 2013, 143, 798–806. [Google Scholar] [CrossRef]
- Niu, X.; Gao, J.; Miao, Q.; Dong, M.; Wang, G.; Fan, W.; Qin, Z.; Wang, J. Influence of preparation method on the performance of Zn-containing HZSM-5 catalysts in methanol-to-aromatics. Microporous Mesoporous Mater. 2014, 197, 252–261. [Google Scholar] [CrossRef]
- Niu, X.; Gao, J.; Wang, K.; Miao, Q.; Dong, M.; Wang, G.; Fan, W.; Qin, Z.; Wang, J. Influence of crystal size on the catalytic performance of H-ZSM-5 and Zn/H-ZSM-5 in the conversion of methanol to aromatics. Fuel Process. Technol. 2017, 157, 99–107. [Google Scholar] [CrossRef]
- Bi, Y.; Wang, Y.; Chen, X.; Yu, Z.; Xu, L. Methanol aromatization over HZSM-5 catalysts modified with different zinc salts. Chin. J. Catal. 2014, 35, 1740–1751. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Hu, J. Study of methanol to aromatics on ZnHZSM-5 catalyst. J. Fuel Chem. Technol. 2009, 37, 607. [Google Scholar]
- Su, X.; Zan, W.; Bai, X.; Wang, G.; Wu, W. Synthesis of microscale and nanoscale ZSM-5 zeolites: Effect of particle size and acidity of Zn modified ZSM-5 zeolites on aromatization performance. Catal. Sci. Technol. 2017, 7, 1943–1952. [Google Scholar] [CrossRef]
- Klein, J.C.; Hercules, D.M. Surface characterization of model Urushibara catalysts. J. Catal. 1983, 82, 424–441. [Google Scholar] [CrossRef]
- Guisnet, M.; Gnep, N. Aromatization of propane over GaHMFI catalysts. Reaction scheme, nature of the dehydrogenating species and mode of coke formation. Catal. Today 1996, 31, 275–292. [Google Scholar] [CrossRef]
- Lee, W.; Lee, T.; Jang, H.-G.; Cho, S.J.; Choi, J.; Ha, K.-S. Effects of hierarchical zeolites on aromatization of acetylene. Catal. Today 2018, 303, 177–184. [Google Scholar] [CrossRef]
- Lee, T.; Bae, Y.; Jeon, J.; Kim, J.; Choi, J.; Ha, K.-S. Effects of nanosheet catalysts on synthesis of aromatics and light hydrocarbons from acetylene. Catal. Today 2020, 352, 183–191. [Google Scholar] [CrossRef]
- Song, C.; Gim, M.Y.; Lim, Y.H.; Kim, D.H. Enhanced yield of benzene, toulene, and xylene from the co-aromatization of methane and propane over gallium supported on mesoporous ZSM-5 and ZSM-11. Fuel 2019, 251, 404–412. [Google Scholar] [CrossRef]
- le van Mao, R.; Yao, J. Kinetic study of n-butane aromatization on ZSM-5 and gallium bearing ZSM-5 catalysts. Appl. Catal. A Gen. 1991, 79, 77–87. [Google Scholar] [CrossRef]
- Kazansky, V.B.; Pidko, E.A. Intensities of IR Stretching Bands as a Criterion of Polarization and Initial Chemical Activation of Adsorbed Molecules in Acid Catalysis. Ethane Adsorption and Dehydrogenation by Zinc Ions in ZnZSM-5 Zeolite. J. Phys. Chem. B 2005, 109, 2103–2108. [Google Scholar] [CrossRef]
- Barbosa, L.A.M.M.; van Santen, R.A. Study of the Activation of C-H and H-H Chemical Bonds by the [ZnOZn]2+ Oxycation: Influence of the Zeolite Framework Geometry. J. Phys. Chem. B 2003, 107, 14342–14349. [Google Scholar] [CrossRef]
- Hsu, Y.; Lee, T.; Hu, H. Isomerization of ethylbenzene and m-xylene on zeolites. Ind. Eng. Chem. Res. 1988, 27, 942–947. [Google Scholar] [CrossRef]
- Namuangruk, S.; Pantu, P.; Limtrakul, J. Alkylation of benzene with ethylene over faujasite zeolite investigated by the ONIOM method. J. Catal. 2004, 225, 523–530. [Google Scholar] [CrossRef]
- Young, L.; Butter, S.; Kaeding, W. Shape selective reactions with zeolite catalysts: III. Selectivity in xylene isomerization, toluene-methanol alkylation, and toluene disproportionation over ZSM-5 zeolite catalysts. J. Catal. 1982, 76, 418–432. [Google Scholar] [CrossRef]
- Tsai, P.; Anderson, J. Reaction acetylene over ZSM-5-type catalysts. J. Catal. 1983, 80, 207–214. [Google Scholar] [CrossRef]





| Component | Vol.% |
|---|---|
| H2 | 36.70 |
| C2H2 | 7.50 |
| C2H4 | 1.13 |
| C2H6 | 1.67 |
| C3H8 | 0.803 |
| n-C4H10 | 2.06 |
| N2 | 50.137 |
| Catalysts | Zn Loading (wt.%) a | Si/Al Ratio a | SBET (m2 g−1) | Sext b (m2·g−1) | Vtotal c (cm3·g−1) | Vmicro d (cm3·g−1) |
|---|---|---|---|---|---|---|
| ZSM-5 | - | 17.0 | 377.2 | 29.0 | 0.17 | 0.15 |
| 0.25Zn/ZSM-5 | 0.23 | 16.5 | 378.3 | 29.4 | 0.21 | 0.15 |
| 0.5Zn/ZSM-5 | 0.49 | 16.9 | 377.0 | 29.7 | 0.18 | 0.15 |
| 1Zn/ZSM-5 | 1.01 | 16.7 | 362.6 | 30.9 | 0.19 | 0.15 |
| Catalysts | Acidity (mmol-NH3/gcat) a | |||
|---|---|---|---|---|
| Weak | Medium | Strong | Total Acidity | |
| ZSM-5 | 0.505 (54%) | 0.091 (10%) | 0.331 (36%) | 0.928 |
| 0.25Zn/ZSM-5 | 0.103 (12%) | 0.410 (48%) | 0.345 (40%) | 0.858 |
| 0.5Zn/ZSM-5 | 0.171 (24%) | 0.296 (41%) | 0.254 (35%) | 0.720 |
| 1Zn/ZSM-5 | 0.098 (15%) | 0.241 (36%) | 0.321 (49%) | 0.659 |
| Catalysts | TOS (min) | Total Carbon Conversion (%) | Aromatics Selectivity (%) | BTX Yield (%) | Total Aromatics Yield (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Benzene | Toluene | Xylenes | EtBz | Styrene | C9 | C10 | |||||
| ZSM-5 | 10 | 64.8 | 35.7 | 15.9 | 1.32 | 0.07 | 0.44 | 0.94 | 1.77 | 41.8 | 44.4 |
| 50 | 67.1 | 34.3 | 18.6 | 1.64 | 0.09 | 0.57 | 1.87 | 7.16 | 43.6 | 51.4 | |
| 0.25Zn/ZSM-5 | 10 | 65.7 | 36.9 | 18.1 | 1.47 | 0.08 | 0.56 | 1.21 | 1.39 | 44.7 | 47.3 |
| 50 | 65.1 | 33.9 | 19.7 | 1.67 | 0.10 | 0.64 | 1.71 | 6.06 | 43.8 | 50.6 | |
| 0.5Zn/ZSM-5 | 10 | 63.0 | 33.6 | 18.9 | 1.78 | 0.11 | 0.93 | 2.74 | 6.01 | 42.3 | 49.9 |
| 50 | 63.8 | 32.6 | 20.0 | 1.83 | 0.15 | 1.10 | 2.85 | 8.34 | 42.9 | 52.8 | |
| 1Zn/ZSM-5 | 10 | 63.4 | 31.7 | 15.9 | 1.09 | 0.07 | 0.53 | 1.67 | 2.10 | 38.5 | 42.0 |
| 50 | 63.2 | 29.5 | 18.0 | 1.47 | 0.11 | 0.75 | 1.79 | 3.11 | 38.8 | 43.4 | |
| ZSM-5 + 0.5Zn/ZSM-5 | 10 | 66.3 | 37.5 | 17.7 | 1.47 | 0.08 | 0.56 | 1.40 | 2.45 | 44.8 | 48.3 |
| 50 | 65.9 | 33.3 | 18.8 | 1.61 | 0.10 | 0.62 | 1.90 | 6.13 | 42.8 | 49.7 | |
| Catalysts | TOS (min) | Conversion (%) | |||||
|---|---|---|---|---|---|---|---|
| H2 | C2H6 | C2H4 | C2H2 | C3H8 | n-C4H10 | ||
| ZSM−5 | 10 | −14.7 | −28.1 | −124.6 | 99.9 | 97.2 | 100 |
| 50 | −3.84 | −24.8 | −121.2 | 99.9 | 95.3 | 100 | |
| 0.25Zn/ZSM−5 | 10 | −10.6 | −25.3 | −121.7 | 99.9 | 97.2 | 100 |
| 50 | −3.43 | −25.9 | −129.0 | 99.9 | 94.1 | 100 | |
| 0.5Zn/ZSM−5 | 10 | −12.6 | −22.5 | −137.5 | 99.9 | 93.1 | 99.7 |
| 50 | −1.33 | −27.2 | −139.2 | 99.8 | 86.9 | 99.0 | |
| 1Zn/ZSM−5 | 10 | −7.33 | −37.0 | −133.6 | 99.9 | 97.2 | 100 |
| 50 | −1.54 | −32.5 | −144.5 | 99.8 | 90.5 | 100 | |
| ZSM−5 + 0.5Zn/ZSM−5 | 10 | −16.6 | −21.8 | −116.7 | 99.9 | 97.5 | 100 |
| 50 | −2.89 | −24.4 | −126.3 | 99.9 | 94.7 | 100 | |
| DBD Plasma | Catalytic Aromatization of Light Hydrocarbons | Methane-to-BTX Yield (%) | Methane-to-Aromatics Yield (%) | ||
|---|---|---|---|---|---|
| Methane Conversion (%) | Catalyst | TOS (min) | C2−C4 Conversion (%) | ||
| 54.8 | 0.5Zn/ZSM−5 | 10 | 63.0 | 22.4 | 26.4 |
| 50 | 63.8 | 22.7 | 28.0 | ||
| ZSM−5 + 0.5Zn/ZSM−5 | 10 | 66.3 | 23.7 | 25.6 | |
| 50 | 65.9 | 22.7 | 26.3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, H.; Hong, J.; Ha, K.-S. Efficient Utilization of Hydrocarbon Mixture to Produce Aromatics over Zn/ZSM-5 and Physically Mixed with ZSM-5. Catalysts 2022, 12, 501. https://doi.org/10.3390/catal12050501
Shim H, Hong J, Ha K-S. Efficient Utilization of Hydrocarbon Mixture to Produce Aromatics over Zn/ZSM-5 and Physically Mixed with ZSM-5. Catalysts. 2022; 12(5):501. https://doi.org/10.3390/catal12050501
Chicago/Turabian StyleShim, Hyunjin, Jinju Hong, and Kyoung-Su Ha. 2022. "Efficient Utilization of Hydrocarbon Mixture to Produce Aromatics over Zn/ZSM-5 and Physically Mixed with ZSM-5" Catalysts 12, no. 5: 501. https://doi.org/10.3390/catal12050501