Electrocatalytic Oxygen Reduction Reaction by the Pd/Fe-N-C Catalyst and Application in a Zn–Air Battery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
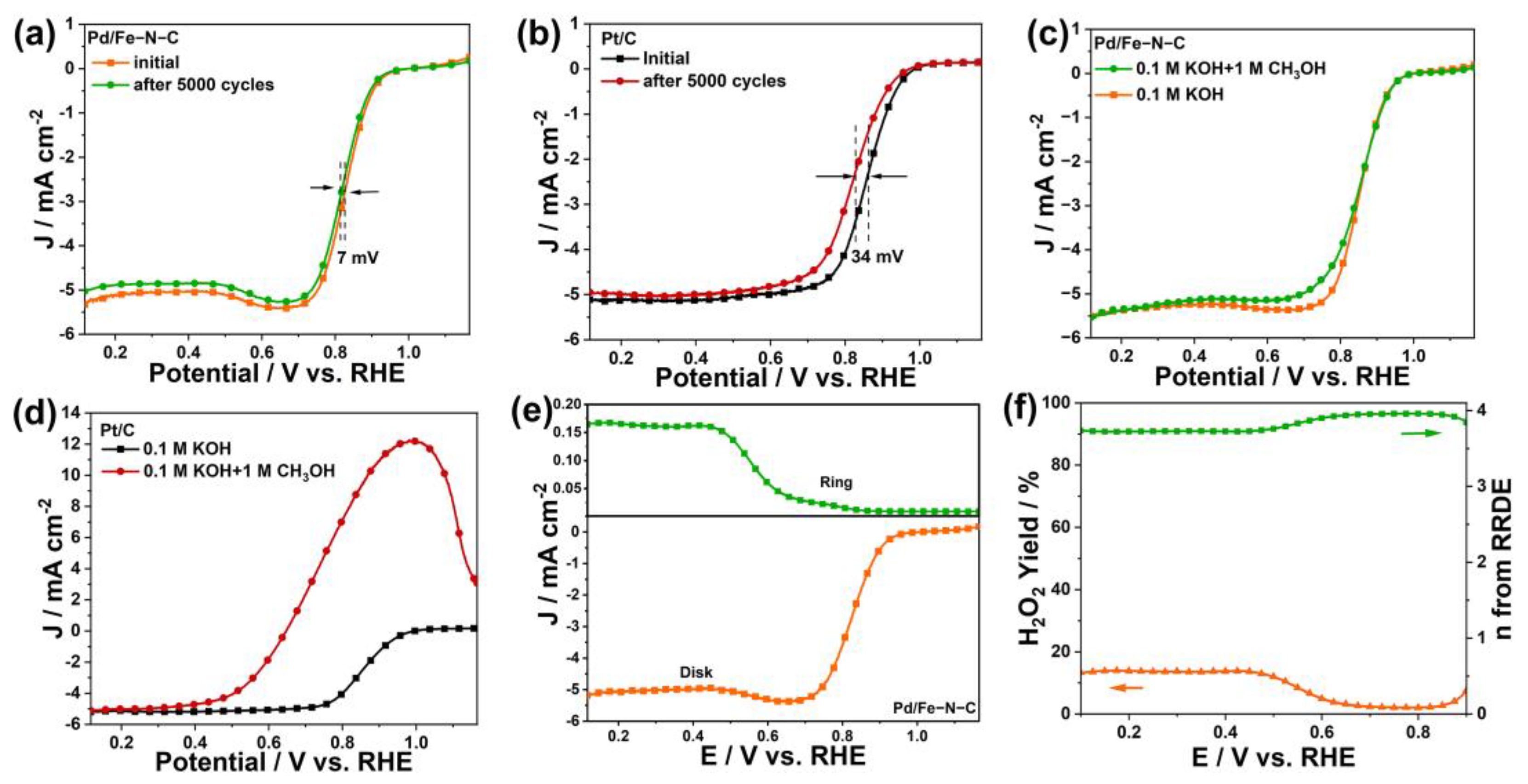
2.2. Evaluation of the ORR Catalytic Performance
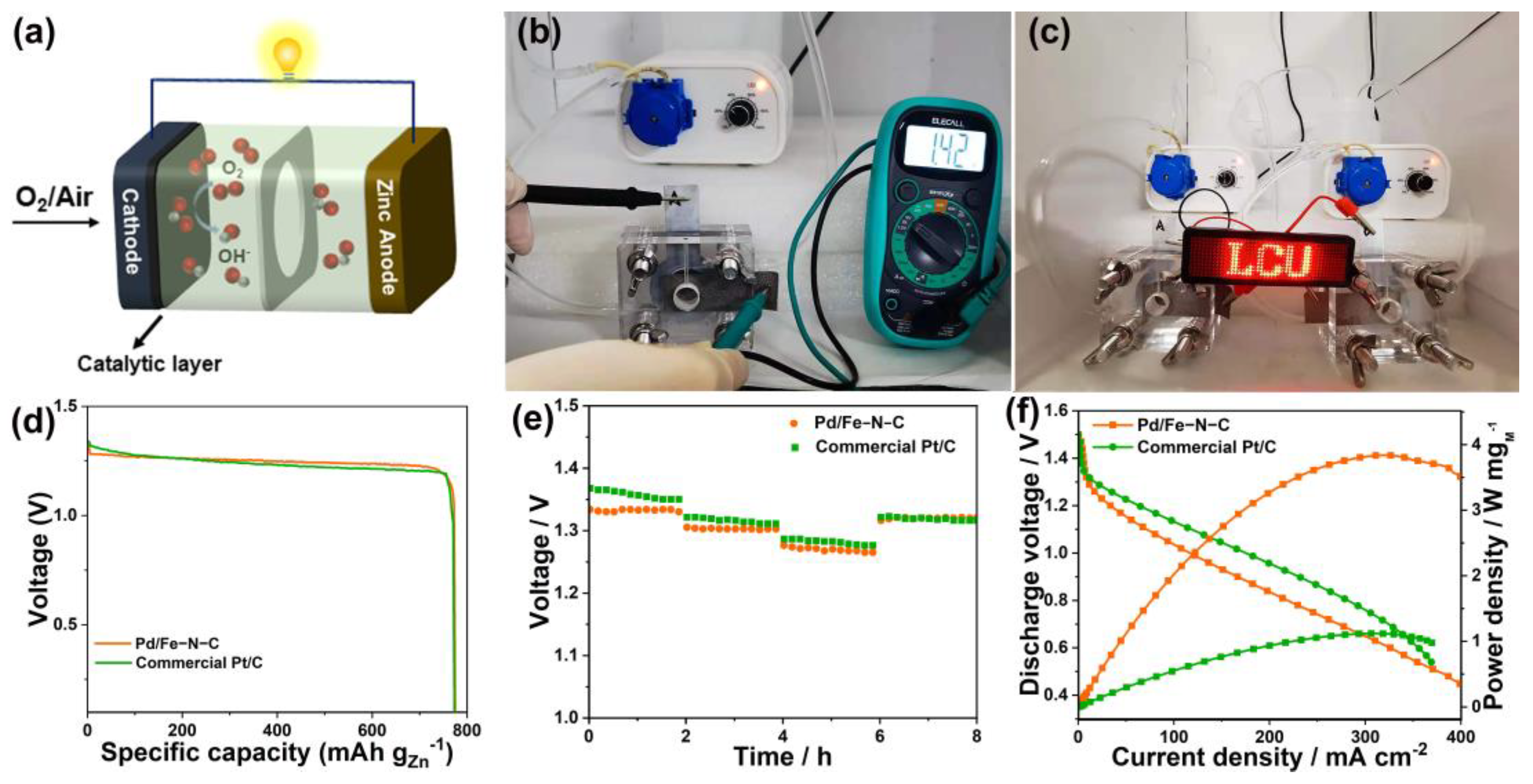
2.3. Zn–Air Battery
3. Experimental Section
3.1. Chemicals
3.2. Synthesis of Pd/Fe-N-C
3.3. Electrochemical Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wu, Y.; Xiao, Z.; Jin, Z.; Li, X.; Chen, Y. The cobalt carbide/bimetallic CoFe phosphide dispersed on carbon nanospheres as advanced bifunctional electrocatalysts for the ORR, OER, and rechargeable Zn-air batteries. J. Colloid Interf. Sci. 2021, 590, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.; Jeon, S. The individual role of pyrrolic, pyridinic and graphitic nitrogen in the growth kinetics of Pd NPs on N-rGO followed by a comprehensive study on ORR. Int. J. Hydrogen Energy 2018, 43, 5690–5702. [Google Scholar] [CrossRef]
- Xiang, W.; Zhao, Y.; Jiang, Z.; Li, X.; Zhang, H.; Sun, Y.; Ning, Z.; Du, F.; Gao, P.; Qian, J.; et al. Palladium single atoms supported by interwoven carbon nanotube and manganese oxide nanowire networks for enhanced electrocatalysis. J. Mater. Chem. A 2018, 6, 23366–23377. [Google Scholar] [CrossRef]
- Nunes, M.; Fernandes, D.M.; Morales, M.V.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A.; Freire, C. Cu and Pd nanoparticles supported on a graphitic carbon material as bifunctional HER/ORR electrocatalysts. Catal. Today 2020, 357, 279–290. [Google Scholar] [CrossRef]
- Fu, Y.; Han, L.; Zheng, P.; Peng, X.; Xian, X.; Liu, J.; Zeng, X.; Dong, P.; Xiao, J.; Zhang, Y. Vanadium nitride supported on N-doped carbon as high-performance ORR catalysts for Zn-air batteries. Catalysts 2022, 12, 877. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, D.; Zhao, X.; Chu, Z.; Zhang, L.; Cao, Y.; Si, W. Pt/Pd decorate MOFs derived Co-N-C materials as high-performance catalysts for oxygen reduction reaction. Catalysts 2022, 12, 482. [Google Scholar] [CrossRef]
- Tang, Z.; Wu, W.; Wang, K. Oxygen reduction reaction catalyzed by noble metal clusters. Catalysts 2018, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.; Wang, L.; Hou, F.; Dou, S.X.; Liang, J. Electrocatalytic oxygen reduction to produce hydrogen peroxide: Rational design from single-atom catalysts to devices. Electrochem. Energy R. 2022, 5, 7. [Google Scholar] [CrossRef]
- Liu, H.; Koenigsmann, C.; Adzic, R.R.; Wong, S.S. Probing ultrathin one-dimensional Pd-Ni nanostructures as oxygen reduction reaction catalysts. ACS Catal. 2014, 4, 2544–2555. [Google Scholar] [CrossRef]
- Jiao, W.; Chen, C.; You, W.; Zhang, J.; Liu, J.; Che, R. Yolk-shell Fe/Fe4 N@Pd/C magnetic nanocomposite as an efficient recyclable ORR electrocatalyst and SERS substrate. Small 2019, 15, 1805032. [Google Scholar] [CrossRef]
- Liu, J.; Fan, X.; Sun, C.Q.; Zhu, W. DFT study on intermetallic Pd-Cu alloy with cover layer Pd as efficient catalyst for oxygen reduction reaction. Materials 2017, 11, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neergat, M.; Gunasekar, V.; Rahul, R. Carbon-supported Pd-Fe electrocatalysts for oxygen reduction reaction (ORR) and their methanol tolerance. Electroanal. Chem. 2011, 658, 25–32. [Google Scholar] [CrossRef]
- Tian, J.; Wu, W.; Tang, Z.; Wu, Y.; Burns, R.; Tichnell, B.; Liu, Z.; Chen, S. Oxygen reduction reaction and hydrogen evolution reaction catalyzed by Pd-Ru nanoparticles encapsulated in porous carbon nanosheets. Catalysts 2018, 8, 329. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Guo, J.; Fang, J. Nanoscale design of Pd-based electrocatalysts for oxygen reduction reaction enhancement in alkaline media. Small Struct. 2022, 3, 2100188. [Google Scholar] [CrossRef]
- Li, Z.; Zhuang, Z.; Lv, F.; Zhu, H.; Zhou, L.; Luo, M.; Zhu, J.; Lang, Z.; Feng, S.; Chen, W.; et al. The marriage of the FeN4 moiety and MXene boosts oxygen reduction catalysis: Fe 3d electron delocalization matters. Adv. Mater. 2018, 30, 1803220. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lai, Y.J.; Song, L.; Zhou, Z.Y.; Liu, J.G.; Wang, Q.; Yang, X.D.; Chen, C.; Shi, W.; Zheng, Y.P.; et al. S-doping of an Fe/N/C ORR catalyst for polymer electrolyte membrane fuel cells with high power density. Angew. Chem. Int. Edit. 2015, 54, 9907–9910. [Google Scholar] [CrossRef]
- Xiao, M.; Zhu, J.; Ma, L.; Jin, Z.; Ge, J.; Deng, X.; Hou, Y.; He, Q.; Li, J.; Jia, Q.; et al. Microporous framework induced synthesis of single-atom dispersed Fe-N-C acidic ORR catalyst and its in situ reduced Fe-N4 active site identification revealed by X-ray absorption spectroscopy. ACS Catal. 2018, 8, 2824–2832. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, P.; Hua, X.; Luo, W.; Chen, S.; Cheng, G. An Fe-N-C hybrid electrocatalyst derived from a bimetal-organic framework for efficient oxygen reduction. J. Mater. Chem. A 2016, 4, 11357–11364. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Priest, C.; Shi, Q.; Wu, G. Atomically dispersed metal-nitrogen-carbon catalysts for fuel cells: Advances in catalyst design, electrode performance, and durability improvement. Chem. Soc. Rev. 2020, 49, 3484–3524. [Google Scholar] [CrossRef]
- Shen, H.; Gracia-Espino, E.; Ma, J.; Zang, K.; Luo, J.; Wang, L.; Gao, S.; Mamat, X.; Hu, G.; Wagberg, T.; et al. Synergistic effects between atomically dispersed Fe-N-C and C-S-C for the oxygen reduction reaction in acidic media. Angew. Chem. Int. Edit. 2017, 56, 13800–13804. [Google Scholar] [CrossRef]
- Xie, X.; He, C.; Li, B.; He, Y.; Cullen, D.A.; Wegener, E.C.; Kropf, A.J.; Martinez, U.; Cheng, Y.; Engelhard, M.H.; et al. Performance enhancement and degradation mechanism identification of a single-atom Co-N-C catalyst for proton exchange membrane fuel cells. Nat. Catal. 2020, 3, 1044–1054. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, M.; Feng, C. A doping-adsorption-pyrolysis strategy for constructing atomically dispersed cobalt sites anchored on a N-doped carbon framework as an efficient bifunctional electrocatalyst for hydrogen evolution and oxygen reduction. RSC Adv. 2022, 12, 20578–20582. [Google Scholar] [CrossRef] [PubMed]
- Lo Vecchio, C.; Sebastián, D.; Lázaro, M.; Aricò, A.; Baglio, V. Methanol-tolerant M-N-C catalysts for oxygen reduction reactions in acidic media and their application in direct methanol fuel cells. Catalysts 2018, 8, 650. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Tang, H.; Gu, H.; Xi, H.; Wu, P.; Liang, B.; Liu, Q.; Chen, W. Research progress of asymmetrically coordinated single-atom catalysts for electrocatalytic reactions. J. Mater. Chem. A 2022, 10, 14732–14746. [Google Scholar] [CrossRef]
- Sahraie, N.R.; Kramm, U.I.; Steinberg, J.; Zhang, Y.; Thomas, A.; Reier, T.; Paraknowitsch, J.P.; Strasser, P. Quantifying the density and utilization of active sites in non-precious metal oxygen electroreduction catalysts. Nat. Commun. 2015, 6, 8618. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Sun, C.; Yan, Y.; Li, F. Understanding the active sites of Fe-N-C materials and their properties in the ORR catalysis system. RSC Adv. 2022, 12, 9543–9549. [Google Scholar] [CrossRef]
- Shui, H.; Jin, T.; Hu, J.; Liu, H. In situ incorporation strategy for bimetallic FeCo-doped carbon as highly efficient bifunctional oxygen electrocatalysts. ChemElectroChem 2018, 5, 1401–1406. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, W.; Li, Z.; Chen, Y.; Zheng, L.; Gong, Y.; Li, Q.; Shen, R.; Han, Y.; Cheong, W.C.; et al. Isolated Fe and Co dual active sites on nitrogen-doped carbon for a highly efficient oxygen reduction reaction. Chem. Commun. 2018, 54, 4274–4277. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, X.; Zhang, Y.; Lu, Y.; Liu, X.; Zheng, Y.; Zhang, Y.; Lin, J. Fe/Co/N-C/graphene derived from Fe/ZIF-67/graphene oxide three dimensional frameworks as a remarkably efficient and stable catalyst for the oxygen reduction reaction. RSC Adv. 2022, 12, 2425–2435. [Google Scholar] [CrossRef]
- Liu, M.; Li, N.; Cao, S.; Wang, X.; Lu, X.; Kong, L.; Xu, Y.; Bu, X.H. A “pre-constrained metal twins” strategy to prepare efficient dual-metal-atom catalysts for cooperative oxygen electrocatalysis. Adv. Mater. 2022, 34, 2107421. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, H.; Meng, T.; Liao, K.; Meng, W.; Yang, J.; He, D.; Xiong, Y.; Mu, S. Fe, Cu-coordinated ZIF-derived carbon framework for efficient oxygen reduction reaction and Zinc-air batteries. Adv. Funct. Mater. 2018, 28, 1802596. [Google Scholar] [CrossRef]
- Feng, M.; Wu, X.; Cheng, H.; Fan, Z.; Li, X.; Cui, F.; Fan, S.; Dai, Y.; Lei, G.; He, G. Well-defined Fe-Cu diatomic sites for efficient catalysis of CO2 electroreduction. J. Mater. Chem. A 2021, 9, 23817–23827. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Liu, W.; Chang, C.; Tang, H.; Li, Z.; Chen, W.; Jia, C.; Yao, T.; Wei, S.; et al. Design of N-coordinated dual-metal sites: A stable and active Pt-free catalyst for acidic oxygen reduction reaction. J. Am. Chem. Soc. 2017, 139, 17281–17284. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wu, J.; Li, Y.; Li, W.; Zhai, J.; Jiang, Q.; Xu, X.; Gao, Y. Enhanced oxygen reduction with carbon-polyhedron-supported discrete cobalt-nitrogen sites for Zn-air batteries. Chem. Eng. J. 2022, 431, 134084. [Google Scholar] [CrossRef]
- Jung, J.Y.; Kim, S.; Kim, J.-G.; Kim, M.J.; Lee, K.-S.; Sung, Y.-E.; Kim, P.; Yoo, S.J.; Lim, H.-K.; Kim, N.D. Hierarchical porous single-wall carbon nanohorns with atomic-level designed single-atom Co sites toward oxygen reduction reaction. Nano Energy 2022, 97, 107206. [Google Scholar] [CrossRef]
- Liu, D.; Tong, Y.; Yan, X.; Liang, J.; Dou, S.X. Recent advances in carbon-based bifunctional oxygen catalysts for zinc-air batteries. Batter. Supercaps 2019, 2, 743–765. [Google Scholar] [CrossRef]
- Zhu, Z.; Cui, J.; Cao, X.; Yang, L.; Sun, H.; Liang, W.; Li, J.; Li, A. Synthesis and electrocatalytic properties of M (Fe, Co), N co-doped porous carbon frameworks for efficient oxygen reduction reaction. Int. J. Hydrogen Energy 2022, 47, 9504–9516. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, X.; Liu, J.; Zhang, J.; Xu, D.; Peng, W.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Rational design of Fe/N/S-doped nanoporous carbon catalysts from covalent triazine frameworks for efficient oxygen reduction. ChemSusChem 2018, 11, 2402–2409. [Google Scholar] [CrossRef]
- Gao, C.; Mu, S.; Yan, R.; Chen, F.; Ma, T.; Cao, S.; Li, S.; Ma, L.; Wang, Y.; Cheng, C. Recent advances in ZIF-Derived atomic metal-N-C Electrocatalysts for oxygen reduction reaction: Synthetic strategies, active centers, and stabilities. Small 2022, 18, 2105409. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y.; Wang, X.; Lu, Z.; Guo, C.; Shi, Y.; Tan, H.; Shen, L.; Cao, S.; Yan, C. A three-dimensional ordered honeycomb nanostructure anchored with Pt-N active sites via self-assembly of a block copolymer: An efficient electrocatalyst towards the oxygen reduction reaction in fuel cells. J. Mater. Chem. A 2022, 10, 12141–12149. [Google Scholar] [CrossRef]
- Qin, J.; Liu, H.; Zou, P.; Zhang, R.; Wang, C.; Xin, H.L. Altering ligand fields in single-atom sites through second-shell anion modulation boosts the oxygen reduction reaction. J. Am. Chem. Soc. 2022, 144, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, G.; Wang, M.; Wang, Q.; Li, B.; Zhou, H.; Zhu, Y.; Zhang, W.; Omer, M.; Orlovskaya, N.; et al. Improving Pd-N-C fuel cell electrocatalysts through fluorination-driven rearrangements of local coordination environment. Nat. Energy 2021, 6, 1144–1153. [Google Scholar] [CrossRef]
- Deng, Y.; Huangfu, H.; Tang, S.; Li, J. High performance ORR electrocatalysts prepared via one-step pyrolysis of riboflavin. Chin. J. Catal. 2017, 38, 1668–1679. [Google Scholar] [CrossRef]
- Dong, X.; Li, J.; Wei, D.; Li, R.; Qu, K.; Wang, L.; Xu, S.; Kang, W.; Li, H. Pd(II)/Ni(II)-dimethylglyoxime derived Pd-Ni-P@N-doped carbon hybrid nanocatalysts for oxygen reduction reaction. Appl. Surf. Sci. 2019, 479, 273–279. [Google Scholar] [CrossRef]
- Goswami, C.; Saikia, H.; Jyoti Borah, B.; Jyoti Kalita, M.; Tada, K.; Tanaka, S.; Bharali, P. Boosting the electrocatalytic activity of Pd/C by Cu alloying: Insight on Pd/Cu composition and reaction pathway. J. Colloid Interf. Sci. 2021, 587, 446–456. [Google Scholar] [CrossRef]
- Chen, C.; Su, H.; Lu, L.-N.; Hong, Y.-S.; Chen, Y.; Xiao, K.; Ouyang, T.; Qin, Y.; Liu, Z.-Q. Interfacing spinel NiCo2O4 and NiCo alloy derived N-doped carbon nanotubes for enhanced oxygen electrocatalysis. Chem. Eng. J. 2021, 408, 127814. [Google Scholar] [CrossRef]
- Najam, T.; Shah, S.S.A.; Ding, W.; Jiang, J.; Jia, L.; Yao, W.; Li, L.; Wei, Z. An Efficient Anti-poisoning Catalyst against SOx, NOx, and POx: P, N-doped carbon for oxygen reduction in acidic media. Angew. Chem. Int. Edit. 2018, 57, 15101–15106. [Google Scholar] [CrossRef]
- Jiang, X.; Elouarzaki, K.; Tang, Y.; Zhou, J.; Fu, G.; Lee, J.-M. Embedded PdFe@N-carbon nanoframes for oxygen reduction in acidic fuel cells. Carbon 2020, 164, 369–377. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.; Zhang, L.-C.; Chen, H.; Zhu, H.; Wu, Y.; Xu, M.; Bao, S.-J. Highly efficient Fe-N-C oxygen reduction electrocatalyst engineered by sintering atmosphere. J. Power Sources 2020, 449, 227497. [Google Scholar] [CrossRef]
- Cai, H.; Chen, B.; Zhang, X.; Deng, Y.; Xiao, D.; Ma, D.; Shi, C. Highly active sites of low spin FeIIN4 species: The identification and the ORR performance. Nano Res. 2020, 14, 122–130. [Google Scholar] [CrossRef]
- Han, C.; Yi, W.; Feng, S.; Li, Z.; Song, H. Single-atom palladium anchored N-doped carbon towards oxygen electrocatalysis for rechargeable Zn-air batteries. Dalton Trans. 2022, 51, 12314–12323. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Sun, X.-L.; Zhao, J.; Gong, B.-Q.; Bao, Z.-L.; Jia, H.-L.; Guan, M.-Y.; Ma, S.-S. A highly efficient bifunctional electrocatalyst (ORR/OER) derived from GO functionalized with carbonyl, hydroxyl and epoxy groups for rechargeable zinc-air batteries. New J. Chem. 2021, 45, 6535–6542. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wang, Z.; Yang, C.; Zou, G.; Liu, S.; Sun, Z.; Wang, L.; Li, R.; Qu, K.; Kang, W.; et al. Electrocatalytic Oxygen Reduction Reaction by the Pd/Fe-N-C Catalyst and Application in a Zn–Air Battery. Catalysts 2022, 12, 1640. https://doi.org/10.3390/catal12121640
Chen J, Wang Z, Yang C, Zou G, Liu S, Sun Z, Wang L, Li R, Qu K, Kang W, et al. Electrocatalytic Oxygen Reduction Reaction by the Pd/Fe-N-C Catalyst and Application in a Zn–Air Battery. Catalysts. 2022; 12(12):1640. https://doi.org/10.3390/catal12121640
Chicago/Turabian StyleChen, Jiabao, Zhongqing Wang, Chunxiang Yang, Guangchao Zou, Shuhua Liu, Zhiran Sun, Lei Wang, Rui Li, Konggang Qu, Wenjun Kang, and et al. 2022. "Electrocatalytic Oxygen Reduction Reaction by the Pd/Fe-N-C Catalyst and Application in a Zn–Air Battery" Catalysts 12, no. 12: 1640. https://doi.org/10.3390/catal12121640




