Magnetic TiO2/Fe3O4-Chitosan Beads: A Highly Efficient and Reusable Catalyst for Photo-Electro-Fenton Process
Abstract
:1. Introduction
2. Results
2.1. Optimization of Operational Parameters
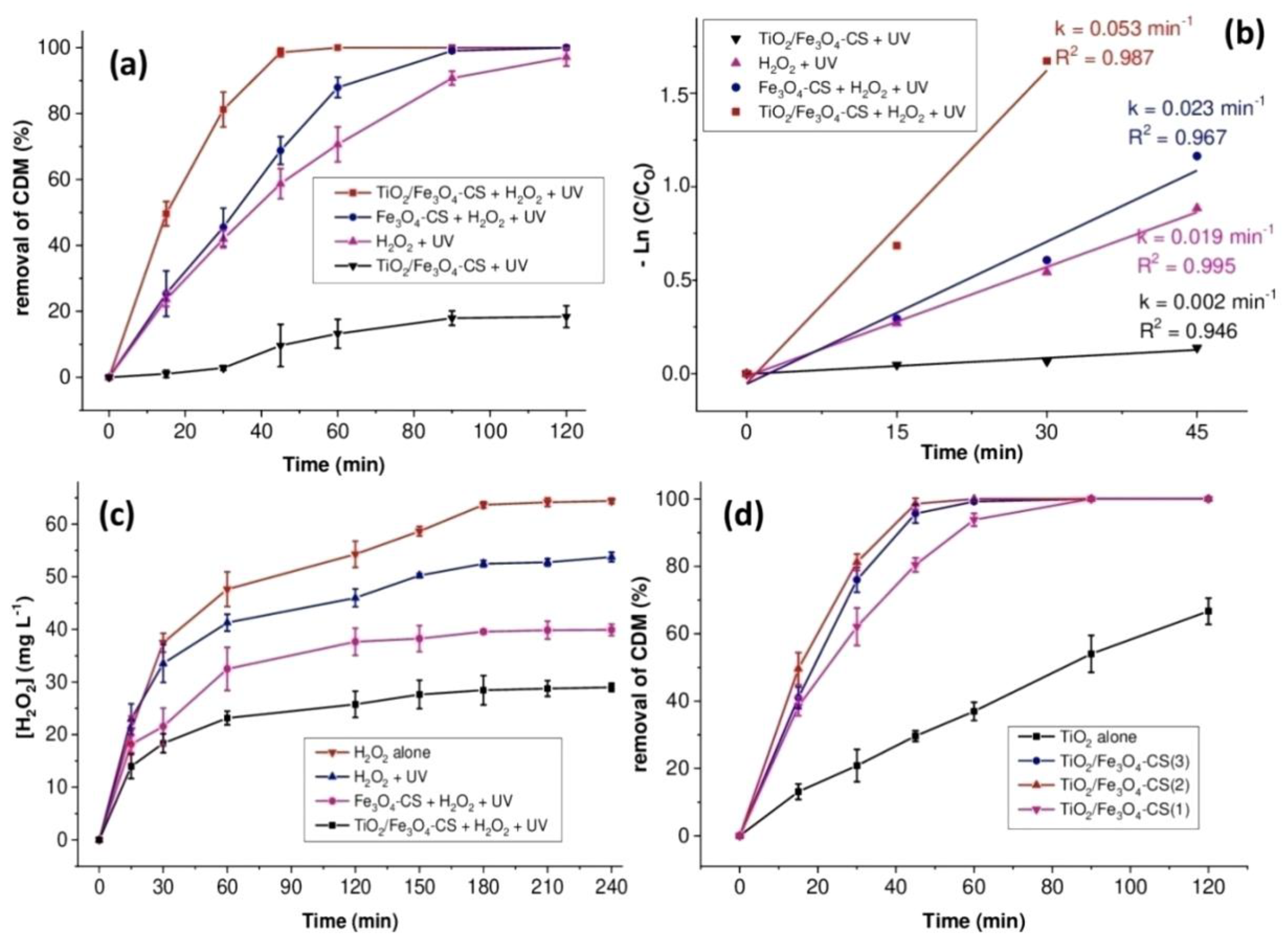
2.1.1. Performance Comparison of Several Processes for the Removal of CDM
2.1.2. Influence of TiO2 in Magnetic Chitosan Beads
2.1.3. Effect of TiO2 Loading Content into Chitosan Beads
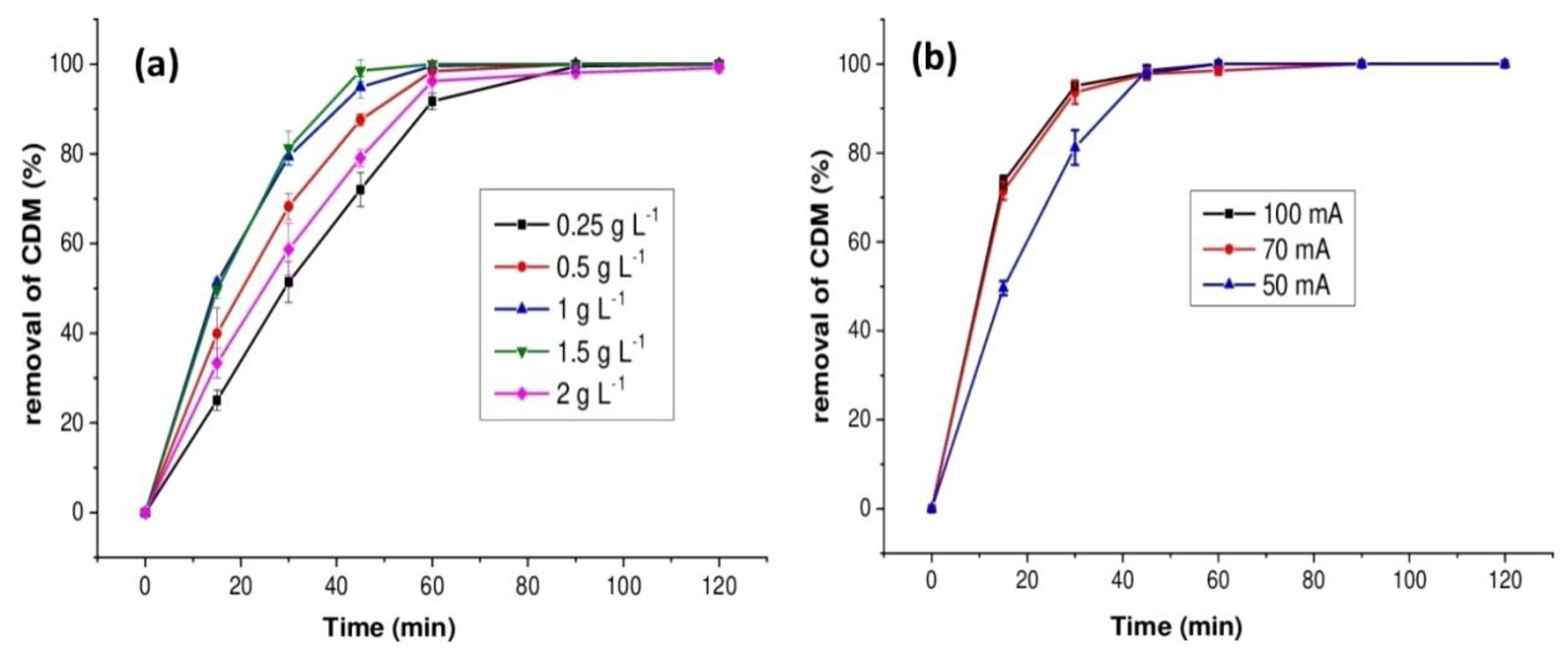
2.1.4. Effect of Catalyst Dosage
2.1.5. Effect of the Current Intensity
2.2. Treatment of Wastewater Doped with Chlordimeform by Photo-Electro-Fenton Process
2.3. Evaluation of Organic Acids and Minerals Produced during Treatment
2.4. Stability of the Catalyst
2.4.1. Catalytic Stability
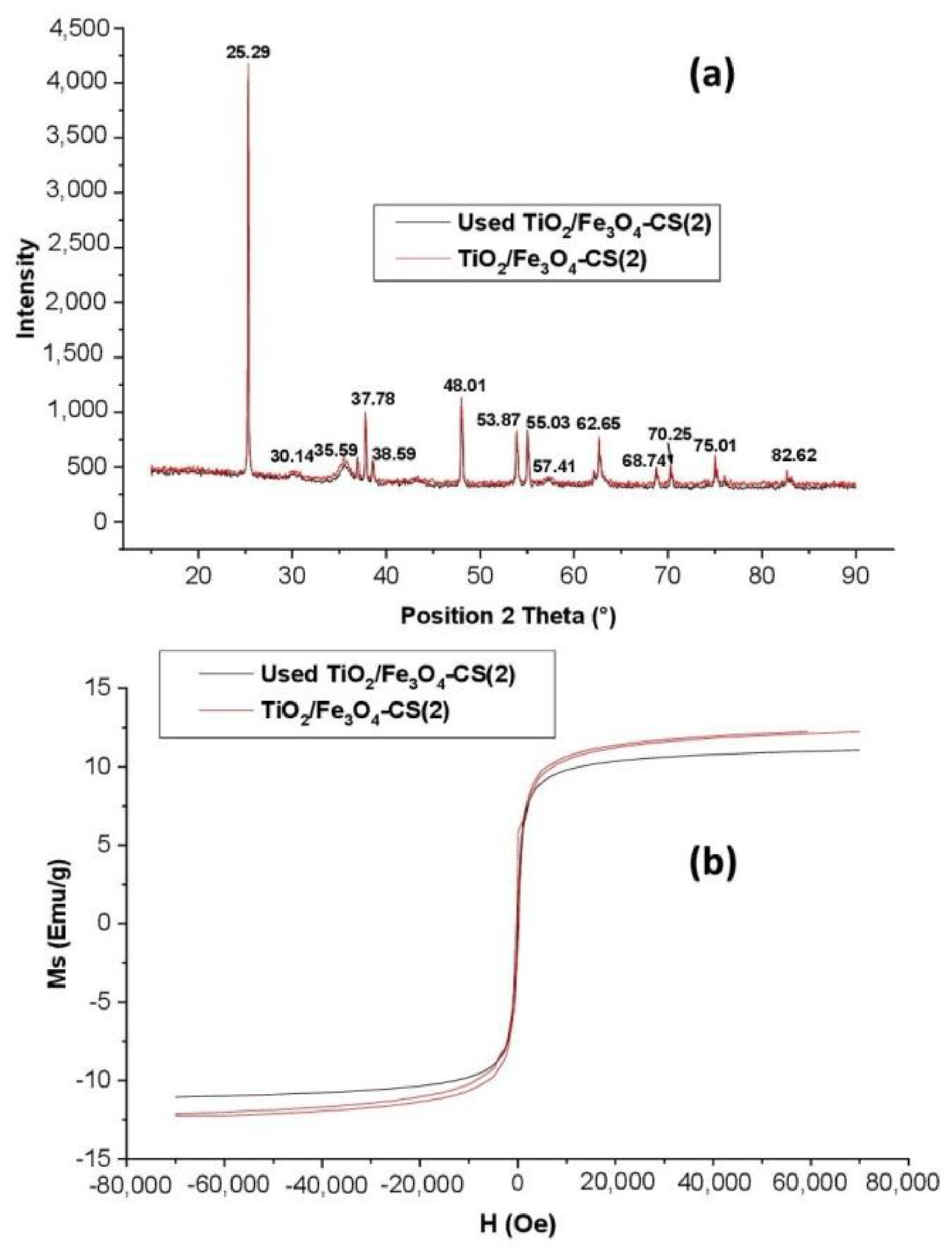
2.4.2. Characterization of Catalyst before and after Use
SEM Analysis
XRD Analysis
Magnetic Properties Analysis
3. Materials and Methods
3.1. Chemical Products
3.2. Preparation of TiO2/Fe3O4-Chitosan Magnetic Beads
3.3. CDM Removal Assays
3.4. Analytical Methods
3.4.1. Determination of CDM Concentration
3.4.2. Determination of Carboxylic Acids Concentrations
3.4.3. Determination of Ions Concentrations
3.4.4. Total Organic Carbon Measurements
3.4.5. Determination of Fe Concentration
3.4.6. Determination of H2O2 Concentration
3.4.7. Characterization of the Synthesized Catalysts
3.5. Specific Energy Consumption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Malakootian, M.; Shahesmaeili, M.A.; Fraji, M.; Amiri, H.; Silva Martinez, S. Advanced oxidation processes for the removal of organophosphorus pesticides in aqueous matrices: A systematic review and meta-analysis. Process. Saf. Environ. Prot. 2020, 134, 292–307. [Google Scholar] [CrossRef]
- Torres, N.H.; de Oliveira Santiago Santos, G.; Ferreira, L.F.R.; Américo-Pinheiro, J.H.P.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Environmental aspects of hormones estriol, 17β-estradiol and 17α-ethinylestradiol: Electrochemical processes as next-generation technologies for their removal in water matrices. Chemosphere 2021, 267, 128888. [Google Scholar] [CrossRef] [PubMed]
- Trellu, C.; Olvera Vargas, H.; Mousset, E.; Oturan, N.; Oturan, M.A. Electrochemical technologies for the treatment of pesticides. Curr. Opin. Electrochem. 2021, 26, 100677. [Google Scholar] [CrossRef]
- Rajasekhar, B.; Venkateshwaran, U.; Durairaj, N.; Divyapriya, G.; Nambi, I.M.; Joseph, A. Comprehensive treatment of urban wastewaters using electrochemical advanced oxidation process. J. Environ. Manag. 2022, 266, 110469. [Google Scholar] [CrossRef] [PubMed]
- Heidari, Z.; Pelalak, R.; Alizadeh, R.; Oturan, N.; Shirazian, S.; et Oturan, M.A. Application of Mineral Iron-Based Natural Catalysts in Electro-Fenton Process: A Comparative Study. Catalysts 2021, 11, 1–18. [Google Scholar] [CrossRef]
- Ganzenko, O.; Trrellu, C.; Oturan, N.; Huguenot, D.; Péchaud, Y.; Van Hullebusch, E.D.; Oturan, M.A. Electro-Fenton treatment of a complex pharmaceutical mixture: Mineralization efficiency and biodegradability enhancement. Chemosphere 2020, 253, 126659. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Aneggi, E.; et Goi, D. Catalytic activity of metals in heterogeneous Fenton-like oxidation of wastewater contaminants: A review. Environ. Chem. Lett. 2021, 19, 2405–2424. [Google Scholar] [CrossRef]
- Guo, W.; Li, T.; Chen, Q.; Wan, J.; Zhang, J.; Wu, B.; Wang, Y. The roles of wavelength in the gaseous toluene removal with OH from UV activated Fenton reagent. Chemosphere 2021, 275, 129998. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. Advanced oxidation of phenol: A comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro-Fenton processes. J. Chem. Eng. 2012, 183, 1–9. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. Removal of phenol by heterogenous photo electro Fenton-like process using nano-zero valent iron. Sep. Purif. Technol. 2012, 98, 130–135. [Google Scholar] [CrossRef]
- Chu, A.C.; Sahu, R.S.; Chou, T.H.; Shih, Y. Magnetic Fe3O4@TiO2 nanocomposites to degrade bisphenol A, one emerging contaminant, under visible and long wavelength UV light irradiation. J. Environ. Chem. Eng. 2021, 9, 105539. [Google Scholar] [CrossRef]
- Sun, Q.; Hong, Y.; Liu, Q.; Dong, L. Synergistic operation of photocatalytic degradation and Fenton process by magnetic Fe3O4 loaded TiO2. Appl. Surf. Sci. 2018, 430, 399–406. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; Rehman, M.N.; Khan, S.A.; Koc, M.; Batool, S.; Hasan, M.; Iqbal, F. Dual Z-scheme core-shell PANI-CeO2-Fe2O3-NiO heterostructured nanocomposite for dyes remediation under sunlight and bacterial disinfection. Environ. Res. 2022, 215, 114140. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; Khan, S.A.; Koc, M.; Batool, S.; Hasan, M.; Iqbal, F. Enhanced sunlight-absorption of Fe2O3 covered by PANI for the photodegradation of organic pollutants and antimicrobial inactivation. Adv. Powder Technol. 2022, 33, 103708. [Google Scholar] [CrossRef]
- Goswami, A.; Jiang, J.-Q.; Petri, M. Treatability of five micro-pollutants using modified Fenton reaction catalyzed by zero-valent iron powder (Fe(0)). J. Environ. Chem. Eng. 2021, 9, 105393. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.; Crittenden, J.C.; Shen, C. Novel RGO/α-FeOOH supported catalyst for Fenton oxidation of phenol at a wide pH range using solar-light-driven irradiation. J. Hazard. Mater. 2017, 239, 321–329. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; He, D.; Pan, X. Application of iron-based materials in heterogeneous advanced oxidation processes for wastewater treatment: A review. J. Chem. Eng. 2021, 407, 127191. [Google Scholar] [CrossRef]
- Dudchenko, N.; Pawar, S.; Perelshtein, I.; Fixler, D. Magnetite Nanoparticles: Synthesis and Applications in Optics and Nanophotonics. Materials 2022, 15, 2601. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.-V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Madhav, V.M.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; Rehman, M.N.; Batool, S.; Hasan, M.; Riaz, M.; Rehman, K.; Iqbal, F. Highly efficient tri-phase TiO2-Y2O3-V2O5 nanaocomposite: Structural, optical, photocatalyst and antibacterial studies. J. Nanostruct. Chem. 2022, 12, 547–564. [Google Scholar] [CrossRef]
- Liu, C.; Dai, H.; Tan, C.; Pan, Q.; Hu, F.; Peng, X. Photo-Fenton degradation of tetracycline over Z-sheme Fe-g-C3N4/Bi2WO6 heterojunctions: Mechanism insight, degradation pathways and DFT calculation. Appl. Catal. B 2022, 310, 121326. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, Q.; Mao, J.; Ouyang, X.; Yuan, Z.; Song, X.; Gong, W.; Mei, S.; Xu, W. Study on Photocatalytic Degradation of Acid Red 73 by Fe3O4@TiO2 Exposed (001) Facets. Appl. Sci. 2022, 12, 3574. [Google Scholar] [CrossRef]
- Rezgui, S.; Díez, A.M.; Monser, L.; Adhoum, N.; Pazos, M.; et Sanromán, M.A. ZnFe2O4-chitosan magnetic beads for the removal of chlordimeform by photo-Fenton process under UVC irradiation. J. Environ. Manag. 2021, 283, 111987. [Google Scholar] [CrossRef] [PubMed]
- Rezgui, S.; Amrane, A.; Fourcade, F.; Assadi, A.; Monser, L.; Adhoum, N. Electro-Fenton catalyzed with magnetic chitosan beads for the removal of Chlordimeform insecticide. Appl. Catal. B 2018, 226, 346–359. [Google Scholar] [CrossRef]
- Lee, M.; Chen, B.-Y.; Den., W. Chitosan as a Natural Polymer for Heterogeneous Catalysts Support: A. Short Review on Its Applications. Appl. Sci. 2015, 5, 1272–1283. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Jia, S.; Wu, Q.; Wu, S.; Ran, J.; Lui, Y.; Hou, J. Studies of the magnetic field intensity on the synthesis of chitosan-coated magnetite nanocomposites by co-precipitation method. Mater. Sci. Eng. C 2012, 32, 381–384. [Google Scholar] [CrossRef]
- Donadel, K.; Felisberto, M.D.V.; Fávere, V.T.; Rigoni, M.; Batistela, N.J.; Laranjeira, C.M.C. Synthesis and characterization of the iron oxide magnetic particles coated with chitosan biopolymer. Mater. Sci. Eng. C 2008, 28, 509–514. [Google Scholar] [CrossRef]
- Moura, F.C.C.; Araujo, M.H.; Costa, R.C.C.; Ardisson, J.D.; Macedo, W.A.A.; Lago, R.M. Efficient use of Fe metal as an electron transfer agent in a heterogeneous Fenton system based on Fe0/Fe3O4 composites. Chemosphere 2005, 60, 1118–1123. [Google Scholar] [CrossRef]
- Sun, Q.; Leng, W.; Li, Z.; Xu, Y. Effect of surface Fe2O3 clusters on the photocatalytic activity of TiO2 for phenol degradation in water. J. Hazard. Mater. 2012, 229–230, 224–232. [Google Scholar] [CrossRef]
- Afzal, S.; Julkapli, N.M.; Mun, L.K. Visible light active TiO2/CS/Fe3O4 for nitrophenol degradation: Studying impact of TiO2, CS and Fe3O4 loading on the optical and photocatalytic performance of nanocomposite. Mater. Sci. Semicond. Process. 2021, 131, 105891. [Google Scholar] [CrossRef]
- Díez, A.M.; Pazos, M.; Sanromán, M.A. Synthesis of magnetic-photo-Fenton catalyst for degradation of emerging pollutant. Catal. Today 2019, 328, 267–273. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, H. Chemical kinetic modeling of organic pollutant degradation in Fenton and solar photo-Fenton processes. J. Taiwan Inst. Chem. Eng. 2021, 123, 175–184. [Google Scholar] [CrossRef]
- Burbano, A.A.; Dionysiou, D.D.; Suidan, M.T.; Richardson, T.L. Oxidation kinetics and effect of pH on the degradation of MTBE with Fenton reagent. Water Res. 2016, 39, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Molamahmood, H.V.; Geng, W.; Wei, Y.; Miao, J.; Yu, S.; Shahi, A.; Chen, C.; Long, M. Catalyzed H2O2 decomposition over iron oxides and oxyhydroxides: Insights from oxygen production and organic degradation. Chemosphere 2022, 291, 133037. [Google Scholar] [CrossRef]
- Li, Q.; Kong, H.; Jia, R.; Shao, J.; He, Y. Enhanced catalytic degradation of amoxicillin with TiO2–Fe3O4 composites via a submerged magnetic separation membrane photocatalytic reactor (SMSMPR). RSC Adv. 2019, 9, 12538–12546. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Kong, H.; Li, P.; Shao, J.; He, Y. Photo-Fenton degradation of amoxicillin via magnetic TiO2-graphene oxide-Fe3O4 composite with a submerged magnetic separation membrane photocatalytic reactor (SMSMPR). J. Hazard. Mater. 2019, 373, 437–446. [Google Scholar] [CrossRef]
- Bai, X.; Lyu, L.; Ma, W.; Ye, Z. Heterogeneous UV/Fenton degradation of bisphenol A catalyzed by synergistic effects of FeCo2O4/TiO2/GO. Environ. Sci. Pollut. Res. 2016, 23, 22734–22743. [Google Scholar] [CrossRef]
- Nwe, T.S.; Sikong, L.; Kokoo, R.; Khangkhamano, M. Photocatalytic activity enhancement of Dy-doped TiO2 nanoparticles hybrid with TiO2 (B) nanobelts under UV and fluorescence irradiation. Curr. Appl. Phys. 2016, 20, 249–254. [Google Scholar] [CrossRef]
- Nam, Y.; Lim, J.H.; Ko, K.C.; Lee, J.K. Photocatalytic activity of TiO2 nanoparticles: A theoretical aspect. J. Mater. Chem. A 2019, 7, 13833–13859. [Google Scholar] [CrossRef]
- Enesca, A.; Isac, L. The Influence of Light Irradiation on the Photocatalytic Degradation of Organic Pollutants. Materials 2020, 11, 2494. [Google Scholar] [CrossRef] [PubMed]
- Boruah, P.K.; Das, M.R. Dual responsive magnetic Fe3O4-TiO2/graphene nanocomposite as an artificial nanozymes for the colorimetric detection and photodegradation of pesticide in an aqueous medium. J. Hazard. Mater. 2020, 385, 121516. [Google Scholar] [CrossRef] [PubMed]
- Pourzad, A.; Sobhi, H.R.; Behbahani, M.; Esrafili, A.; Kalantary, R.R.; Kermani, M. Efficient visible light-induced photocatalytic removal of paraquat using N-doped TiO2@SiO2@Fe3O4 nanocomposite. J. Mol. Liq. 2020, 299, 112467. [Google Scholar] [CrossRef]
- Zahedifar, M.; Seyedi, N. Bare 3D-TiO2/magnetic biochar dots (3D-TiO2/BCDs MNPs): Highly efficient recyclable photocatalyst for diazinon degradation under sunlight irradiation. Phys. E Low-Dimens. Syst. Nanostruct. 2022, 139, 115151. [Google Scholar] [CrossRef]
- Hua, Y.; Wang, S.; Xiao, J.; Cui, C.; Wang, C. Preparation and characterization of Fe3O4/gallic acid/graphene oxide magnetic nanocomposites as highly efficient Fenton catalysts. RSC Adv. 2017, 7, 28979–28986. [Google Scholar] [CrossRef] [Green Version]
- Kasiri, M.B.; Aleboyeh, H.; Aleboyeh, A. Degradation of Acid Blue 74 using Fe-ZSM5 zeolite as a heterogeneous photo-Fenton catalyst. Appl. Catal. B 2008, 84, 9–15. [Google Scholar] [CrossRef]
- Tekbaş, M.; Yatmaz, H.C.; Bektaş, N. Heterogeneous photo-Fenton oxidation of reactive azo dye solutions using iron exchanged zeolite as a catalyst. Microporous Mesoporous Mater. 2008, 115, 594–602. [Google Scholar] [CrossRef]
- Jiang, B.; Niu, Q.; Li, C.; Oturan, N.; Oturan, M.A. Outstanding performance of electro-Fenton process for efficient decontamination of Cr(III) complexes via alkaline precipitation with no accumulation of Cr(VI): Important roles of iron species. Appl. Catal. B 2020, 272, 119002. [Google Scholar] [CrossRef]
- Hammouda, S.B.; Amrane, A.; Fourcade, F.; Assadi, A.; Adhoum, N.; Monser, L. Effective heterogeneous electro-Fenton process for the degradation of a malodorous compound indole using iron loaded alginate beads as a reusable catalyst. Appl. Catal. B 2016, 182, 47–58. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Wei, Y.; Liu, B.; Lin, Y.; Li, G.; Zhang, F. Enhanced degradation of ibuprofen by heterogeneous electro-Fenton at circumneutral pH. Chemosphere 2018, 209, 998–1006. [Google Scholar] [CrossRef]
- Chmayssem, A.; Taha, S.; Hauchard, D. Scaled-up electrochemical reactor with a fixed bed three-dimensional cathode for electro-Fenton process: Application to the treatment of bisphenol A. Electrochim. Acta 2017, 225, 435–442. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R. Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination 2012, 299, 1–15. [Google Scholar] [CrossRef]
- Kang, B.; Dai, Y.; Zhang, H.; Chen, D. Synergetic degradation of chitosan with gamma radiation and hydrogen peroxide. Polym. Degrad. Stab. 2007, 92, 359–362. [Google Scholar] [CrossRef]
- Wang, S.-M.; Huang, Q.-Z.; Wang, Q.-S. Study on the synergetic degradation of chitosan with ultraviolet light and hydrogen peroxide. Carbohydr. Res. 2005, 340, 1143–1147. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Ghanbari, F.; Yousefi, M.; Madihi-Bidgoli, S. Photocatalytic degradation of food dye by Fe3O4–TiO2 nanoparticles in presence of peroxymonosulfate: The effect of UV sources. J. Environ. Chem. Eng. 2017, 5, 2459–2468. [Google Scholar] [CrossRef]
- Maktabifard, M.; Zaborowska, E.; Makinia, J. Achieving energy neutrality in wastewater treatment plants through energy savings and enhancing renewable energy production. Rev. Environ. Sci. Biotechnol. 2018, 17, 655–689. [Google Scholar] [CrossRef] [Green Version]
- Ghazouani, M.; Bousselmi, L.; Akrout, H. Combined electrocoagulation and electrochemical treatment on BDD electrodes for simultaneous removal of nitrates and phosphates. J. Environ. Chem. Eng. 2020, 8, 104509. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M.A. comparative study of electrocoagulation, electrochemical Fenton, electro-Fenton and peroxi-coagulation for decolorization of real textile wastewater: Electrical energy consumption and biodegradability improvement. J. Environ. Chem. Eng. 2015, 3, 499–506. [Google Scholar] [CrossRef]
- Gerek, E.E.; Yılmaz, S.; Koparal, A.S.; Gerek, Ö.N. Combined energy and removal efficiency of electrochemical wastewater treatment for leather industry. J. Water Process. Eng. 2019, 30, 100382. [Google Scholar] [CrossRef]
- Flores, N.; Sirés, I.; Garrido, J.A.; Centellas, F.; Rodriguez, R.M.; Cabot, P.L.; Brillas., E. Degradation of trans-ferulic acid in acidic aqueous medium by anodic oxidation, electro-Fenton and photoelectro-Fenton. J. Hazard. Mater. 2016, 319, 3–12. [Google Scholar] [CrossRef]
- Hammami, S.; Oturan, N.; Bellakhal, N.; Dachraoui, M.; Oturan, M.A. Oxidative degradation of direct orange 61 by electro-Fenton process using a carbon felt electrode: Application of the experimental design methodology. J. Electroanal. Chem. 2007, 610, 75–84. [Google Scholar] [CrossRef]
- Aplin, R.; Feitz, A.J.; Waite, T.D. Effect of Fe(III)-ligand properties on effectiveness of modified photo-Fenton processes. Water Sci. Technol. 2001, 44, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Zazou, H.; Oturan, N.; Zhang, H.; Hamdani, M.; Oturan, M.A. Comparative study of electrochemical oxidation of herbicide 2,4,5-T: Kinetics, parametric optimization and mineralization pathway. Sustain. Environ. Res. 2017, 27, 15–23. [Google Scholar] [CrossRef]
- Mhemdi, A.; Oturan, M.A.; Oturan, N.; Abdelhédi, R.; Ammar, S. Electrochemical advanced oxidation of 2-chlorobenzoic acid using BDD or Pt anode and carbon felt cathode. J. Electroanal. Chem. 2013, 709, 111–117. [Google Scholar] [CrossRef]
- Skoumal, M.; Arias, C.; Cabot, P.L.; Centellas, F.; Garrido, J.A.; Rodriguez, R.M.; Brillas, E. Mineralization of the biocide chloroxylenol by electrochemical advanced oxidation processes. Chemosphere 2008, 71, 1718–1729. [Google Scholar] [CrossRef]
- Arai, M.; Zhao, F. Metal Catalysts Recycling and Heterogeneous/Homogeneous Catalysis. Catalysts 2015, 5, 868–870. [Google Scholar] [CrossRef] [Green Version]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef]
- Rusevova, K.; Kopinke, F.-D.; Georgi, A. Nano-sized magnetic iron oxides as catalysts for heterogeneous Fenton-like reactions—Influence of Fe(II)/Fe(III) ratio on catalytic performance. J. Hazard. Mater. 2012, 241–242, 433–440. [Google Scholar] [CrossRef]
- Gijs, M.A.M. Magnetic bead handling on-chip: New opportunities for analytical applications. Microfluid. Nanofluid. 2004, 1, 22–40. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, M.; Guo, M.; Wang, X. Preparation and properties of a nano TiO2/Fe3O4 composite superparamagnetic photocatalyst. Rare Metals 2009, 28, 423–427. [Google Scholar] [CrossRef]
- Almuaibed, A.M.; Townshend, A. Flow spectrophotometric method for determination of hydrogen peroxide using a cation exchanger for preconcentration. Anal. Chim. Acta 1994, 295, 159–163. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B 2009, 87, 105–145. [Google Scholar] [CrossRef]





| Parameter | Assay | Rate of TOC Removal after 4 h of Treatment |
|---|---|---|
| Performance comparison of several processes | TiO2/Fe3O4-CS(2) + UV-LED | 4.4 ± 1.6 |
| H2O2 + UV-LED (Without catalyst) | 18.2 ± 3.3 | |
| TiO2/Fe3O4-CS(2) + H2O2 + UV-LED | 69.6 ± 2.7 | |
| Influence of TiO2 in magnetic chitosan beads | Fe3O4-CS + H2O2 + UV-LED | 33.9 ± 4.1 |
| TiO2/Fe3O4-CS(2) + H2O2 + UV-LED | 69.6 ± 2.7 | |
| Effect of TiO2 loading content into magnetic chitosan beads | Molar ratio TiO2/Fe = 1 | 38.2 ± 3.5 |
| Molar ratio TiO2/Fe = 2 | 69.6 ± 2.7 | |
| Molar ratio TiO2/Fe = 3 | 54.2 ± 5.6 | |
| Catalytic beads of TiO2 | 24.5 ± 1.9 | |
| Effect of catalyst dosage | 0.25 g L−1 | 40.9 ± 4.1 |
| 0. 5 g L−1 | 60.8 ± 3.2 | |
| 1 gL−1 | 69.6 ± 2.7 | |
| 1.5 g L−1 | 70.2 ± 1.7 | |
| 2 g L−1 | 58.2 ± 6.2 | |
| Effect of current intensity | 50 mA | 68.6 ± 4.9 |
| 70 mA | 76.9 ± 3.7 | |
| 100 mA | 77.7 ± 1.2 |
| Catalysts | Pesticides | Degradation Efficiency (%) | Refs. |
|---|---|---|---|
| Fe3O4-TiO2/reduced graphene oxide | Atrazine | 99% within 40 min | [42] |
| N-doped TiO2@SiO2@Fe3O4 nanocomposite | Paraquat | 98.7% within 180 min | [43] |
| Bare 3D-TiO2/magnetic biochar dots | Diazinon | 98.5% within 30 min | [44] |
| TiO2/Fe3O4-CS | Chlordimeform | 100% removal within 60 min | Present work |
| Parameter | Value |
|---|---|
| Total organic carbon TOC (mg L−1) | 52.7 |
| Chemical oxygen demand DCO (mg L−1) | 35 |
| Biological oxygen demand BOD5 (mg L−1) | 2 |
| Conductivity (mS cm−1) | 3.33 |
| pH | 7.31 |
| Turbidity (mg L−1) | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezgui, S.; Díez, A.M.; Monser, L.; Adhoum, N.; Pazos, M.; Sanromán, M.Á. Magnetic TiO2/Fe3O4-Chitosan Beads: A Highly Efficient and Reusable Catalyst for Photo-Electro-Fenton Process. Catalysts 2022, 12, 1425. https://doi.org/10.3390/catal12111425
Rezgui S, Díez AM, Monser L, Adhoum N, Pazos M, Sanromán MÁ. Magnetic TiO2/Fe3O4-Chitosan Beads: A Highly Efficient and Reusable Catalyst for Photo-Electro-Fenton Process. Catalysts. 2022; 12(11):1425. https://doi.org/10.3390/catal12111425
Chicago/Turabian StyleRezgui, Soumaya, Aida M. Díez, Lotfi Monser, Nafaa Adhoum, Marta Pazos, and M. Ángeles Sanromán. 2022. "Magnetic TiO2/Fe3O4-Chitosan Beads: A Highly Efficient and Reusable Catalyst for Photo-Electro-Fenton Process" Catalysts 12, no. 11: 1425. https://doi.org/10.3390/catal12111425







