Ratiometric Electrochemical Biosensing of Methyltransferase Activity
Abstract
:1. Introduction
2. Results and Discussion
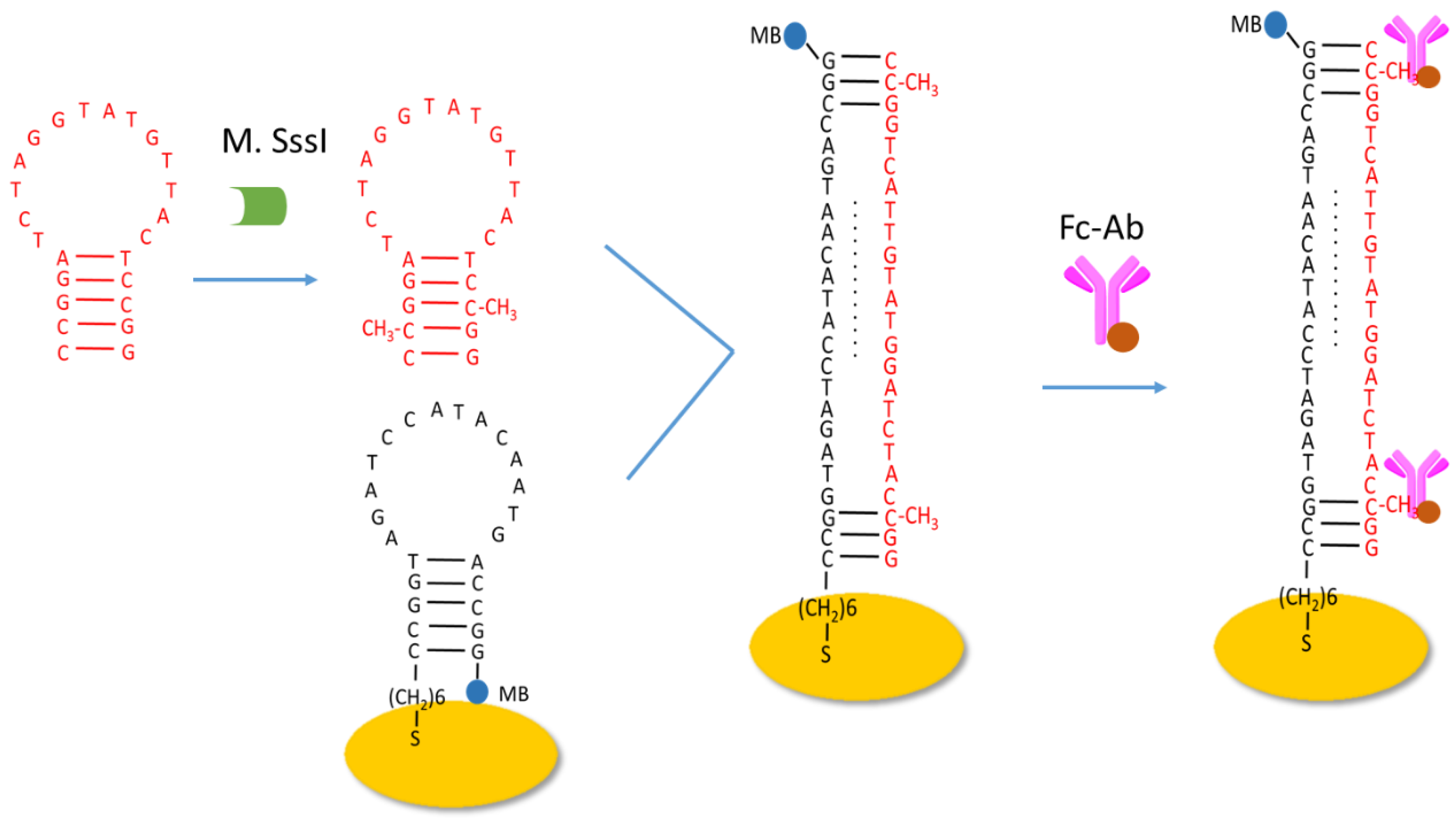
2.1. Principle of the Proposed Method
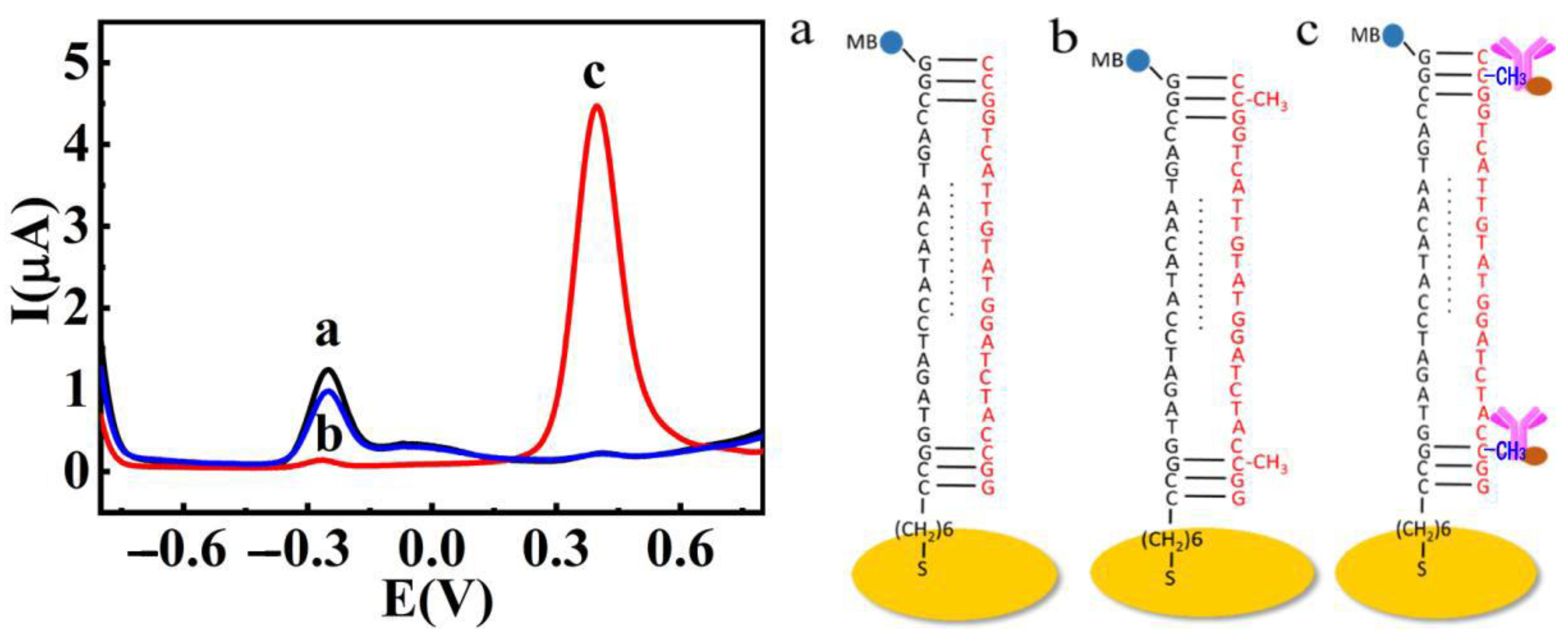
2.2. Feasibility of the Ratiometric Electrochemical Biosensor
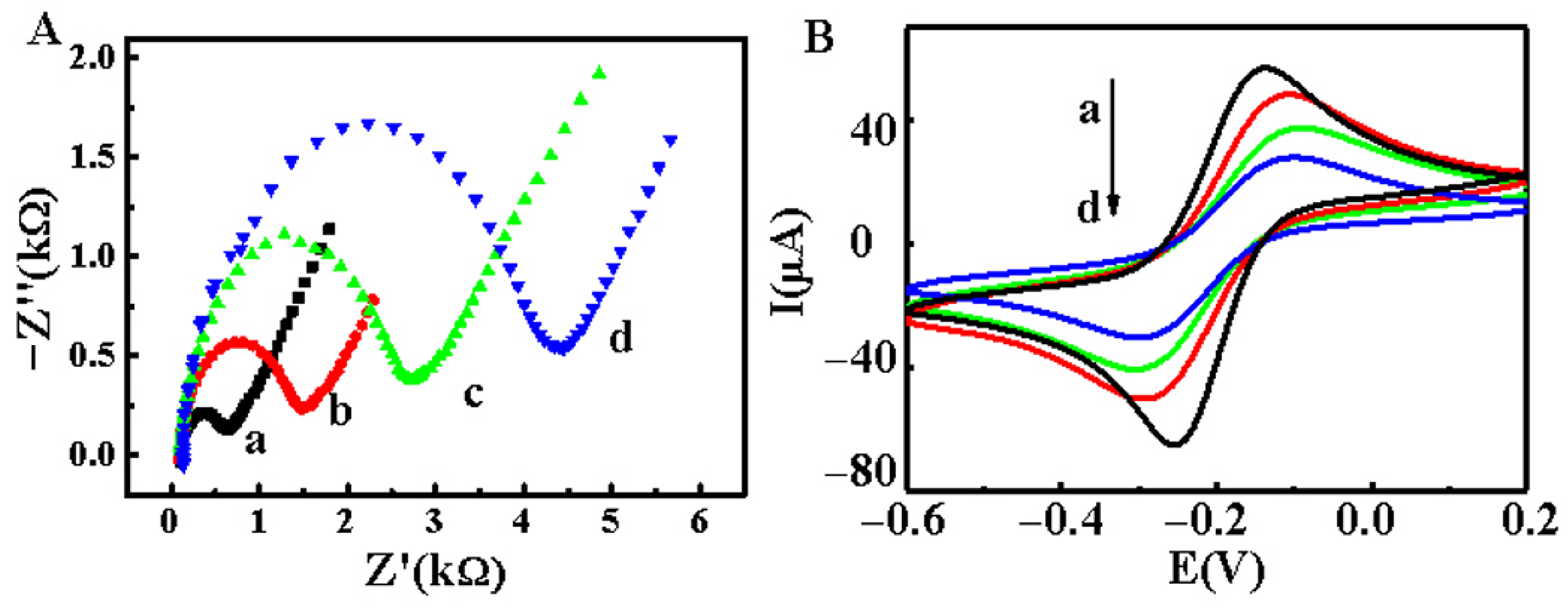
2.3. Characterization of the Ratiometric Electrochemical Biosensor
2.4. Optimization of Experimental Conditions for DNA Biosensor
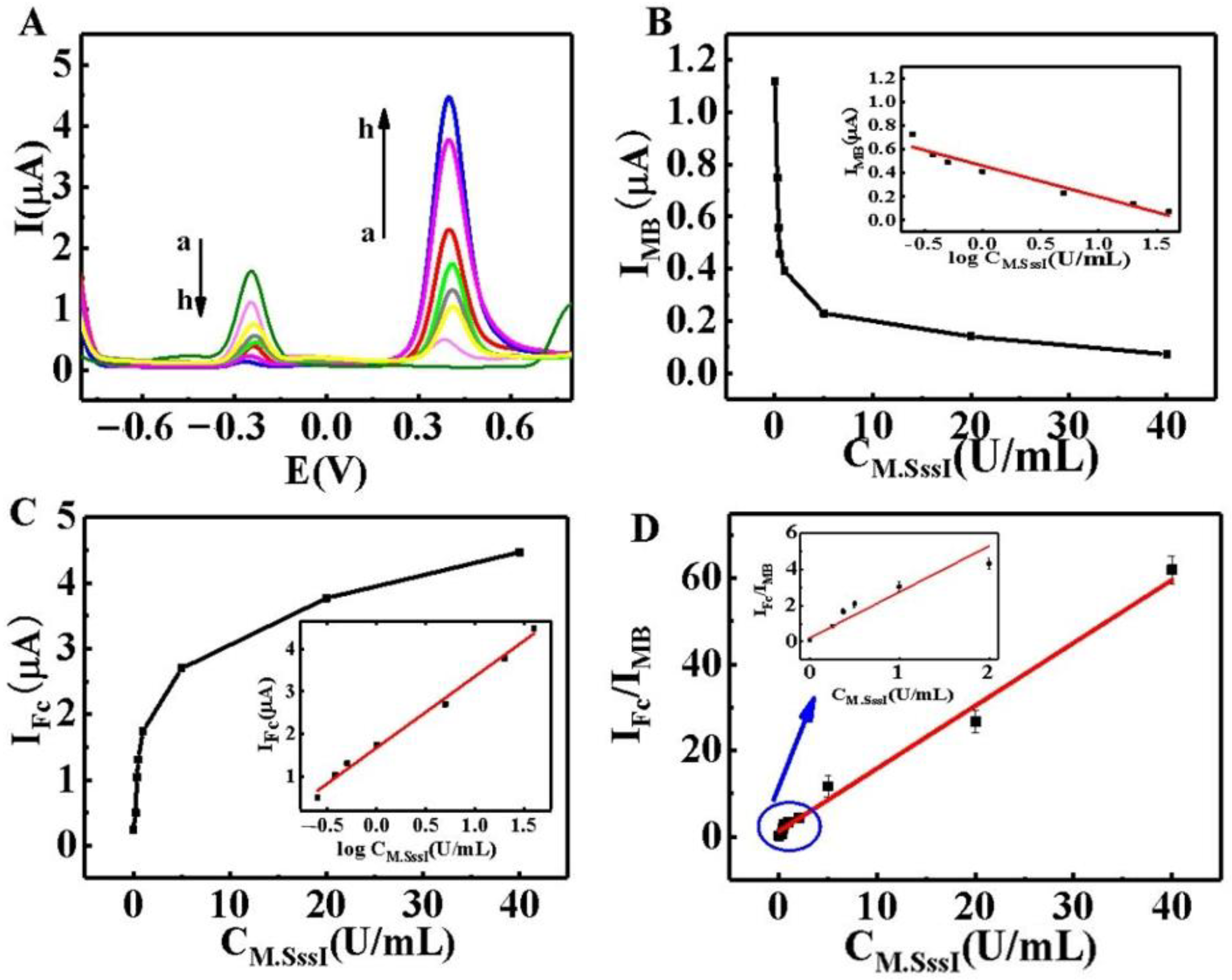
2.5. Assay Performance
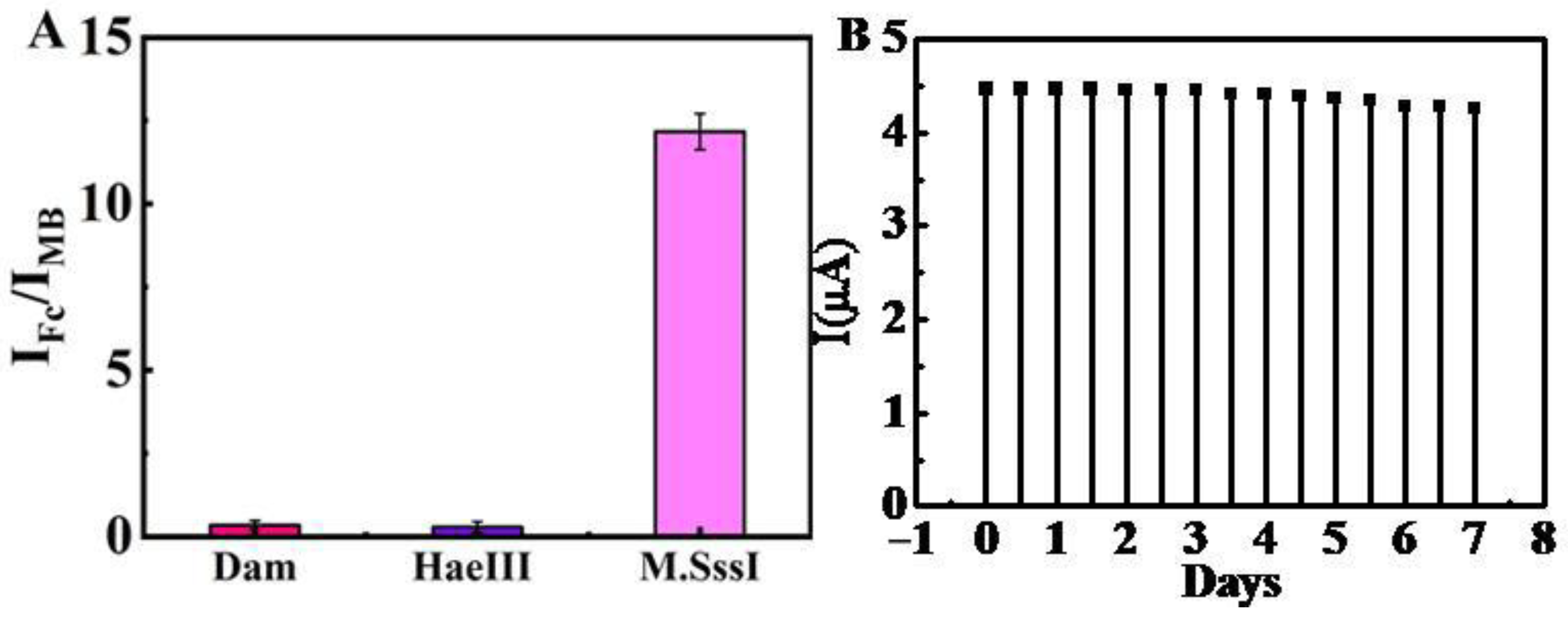
2.6. Reproducibility, Stability, and Specificity of the Electrochemical Biosensor
3. Materials and Methods
3.1. Materials and Reagents
3.2. Apparatus
3.3. Preparation of FcDA Labeled Antibody (Fc-Ab)
3.4. Methylation of Hairpin Probe
3.5. Fabrication of the Ratiometric Electrochemical Biosensor
3.6. Measurement Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Holliday, R. The inheritance of epigenetic defects. Science 1987, 238, 163–170. [Google Scholar] [CrossRef]
- Egger, G.; Liang, G.; Aparicio, A.; Jones, P.A. Epigenetics in human disease and prospects for epigenetic therapy. Nature 2004, 429, 457–463. [Google Scholar] [CrossRef]
- Heithoff, D.M.; Sinsheimer, R.L.; Low, D.A.; Mahan, M.J. An essential role for DNA adenine methylation in bacterial virulence. Science 1999, 284, 967–970. [Google Scholar] [CrossRef]
- Robertson, K.D. DNA methylation and human disease. Nat. Rev. Genet. 2005, 6, 597–610. [Google Scholar] [CrossRef]
- Razin, A.; Cedar, H. DNA methylation and gene expression. Microbiol. Rev. 1991, 55, 451–458. [Google Scholar] [CrossRef]
- Li, E.; Beard, C.; Jaenisch, R. Role for DNA methylation in genomic imprinting. Nature 1993, 366, 362–365. [Google Scholar] [CrossRef]
- Csankovski, G.; Nagy, A.; Jaenisch, R.J. Synergism of Xist RNA, DNA methylation, and histone hypoacetylation in maintaining X chromosome inactivation. Cell Biol. 2001, 153, 773–784. [Google Scholar] [CrossRef]
- Branciamore, S.; Chen, Z.X.; Riggs, A.D.; Rodin, S.N. Rodin, CpG island clusters and pro-epigenetic selection for CpGs in protein-coding exons of HOX and other transcription factors. Proc. Natl. Acad. Sci. USA 2010, 107, 15485–15490. [Google Scholar] [CrossRef] [Green Version]
- Badran, A.H.; Furman, J.L.; Ma, A.S.; Comi, T.J.; Porter, J.; Ghosh, R.I. Global CpG Methylation Status Native DNA Utilizing Bipartite Split-Luciferase Sensor. Anal. Chem. 2011, 83, 7151–7157. [Google Scholar] [CrossRef] [Green Version]
- Robertson, K.D. DNA methylation, methyltransferases, and cancer. Oncogene 2001, 20, 3139–3155. [Google Scholar] [CrossRef]
- Lyko, F.; Brown, R.J. DNA methyltransferase inhibitors and the development of epigenetic cancer therapies. Natl. Cancer Inst. 2005, 97, 1498–1506. [Google Scholar] [CrossRef] [Green Version]
- Choy, J.S.; Wei, S.; Lee, J.Y.; Tan, S.; Chu, S.; Lee, T.H. DNA methylation increases nucleosome compaction and rigidity. J. Am. Chem. Soc. 2010, 132, 1782–1783. [Google Scholar] [CrossRef] [Green Version]
- Frigola, J.; Song, J.; Stirzaker, C.; Hinshelwood, R.A.; Peinado, M.A.; Clark, S.J. Epigenetic remodeling in colorectal cancer results in coordinate gene suppression across an entire chromosome band. Nat. Genet. 2006, 38, 540–549. [Google Scholar] [CrossRef]
- Lopez Torres, A.; Yanez Barrientos, E.; Wrobel, K. Selective Derivatization of Cytosine and Methylcytosine Moieties with 2-Bromoacetophenone for Submicrogram DNA Methylation Analysis by Reversed Phase HPLC with Spectrofluorimetric Detection. Anal. Chem. 2011, 83, 7999–8005. [Google Scholar] [CrossRef]
- Lyko, F.; Ramsahoye, B.H.; Jaenisch, R. DNA methylation in Drosophila melanogaster. Nature 2000, 408, 538–540. [Google Scholar] [CrossRef]
- McLaughlin, L.W.; Benseler, F.; Graeser, E.; Piel, N.; Scholtissek, S. Effects of functional group changes in the EcoRI recognition site on the cleavage reaction catalyzed by the endonuclease. Biochemistry 1987, 26, 7238–7245. [Google Scholar] [CrossRef]
- Li, Z.M.; Zhang, X.; Pi, T.; Bu, J.; Deng, R.H.; Chi, B.Z.; Zheng, X.J. Colorimetric determination of the activity of methyltransferase based on nicking enzyme amplification and the use of gold nanoparticles conjugated to graphene oxide. Microchim. Acta 2019, 186, 594. [Google Scholar] [CrossRef]
- Kermani, H.A.; Hosseini, M.; Miti, A.; Dadmehr, M.; Zuccheri, G.; Hosseinkhani, S.; Ganjali, M.R. A colorimetric assay of DNA methyltransferase activity based on peroxidase mimicking of DNA template Ag/Pt bimetallic nanoclusters. Anal. Bioanal. Chem. 2018, 410, 4943–4952. [Google Scholar] [CrossRef]
- Bi, S.; Zhao, T.; Luo, B.; Zhu, J.J. Hybridization chain reaction-based branched rolling circle amplification for chemiluminescence detection of DNA methylation. Chem. Commun. 2013, 49, 6906–6908. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, M.; Fan, Z.; Qin, X.; Zhu, X.; Ji, H.; Qin, Y.; Wang, Q.; Wu, L. Ultrasensitive DNA methyltransferase activity sensing and inhibitor evaluation with highly photostable upconversion nanoparticle transducer. Microchim. Acta 2021, 188, 169. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Li, W.Y.; Zhou, H.P.; Li, Y.X.; Yu, C. Nucleic Acid-Induced Tetraphenylethene Probe Noncovalent Self-Assembly and the Superquenching of Aggregation-Induced Emission. Anal. Chem. 2014, 86, 9866–9872. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, X.-L.; Jiang, W.-W.; Zeng, S.-H.; Pi, T.; Zheng, X.-J.; Li, Z.-M. Fluorescence-based Polymerase Amplification for the Sensitive Detection of DNA Methyltransferase Activity. Anal. Sci. 2018, 34, 959–964. [Google Scholar] [CrossRef] [Green Version]
- Quach, Q.H.; Chung, B.H. A signal-on fluorescent assay for DNA methyltransferase activity using a methylation-resistant endonuclease. Analyst 2014, 139, 2674–2677. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Chen, Y.; Li, W.Y.; Yu, C. Polymer-Induced Perylene Probe Excimer Formation and Selective Sensing of DNA Methyltransferase Activity through the Monomer–Excimer Transition. Anal. Chem. 2014, 86, 4371–4378. [Google Scholar] [CrossRef]
- Hu, H.; Zhou, F.; Wang, B.J.; Chang, X.; Dai, T.Y.; Tian, R.F.; Wan, Y.F.; Wang, X.Y.; Wang, G.F. Autonomous operation of 3D DNA walkers in living cells for microRNA imaging. Nanoscale 2021, 13, 1863–1868. [Google Scholar] [CrossRef]
- Shen, Q.M.; Han, L.; Fan, G.C.; Abdel-Halim, E.S.; Jiang, L.P.; Zhu, J.J. Highly sensitive photoelectrochemical assay for DNA methyltransferase activity and inhibitor screening by exciton energy transfer coupled with enzyme cleavage biosensing strategy. Biosens. Bioelectron. 2015, 64, 449–455. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Xu, Z.N.; Wang, M.; Sun, B.; Yin, H.S.; Ai, S.Y. DNA methyltransferase activity assay based on visible light-activated photoelectrochemical biosensor. Biosens. Bioelectron. 2014, 53, 263–267. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, X.; Yin, H.S.; Zhou, Y.L.; Yang, Y.; Ai, S.Y. Photoelectrochemical immunosensor for methylated RNA detection based on WS2 and poly(U) polymerase–triggered signal amplification. Microchim. Acta 2020, 187, 596. [Google Scholar] [CrossRef]
- Deng, H.M.; Yang, X.J.; Yeo, S.P.X.; Gao, Z.Q. Highly Sensitive Electrochemical Methyltransferase Activity Assay. Anal. Chem. 2014, 86, 2117–2123. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Z.H.; Zhang, L.; Zhang, N.; Zhang, K.Y.; Xu, J.G.; Wang, H.Y.; Shi, H.W.; Qin, M.; Ren, L. DNA based signal amplified molecularly imprinted polymer electrochemical sensor for multiplex detection. RSC Adv. 2016, 6, 49597–49603. [Google Scholar] [CrossRef]
- Li, W.; Wu, P.; Zhang, H.; Cai, C.X. Signal Amplification of Graphene Oxide Combining with Restriction Endonuclease for Site-Specific Determination of DNA Methylation and Assay of Methyltransferase Activity. Anal. Chem. 2012, 84, 7583–7590. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Moriam, S.; Umer, M.; Nguyen, N.T.; Shiddiky, M.J.A. DNA methylation detection: Recent developments in bisulfite free electrochemical and optical approaches. Analyst 2018, 143, 4802–4818. [Google Scholar] [CrossRef]
- Muren, N.B.; Barton, J.K. Electrochemical Assay for the Signal-On Detection of Human DNA Methyltransferase Activity. J. Am. Chem. Soc. 2013, 135, 16632–16640. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.F.; Wan, J.; Zhang, X.J. TTE DNA–Cu NPs: Enhanced fluorescence and application in a target DNA triggered dual-cycle amplification biosensor. Chem. Commun. 2017, 53, 5629–5632. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Z.N.; Chen, L.J.; Yin, H.S.; Ai, S.Y. Electrochemical Immunosensing Platform for DNA Methyltransferase Activity Analysis and Inhibitor Screening. Anal. Chem. 2012, 84, 9072–9078. [Google Scholar] [CrossRef]
- Lu, L.; Liu, B.; Leng, J.; Ma, X. Electrochemical determination of the activity of DNA methyltransferase based on the methyl binding domain protein and a customized modular detector. Microchim. Acta 2019, 186, 229. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, L.J.; Zhao, Z.; Yang, X.Y.; Wang, L.; Liu, S.F. Immuno-DNA binding directed template-free DNA extension and enzyme catalysis for sensitive electrochemical DNA methyltransferase activity assay and inhibitor screening. Analyst 2020, 145, 3064–3072. [Google Scholar] [CrossRef]
- Ricci, F.; Plaxco, K.W. E-DNA sensors for convenient, label-free electrochemical detection of hybridization. Microchim. Acta 2008, 163, 149–155. [Google Scholar] [CrossRef]
- Bakker, E.; Qin, Y. Electrochemical Sensors. Anal. Chem. 2006, 78, 3965–3984. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.G.; Lai, R.Y. A reagentless and reusable electrochemical DNA sensor based on target hybridization-induced stem-loop probe formation. Chem. Commun. 2012, 48, 10523–10525. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, Y.; Lei, J.P.; Zhang, L.; Ju, H.X. Design and Biosensing of Mg2+ Dependent DNAzyme Triggered Ratiometric Electrochemiluminescence. Anal. Chem. 2014, 86, 5158–5163. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Q.H.; Lei, J.P.; Xu, N.; Ju, H.X. DNA-regulated silver nanoclusters for label-free ratiometric fluorescence detection of DNA. Chem. Commun. 2014, 50, 13698–13701. [Google Scholar] [CrossRef]
- Zhang, X.X.; Xiao, K.Y.; Cheng, L.W.; Chen, H.; Liu, B.H.; Zhang, S.; Kong, J.L. Visual and Highly Sensitive Detection of Cancer Cells by a Colorimetric Aptasensor Based on Cell-Triggered Cyclic Enzymatic Signal Amplification. Anal. Chem. 2014, 86, 5567–5572. [Google Scholar] [CrossRef]
- Yu, P.; Zhou, J.W.; Wu, L.; Xiong, E.H.; Zhang, X.H.; Chen, J.H. A ratiometric electrochemical aptasensor for sensitive detection of protein based on aptamer–target–aptamer sandwich structure. J. Electroanal. Chem. 2014, 732, 61–65. [Google Scholar] [CrossRef]
- Du, Y.; Lim, B.J.; Li, B.L.; Jiang, Y.S.; Sessler, J.L.; Ellington, A.D. Ellington, Reagentless, Ratiometric Electrochemical DNA Sensors with Improved Robustness and Reproducibility. Anal. Chem. 2014, 86, 8010–8016. [Google Scholar] [CrossRef]
- Ren, K.W.; Wu, J.; Yan, F.; Ju, H.X. Ratiometric electrochemical proximity assay for sensitive one-step protein detection. Sci. Rep. 2014, 4, 4360–4365. [Google Scholar] [CrossRef] [Green Version]
- Ren, K.W.; Wu, J.; Yan, F.; Zhang, Y.; Ju, H.X. Immunoreaction-triggered DNA assembly for one-step sensitive ratiometric electrochemical biosensing of protein biomarker. Biosens. Bioelectron. 2015, 66, 345–349. [Google Scholar] [CrossRef]
- Xiong, E.H.; Wu, L.; Zhou, J.W.; Yu, P.; Zhang, X.H.; Chen, J.H. A ratiometric electrochemical biosensor for sensitive detection of Hg2+ based on thymine–Hg2+–thymine structure. Anal. Chim. Acta 2015, 853, 242–248. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, X.H.; Liu, W.; Xiong, E.H.; Chen, J.H. Sensitive Electrochemical Aptasensor by Coupling “Signal-on’’ and “Signal-off’’ Strategies. Anal. Chem. 2013, 85, 8397–8402. [Google Scholar] [CrossRef]
- Grabowska, I.; Malecka, K.; Stachyra, A.; Góra-Sochacka, A.; Sirko, A.; Zagórski-Ostoja Radecka, W.H.; Radecki, J. Single Electrode Genosensor for Simultaneous Determination of Sequences Encoding Hemagglutinin and Neuraminidase of Avian Influenza Virus Type H5N1. Anal. Chem. 2013, 85, 10167–10173. [Google Scholar] [CrossRef]
- Yang, W.W.; Lai, R.Y. Integration of two different sensing modes in an electrochemical DNA sensor for approximation of target mismatch location. Electrochem. Commun. 2011, 13, 989–992. [Google Scholar] [CrossRef]
- Miodek, A.; Regan, E.M.; Bhalla, N.; Hopkins, N.A.E.; Goodchild, S.A.; Estrela, P. Optimisation and Characterisation of Anti-Fouling Ternary SAM Layers for Impedance-Based Aptasensors. Sensors 2015, 15, 25015–25032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.L.; Xu, Q.; James, T.; Davis, J.J. Redox and Label-Free Array Detection of Protein Markers in Human Serum. Anal. Chem. 2014, 86, 5553–5558. [Google Scholar] [CrossRef] [PubMed]
- Žutić, V.; Svetličić, V.; Clavilier, J.; Chevalet, J. Supramolecular phenomena in organic redox films at electrodes: Part II. The methylene blue/leucomethylene blue redox couple at the gold electrode. J. Electroanal. Chem. Interfacial Electrochem. 1987, 219, 183–195. [Google Scholar] [CrossRef]
- Zhan, R.; Song, S.; Liu, Y.; Dong, S. Mechanisms of methylene blue electrode processes studied by in situ electron paramagnetic resonance and ultraviolet–visible spectroelectrochemistry. J. Chem. Soc. Faraday FTrans. 1990, 86, 3125–3127. [Google Scholar] [CrossRef]
- Ahmad, H.M.N.; Dutta, G.; Csoros, J.; Si, B.; Yang, R.; Halpern, J.M.; Seitz, W.R.; Song, E. Stimuli-Responsive Templated Polymer as a Target Receptor for a Conformation-Based Electrochemical Sensing Platform. ACS Appl. Polym. Mater. 2021, 3, 329–341. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, S.; Yu, J.C.; Zhu, X.J.; Yin, Y.M.; Li, G.X. Protein Detection Based on Small Molecule-Linked DNA. Anal. Chem. 2012, 84, 4314–4320. [Google Scholar] [CrossRef]
- Koo, K.M.; Carrascosa, L.G.; Shiddiky, M.J.; Trau, M. Poly(A) extensions of miRNAs for amplification-free electrochemical detection on screen-printed gold electrodes. Anal. Chem. 2016, 88, 2000–2005. [Google Scholar] [CrossRef] [Green Version]
- Ferapontova, E.E. Hybridization Biosensors Relying on Electrical Properties of Nucleic Acids. Eletroanalysis 2017, 29, 6–13. [Google Scholar] [CrossRef]

| Strategy | Detection Method | Signal | Detection Limit (U/mL) | Linear Range (U/mL) | Reference |
|---|---|---|---|---|---|
| HRP mimicking DNA zyme | Colorimetry | Signal on | 0.4 | 0.8–24 | [17] |
| HRP mimicking DNA zyme | Colorimetry | Signal on | 6 | 6–100 | [18] |
| RCA | Chemiluminescence | Signal on | 0.52 | 1–10 | [19] |
| Nicking enzyme-assisted signal amplification | Fluorescence | Signal on | 0.06 | 0.1–4 | [20] |
| without any amplification | Electrochemistry | Signal off | 0.18 | 0.25–10 | [36] |
| This work | Electrochemistry | Dual signal | 0.083 | 0–40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Guo, Z.; Tian, R.; Zhang, K.; Wang, H.; Li, F.; Shi, H.; Wang, Z. Ratiometric Electrochemical Biosensing of Methyltransferase Activity. Catalysts 2022, 12, 1362. https://doi.org/10.3390/catal12111362
Wang C, Guo Z, Tian R, Zhang K, Wang H, Li F, Shi H, Wang Z. Ratiometric Electrochemical Biosensing of Methyltransferase Activity. Catalysts. 2022; 12(11):1362. https://doi.org/10.3390/catal12111362
Chicago/Turabian StyleWang, Cong, Zhihua Guo, Ruifen Tian, Keying Zhang, Hongyan Wang, Fajun Li, Hongwei Shi, and Zhicheng Wang. 2022. "Ratiometric Electrochemical Biosensing of Methyltransferase Activity" Catalysts 12, no. 11: 1362. https://doi.org/10.3390/catal12111362





