In Situ Formation of Z-Scheme Bi2WO6/WO3 Heterojunctions for Gas-Phase CO2 Photoreduction with H2O by Photohydrothermal Treatment
Abstract
:1. Introduction
2. Results and Discussions
2.1. CO2 Photoreduction Performance of Bi2WO6/WO3 under Real and Simulated Light
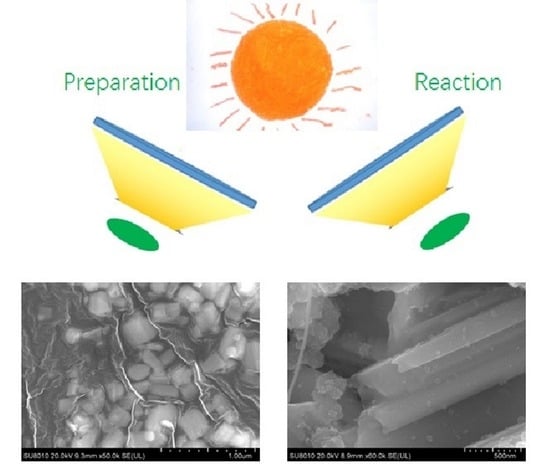
2.2. Characterization of Bi2WO6 and Bi2WO6/WO3 Samples
2.3. Discussions
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shih, C.F.; Zhang, T.; Li, J.; Bai, C. Powering the Future with Liquid Sunshine. Joule 2018, 2, 1925–1949. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, L.A.; Loomis, J.; Bhatia, B.; Bierman, D.M.; Wang, E.N.; Chen, G. Concentrating Solar Power. Chem. Rev. 2015, 115, 12797–12838. [Google Scholar] [CrossRef] [PubMed]
- Devens, G.; Moore, T.A.; Moore, A.L. Solar fuels via artificial photosynthesis. Acc. Chem. Res. 2009, 42, 1890–1898. [Google Scholar]
- He, J.; Janáky, C. Recent Advances in Solar-Driven Carbon Dioxide Conversion: Expectations versus Reality. ACS Energy Lett. 2020, 5, 1996–2014. [Google Scholar] [CrossRef]
- Ulmer, U.; Dingle, T.; Duchesne, P.N.; Morris, R.H.; Tavasoli, A.; Wood, T.; Ozin, G.A. Fundamentals and applications of photocatalytic CO2 methanation. Nat. Commun. 2019, 10, 3169–3181. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Toward Solar Fuels: Photocatalytic Conversion of Carbon Dioxide to Hydrocarbons. ACS Nano 2010, 4, 1259–1278. [Google Scholar] [CrossRef]
- Jiao, X.; Zheng, K.; Liang, L.; Li, X.; Sun, Y.; Xie, Y. Fundamentals and challenges of ultrathin 2D photocatalysts in boosting CO2 photoreduction. Chem. Soc. Rev. 2020, 49, 6592–6604. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Wu, J.C. Recent developments in the design of photoreactors for solar energy conversion from water splitting and CO2 reduction. Appl. Catal. A 2018, 550, 122–141. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef]
- Teramura, K.; Tanaka, T. Necessary and sufficient conditions for the successful three-phase photocatalytic reduction of CO2 by H2O over heterogeneous photocatalysts. Phys. Chem. Chem. Phys. 2018, 20, 8423–8437. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef] [Green Version]
- Rossetti, I.; Villa, A.; Pirola, C.; Prati, L.; Ramis, G. A novel high-pressure photoreactor for CO2 photoconversion to fuels. RSC Adv. 2014, 4, 28883–28885. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.-V.; Wu, J.C.; Chiou, C.-H. Photoreduction of CO2 over Ruthenium dye-sensitized TiO2-based catalysts under concentrated natural sunlight. Catal. Commun. 2008, 9, 2073–2076. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Cui, G.; Liu, H.; Abanades, S.; Lu, H. Improvement of CO2 Photoreduction Efficiency by Process Intensification. Catalysts 2021, 11, 912. [Google Scholar] [CrossRef]
- Ghoussoub, M.; Xia, M.; Duchesne, P.N.; Segal, D.; Ozin, G. Principles of photothermal gas-phase heterogeneous CO2 catalysis. Energy Environ. Sci. 2019, 12, 1122–1142. [Google Scholar] [CrossRef]
- Shi, L.; Wang, X.Z.; Hu, Y.W.; He, Y.R. Investigation of photocatalytic activity through photo-thermal heating enabled by Fe3O4/TiO2 composite under magnetic field. Sol. Energy 2020, 196, 505–512. [Google Scholar] [CrossRef]
- Wong, C.L.; Tan, Y.N.; Mohamed, A.R. A review on the formation of titania nanotube photocatalysts by hydrothermal, treatment. J. Environ. Manag. 2011, 92, 1669–1680. [Google Scholar] [CrossRef]
- Dusselier, M.; Deimund, M.A.; Schmidt, J.E.; Davis, M.E. Methanol-to-Olefins Catalysis with Hydrothermally Treated Zeolite SSZ-39. ACS Catal. 2015, 5, 6078–6085. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Li, H.; Gao, J.; Ding, S.; Liu, X.; Jia, H.; Xue, J. Effect of oxygen vacancies on the photocatalytic CO2 reduction performance of Bi2WO6: DFT and experimental studies. Appl. Surf. Sci. 2021, 579, 152135. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.J.; Yu, J.G.; Cao, S.W. Ultrathin 2D/2D Graphdiyne/Bi2WO6 Heterojunction for Gas-Phase CO2 Photoreuction. ACS Appl. Energy Mater. 2021, 4, 8734–8738. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, D.; Zhang, Q.; Lin, Y.; Peng, F. Enhanced photocatalytic CO2 reduction in H2O vapor by atomically thin Bi2WO6 nanosheets with hydrophobic and nonpolar surface. Appl. Catal. B 2020, 283, 119630. [Google Scholar] [CrossRef]
- Ma, H.; He, Y.; Chen, P.; Wang, H.; Sun, Y.; Li, J.; Dong, F.; Xie, G.; Sheng, J. Ultrathin Two-Dimensional Bi-Based photocatalysts: Synthetic strategies, surface defects, and reaction mechanisms. Chem. Eng. J. 2021, 417, 129305. [Google Scholar] [CrossRef]
- Xiong, J.; Di, J.; Li, H. Interface engineering in low-dimensional bismuth-based materials for photoreduction reactions. J. Mater. Chem. A 2020, 9, 2662–2677. [Google Scholar] [CrossRef]
- Ribeiro, C.S.; Tan, J.Z.; Maroto-Valer, M.M.; Lansarin, M.A. Photocatalytic reduction of CO2 over Bi2WO6 in a continuous-flow differential photoreactor: Investigation of operational parameters. J. Environ. Chem. Eng. 2021, 9, 105097. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, X.; Zhang, C.; Li, R.; Liu, J.; Wang, Y.; Wang, Y.; Fan, C.; Zhao, Q. Synergetic effect of Bi2WO6 micro-spheres and activated carbon mm-spheres for enhancing photoreduction activity of CO2 to CO. Mater. Lett. 2019, 264, 127201. [Google Scholar] [CrossRef]
- Xu, Q.C.; Wellia, D.V.; Ng, Y.H.; Amal, R.; Tan, T.T.Y. Synthesis of Porous and Visible-Light Absorbing Bi2WO6/TiO2 Heterojunction Films with Improved Photoelectrochemical and Photocatalytic Performances. J. Phys. Chem. C 2011, 115, 7419–7428. [Google Scholar] [CrossRef]
- Zhou, H.R.; Wen, Z.P.; Liu, J.; Ke, J.; Duan, X.G.; Wang, S.B. Z-scheme plasmonic Ag decorated WO3/Bi2WO6 hybrids for enhanced photocatalytic abatement of chlorinated-VOCs under solar light irradiation. Appl. Catal. B 2019, 242, 76–84. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef]
- Wang, L.X.; He, S.X.; Wang, L.; Lei, Y.; Meng, X.J.; Xiao, F.S. Cobalt–Nickel Catalysts for Selective Hydrogenation of Carbon Dioxide into Ethanol. ACS Catal. 2019, 9, 11335–11340. [Google Scholar] [CrossRef]
- Huang, J.; Tan, G.Q.; Ren, H.J.; Yang, W.; Xu, C.; Zhao, C.C.; Xia, A. Photoelectric Activity of a Bi2O3/Bi2WO6−xF2x Heterojunction Prepared by a Simple One-Step Microwave Hydrothermal Method. ACS Appl. Mater. Interfaces 2014, 6, 21041–21050. [Google Scholar] [CrossRef]
- Ng, C.; Iwase, A.; Ng, Y.H.; Amal, R. Transforming Anodized WO3 Films into Visible-Light-Active Bi2WO6 Photoelectrodes by Hydrothermal Treatment. J. Phys. Chem. Lett. 2012, 3, 913–918. [Google Scholar] [CrossRef]
- Mehta, N.; Kumar, A. Structural characterization of light-induced crystal growth in Se98Sb2 chalcogenide glass. J. Non-Cryst. Solids 2012, 358, 776–781. [Google Scholar] [CrossRef]
- Low, J.; Jiang, C.; Cheng, B.; Swelm, W.; Al-Ghamdi, A.A.; Yu, J. A Review of Direct Z-Scheme Photocatalysts. Small Methods 2017, 1, 1700080. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Gao, Z.H.; Liu, H.Y.; Abanades, S.; Lu, H.F. High Photothermally Active Fe2O3 Film for CO2 Photoreduction with H2O Driven by Solar Light. ACS Appl. Energy Mater. 2019, 2, 8376–8380. [Google Scholar] [CrossRef]
- Aranda-Aguirre, A.; de Oca, J.M.; Corzo, A.; Garcia-Segura, S.; Alarcon, H. Mixed metal oxide Bi2O3/Bi2WO6 thin films for the photoelectrocatalytic degradation of histamine. J. Electronanaly. Chem. 2022, 919, 116528. [Google Scholar] [CrossRef]
- Chung, H.Y.; Wong, R.J.; Amal, R.; Ng, Y.H. Engineering the Interfacial Contact between Bi2WO6 and WO3 Heterojunction Photoanode for Improved Charge Transportation. Energy Fuels 2022, 36, 11550–11558. [Google Scholar] [CrossRef]
- He, G.H.; He, G.L.; Li, A.J.; Li, X.; Wang, X.J.; Fang, Y.P.; Xu, Y.H. Synthesis and visible light photocatalytic behavior of WO3 (core)/ Bi2WO6 (shell). J. Mole. Catal. A 2014, 385, 106–111. [Google Scholar] [CrossRef]
- Gui, M.S.; Zhang, W.D.; Chang, Y.Q.; Yu, Y.X. One-step hydrothermal preparation strategy for nanostructured WO3/Bi2WO6 heterojunction with high visible light photocatalytic activity. Chem. Eng. J. 2012, 197, 283–288. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.X.; Li, L. Facet-engineered surface and interface design of WO3/ Bi2WO6 photocatalyst with direct Z-scheme heterojunction for efficient salicylic acid removal. Appl. Sur. Sci. 2020, 508, 144796. [Google Scholar] [CrossRef]
- Tian, Y.L.; Chang, B.B.; Lu, J.L.; Fu, J.; Xi, F.N.; Dong, X.P. Hydrothermal Synthesis of Graphitic Carbon Nitride–Bi2WO6 Heterojunctions with Enhanced Visible Light Photocatalytic Activities. ACS Appl. Mater. Interfaces 2013, 5, 7079–7085. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Li, Y.F.; Liu, L.; Zhou, Z.; Chen, W. Bi2O3−Bi2WO6 Composite Microspheres: Hydrothermal Synthesis and Photocatalytic Performances. J. Phys. Chem. C 2011, 115, 5220–5225. [Google Scholar] [CrossRef]









| CR | Yield/μmol·g−1 | STCaverage/% | CO2 Conversion/% | |||
|---|---|---|---|---|---|---|
| CH4 | C2H4 | C2H6 | UV | Total | ||
| 400 | 830.67 | 282.09 | 140.54 | 0.42 | 0.03 | 1.12 |
| 600 | 1524.70 | 313.52 | 273.31 | 0.72 | 0.05 | 1.89 |
| 800 | 368.95 | 83.05 | 84.05 | 0.28 | 0.02 | 0.63 |
| CR | Yield/μmol·g−1 | STCmax/% | |||
|---|---|---|---|---|---|
| CH4 | C2H4 | C2H6 | UV | Total | |
| 400 | 198.31 | 152.42 | 43.33 | 0.84 | 0.06 |
| 600 | 430.18 | 127.79 | 288.29 | 1.73 | 0.12 |
| 800 | 117.50 | 30.42 | 22.73 | 0.28 | 0.02 |
| Sample | Surface Area (m2/g) | Pore Volume (cc/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| Bi2WO6/WO3 | 25.8 | 0.101 | 14.7 |
| Bi2WO6/WO3 (CR 400) | 26.3 | 0.118 | 17.9 |
| Bi2WO6/WO3 (CR600) | 17.0 | 0.217 | 51.2 |
| Bi2WO6/WO3 (CR800) | 5.6 | 0.119 | 85.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, D.; Lyu, L.; Cui, G.; Lu, H. In Situ Formation of Z-Scheme Bi2WO6/WO3 Heterojunctions for Gas-Phase CO2 Photoreduction with H2O by Photohydrothermal Treatment. Catalysts 2022, 12, 1237. https://doi.org/10.3390/catal12101237
Zhang Z, Zhang D, Lyu L, Cui G, Lu H. In Situ Formation of Z-Scheme Bi2WO6/WO3 Heterojunctions for Gas-Phase CO2 Photoreduction with H2O by Photohydrothermal Treatment. Catalysts. 2022; 12(10):1237. https://doi.org/10.3390/catal12101237
Chicago/Turabian StyleZhang, Zekai, Ding Zhang, Lin Lyu, Guokai Cui, and Hanfeng Lu. 2022. "In Situ Formation of Z-Scheme Bi2WO6/WO3 Heterojunctions for Gas-Phase CO2 Photoreduction with H2O by Photohydrothermal Treatment" Catalysts 12, no. 10: 1237. https://doi.org/10.3390/catal12101237