Photocatalytic Bacterial Inactivation of Acinetobacter baumannli on Cu/TiO2/Diatomite
Abstract
:1. Introduction
2. Results and Discussion
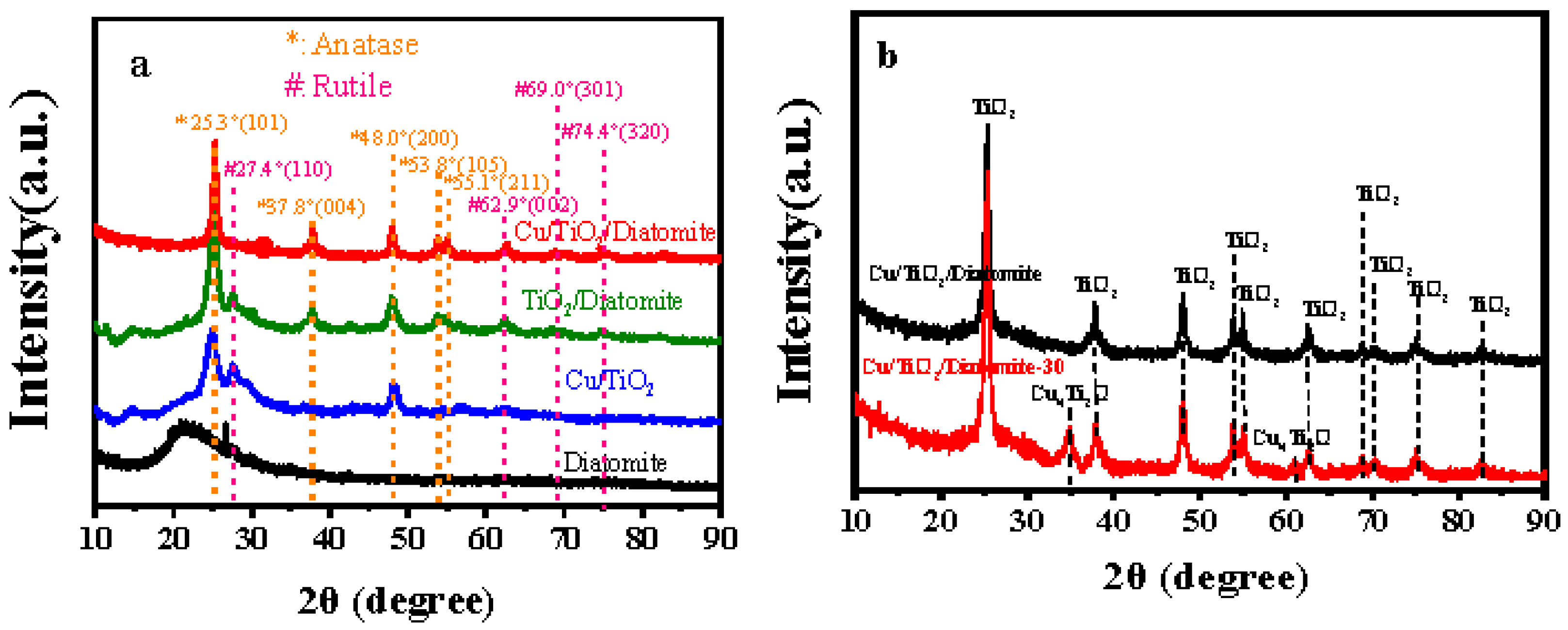
2.1. XRD Analysis
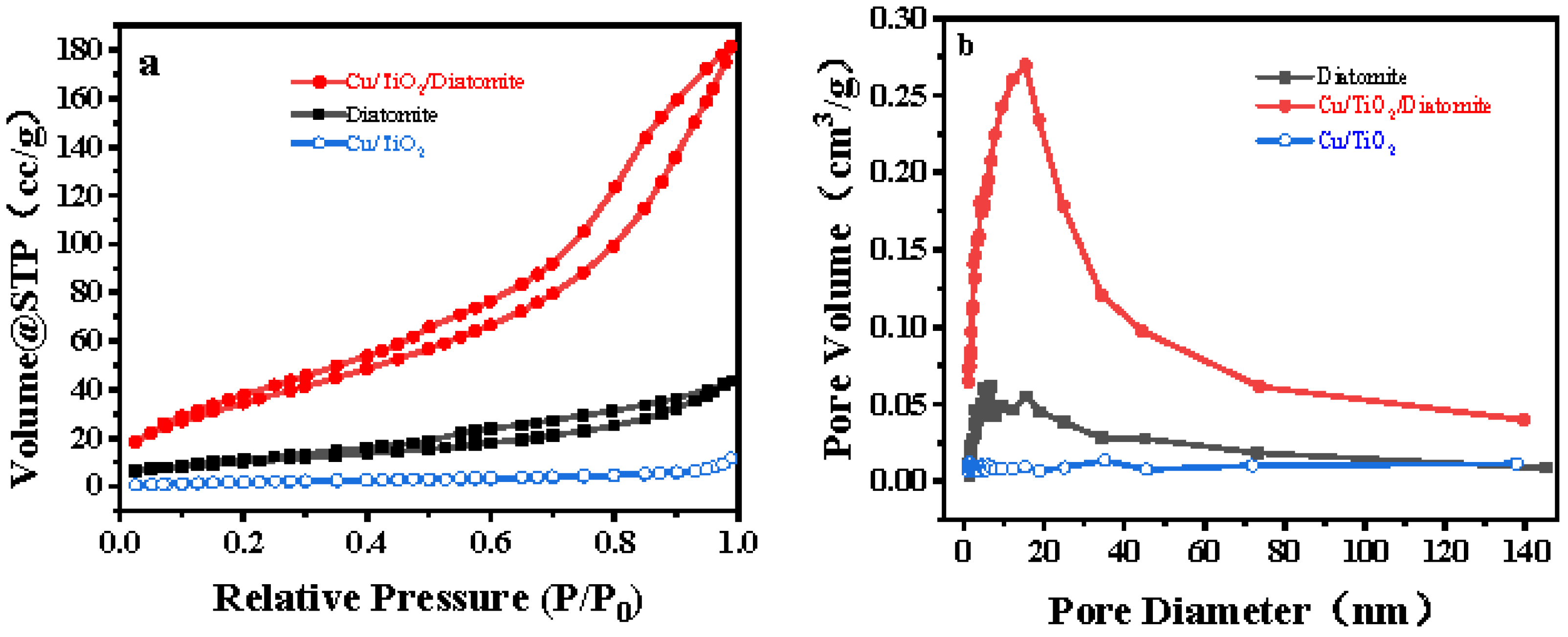
2.2. BET Analysis
2.3. Energy Dispersive Spectrometer (EDS) and SEM Mapping
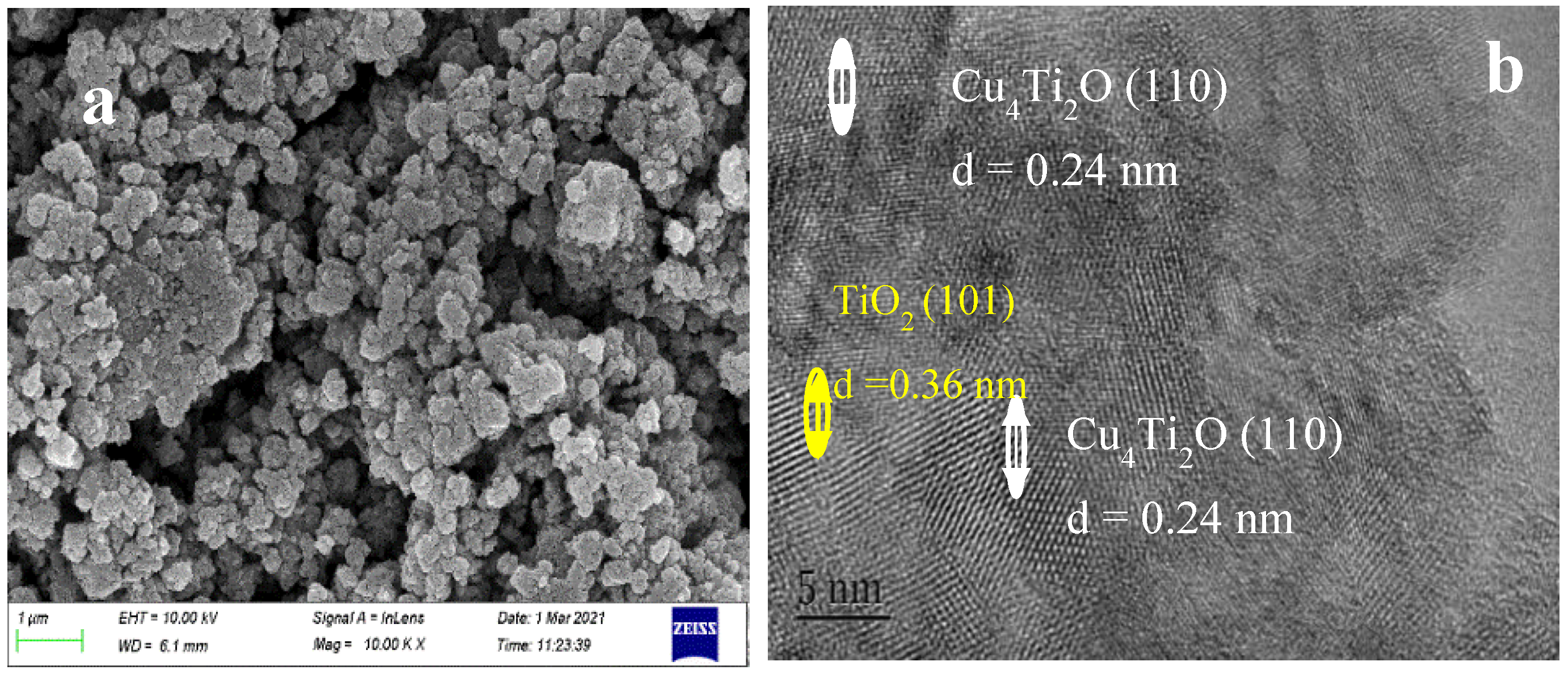
2.4. SEM and TEM Analysis
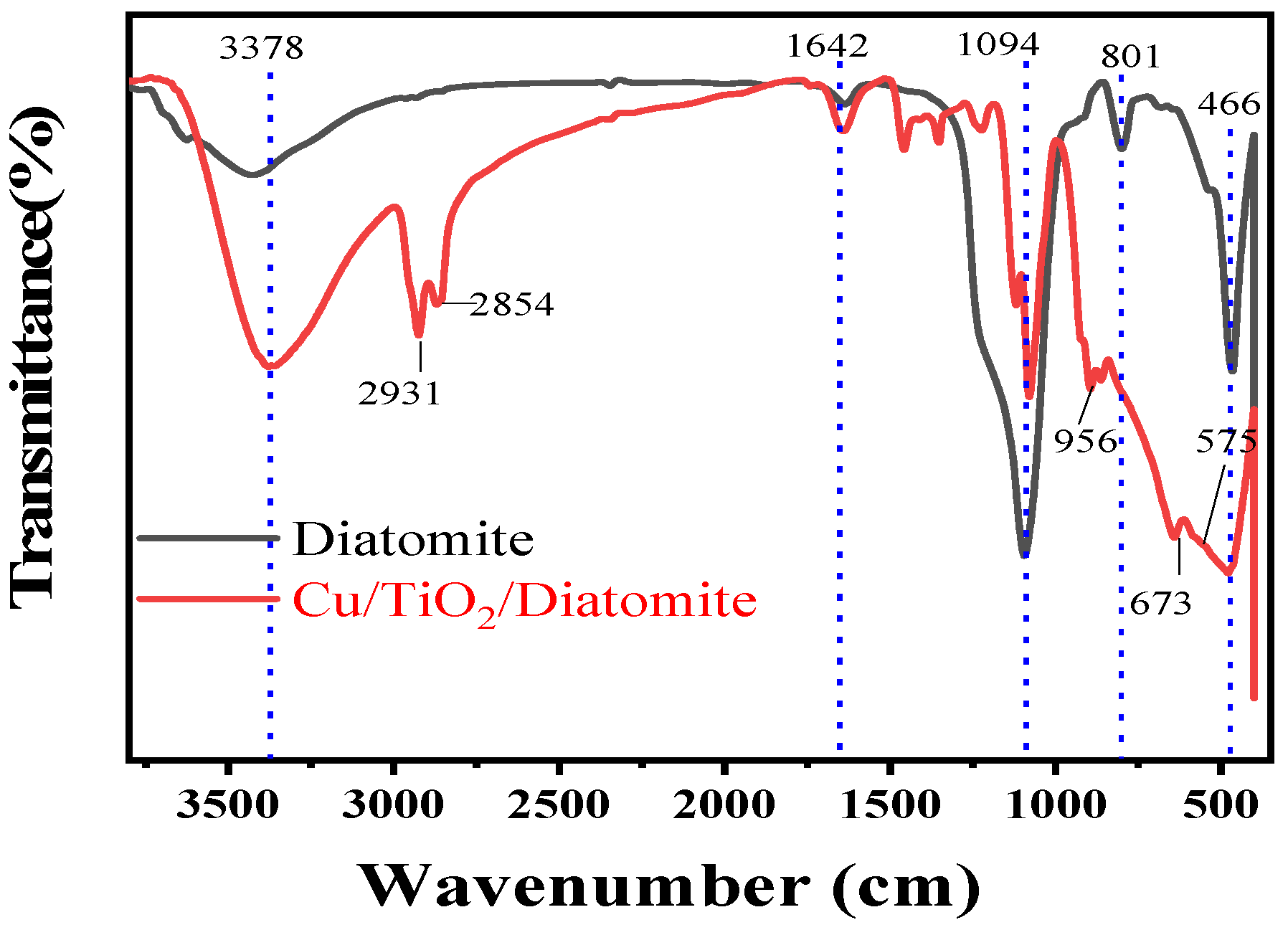
2.5. FTIR Analysis
2.6. XPS Analysis
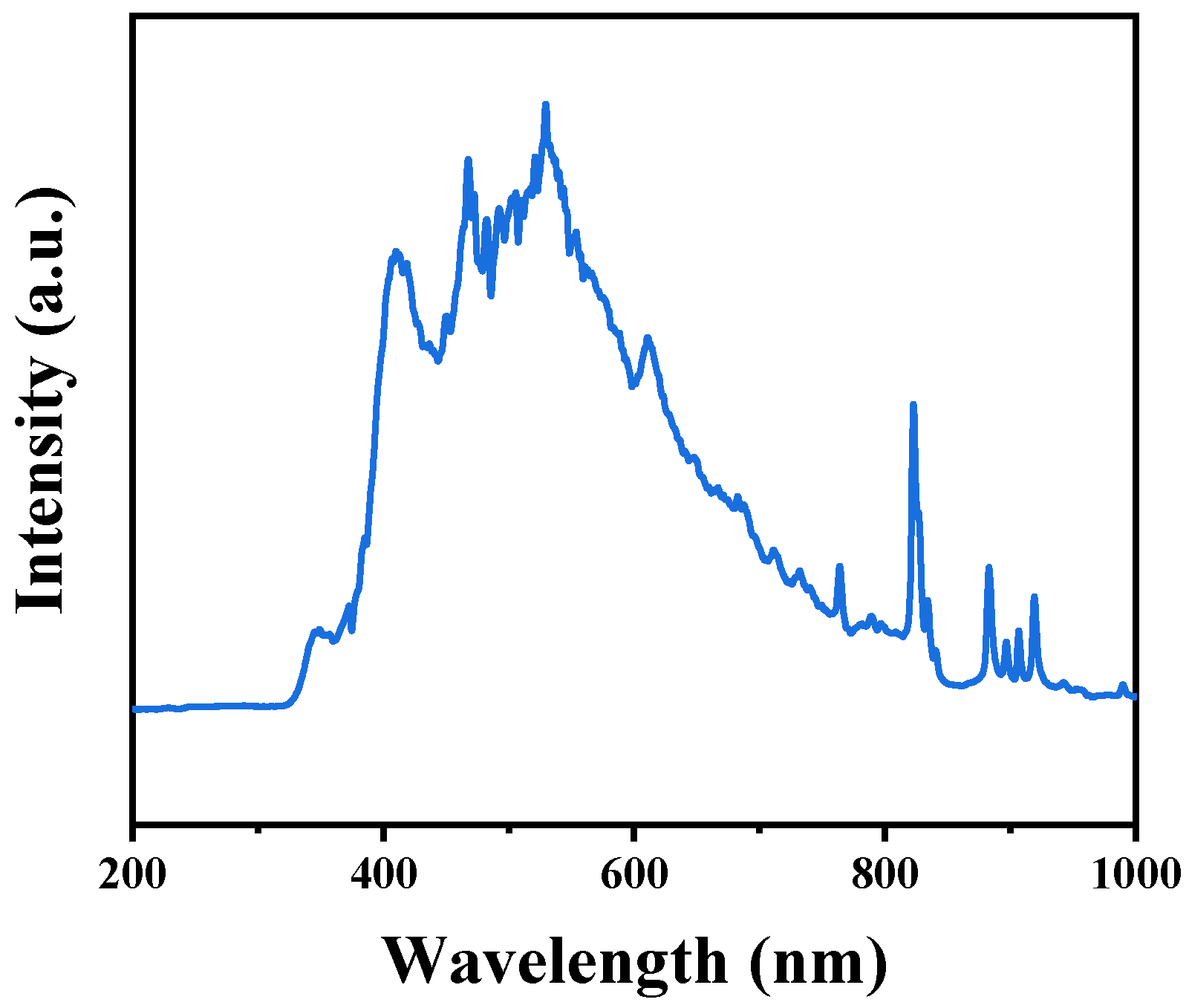
2.7. DRS Analysis
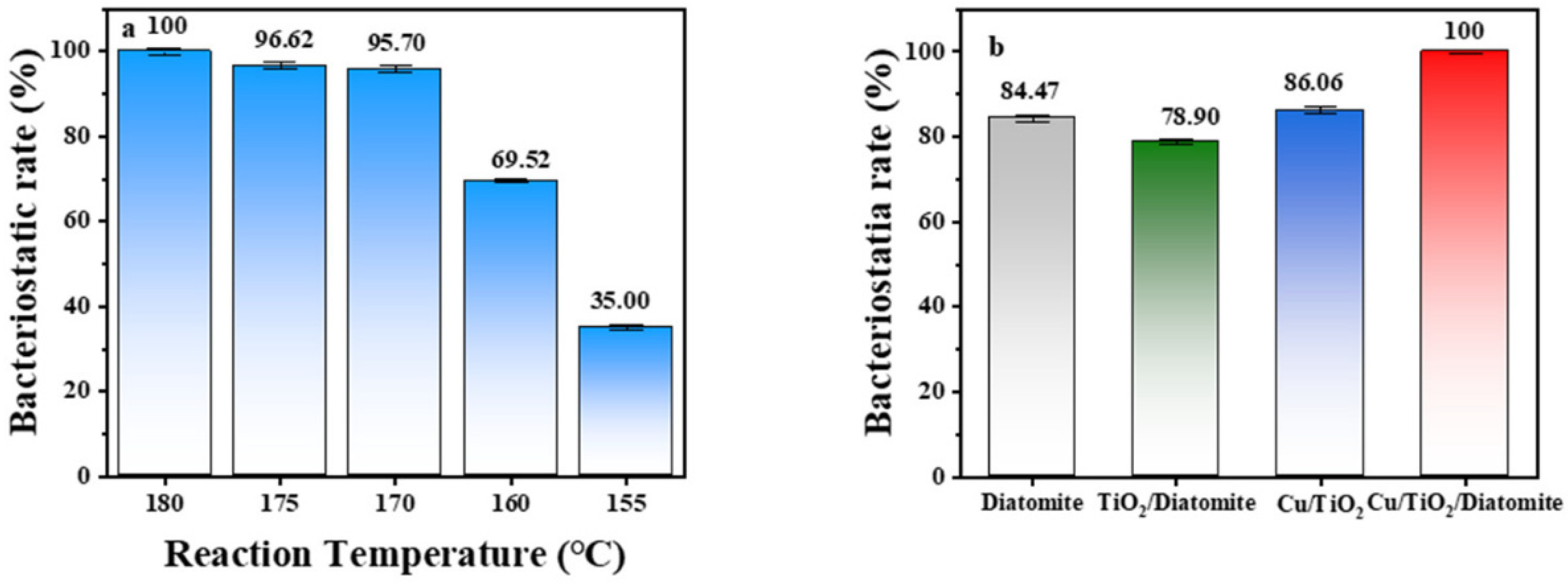
2.8. Antibacterial Activity
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Synthesis of Cu/TiO2/Diatomite
3.3. Synthesis of Cu/TiO2/Diatomite-30
3.4. Antibacterial Study
3.5. Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grinter, R.; Morris, F.C.; Dunstan, R.A.; Leung, P.M.; Kropp, A.; Belousoff, M.; Gunasinghe, S.D.; Scott, N.E.; Beckham, S.; Peleg, A.Y.; et al. BonA from Acinetobacter baumannii forms a divisome- localized decamer that supports outer envelope function. mBio 2021, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.E.; Glaza, P.; Leus, I.V.; Trinh, A.; Su, C.-C.; Cui, M.; Zgurskaya, H.I.; Yu, E.W. Cryoelectron Microscopy Structures of AdeB Illuminate Mechanisms of Simultaneous Binding and Exporting of Substrates. mBio 2021, 12, e03690-20. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Razo-Gutierrez, C.; Le, C.; Courville, R.; Pimentel, C.; Liu, C.; Fung, S.E.; Tuttobene, M.R.; Phan, K.; Vila, A.J.; et al. Cerebrospinal fluid (CSF)) augments metabolism and virulence expression factors in Acinetobacter baumannii. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Knig, P.; Averhoff, B.; Müller, V. K+ and its role in virulence of Acinetobacter baumannii. Int. J. Med. Microbiol. 2021, 311, 151516–151524. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Y.; Lin, L.; Zhao, M.; Zhang, L.; Yan, J.; Wang, Y.; Zeng, J.; Zhang, Y. An in situ ion exchange grown visible-light-driven Z-scheme AgVO3/AgI graphene microtube for enhanced photocatalytic performance. New J. Chem. 2020, 44, 1579–1587. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Yu, X.; Gao, F.; Shen, Z.; Zhang, X.; Ge, S.; Liu, G.; Gu, Z.; Chen, C. An All Mrganic Semiconductor C3N4/PDINH Heterostructure with Advanced Antibacterial Photocatalytic Therapy Activity. Adv. Mater. 2019, 31, 190–196. [Google Scholar]
- Zheng, X.; Shen, Z.; Cheng, C.; Shi, L.; Cheng, R.; Yuan, D.H. Photocatalytic Disinfection Performance in Virus and Virus/Bacteria System by Cu-TiO2, Nanofibers under Visible Light. Environ. Pollut. 2018, 237, 452–459. [Google Scholar] [CrossRef]
- Magda, K.; Pawe, M.; Joanna, Z.; Kobylański, M.; Klimczuk, T.; Lisowski, W.; Trykowski, G.; Nowaczyk, G.; Zaleska-Medynska, A. Electrochemically Obtained TiO2/CuxOy Nanotube Arrays Presenting a Photocatalytic Response in Processes of Pollutants Degradation and Bacteria Inactivation in Aqueous Phase. Catalysts 2018, 8, 237–257. [Google Scholar]
- Ikram, M.; Umar, E.; Raza, A.; Haider, A.; Naz, S.; Ul-Hamid, D.A.; Haider, J.; Shahzadi, I.; Hassan, J.; Ali, S. Dye degradation performance, bactericidal behavior and molecular docking analysis of Cu-doped TiO2 nanoparticles. RSC Adv. 2020, 10, 24215–24233. [Google Scholar] [CrossRef]
- Miao, Y.; Lian, Z.; Huo, Y.; Li, H. Microwave Assisted Ionothermal Synthesis of Hierarchical Microcube-Like BiOBr with Enhanced Photocatalytic Activity. Chin. J. Catal. 2018, 39, 1411–1417. [Google Scholar] [CrossRef]
- Masanori, H.; Keisuke, O.; Hiroyuki, I. Direct Formation of Anatase (TiO2)/Silica (SiO2) Composite Nanoparticles with High Phase Stability of 1300 °C from Acidic Solution by Hydrolysis under Hydrothermal Condition. Chem. Mater. 2004, 16, 3725–3732. [Google Scholar]
- Tassadit, B.; Boualem, H.; Alain, C.; Balard, H.; Calvet, R. Physicochemical characterization of a diatomaceous upon an acid treatment: A focus on surface properties by inverse gas chromatography. Powder Technol. 2016, 294, 498–550. [Google Scholar]
- Miao, Y.; Xu, X.; Liu, K.; Wang, N. Preparation of Novel Cu/TiO2 Mischcrystal Composites and Antibacterial Activities for Escherichia Coli under Visible Light. Ceram. Int. 2017, 43, 9658–9663. [Google Scholar] [CrossRef]
- Dao, T.; Ha, T.; Nguyen, T.D.; Le, H.N.; Ha-Thuc, C.N.; Nguyen, T.M.L.; Perre, P.; Nguyen, D.M. Effectiveness of photocatalysis of Montmorillonite-supported TiO2 and TiO2 nanotubes for rhodamine B degradation. Chemosphere 2021, 280, 130802–130813. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Baskin, A.; Liu, Z.; Prendergast, D.; Crumlin, E.J. Addressing the sensitivity of signals from solid/liquid ambient pressure XPS (APXPS) measurement. J. Chem. Phys. 2020, 153, 04470901–04470909. [Google Scholar] [CrossRef]
- Bengotni, L.; Trari, B.; Lebeau, B.; Michelin, L.; Josien, L.; Bengueddach, A.; Hamacha, R. Effect of diatomite addition on crystalline phase formation of TiO2 and photocatalytic degradation of MDMA. New J. Chem. 2021, 45, 13463–13474. [Google Scholar] [CrossRef]
- Zhang, L.W.; Mohamed, H.H.; Dillert, R.; Bahnemann, D. Kinetics and mechanisms of charge transfer processes in photocatalytic systems: A review. Photochem. Photobiol. 2012, 13, 263–276. [Google Scholar] [CrossRef]
- Zhu, B.J.; Chen, S.H.; Li, C.X.; Jiang, G.; Liu, F.; Zhao, R.; Liu, C. Non-metallic hollow porous sphere loaded CN/catalytic ozonation synergistic photocatalytic system: Enhanced treatment of emerging pollutants by three-stage cyclic reaction mechanism. Appl. Catal. B Environ. 2022, 318, 121881–121893. [Google Scholar] [CrossRef]
- Zhang, L.H.; Han, B.; Cheng, P.F.; Hu, Y.H. In-situ FTIR-DRS investigation on shallow trap state of Cu-doped TiO2 photocatalyst. Catal. Today 2020, 341, 24–25. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, K.R. Preparation and characterization of nitrogen-doped TiO2/diatomite integrated photocatalytic pellet for the adsorption-degradation of tetracycline hydrochloride using visible light. Chem. Eng. J. 2016, 32, 682–696. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Q.; Zhou, C.; Jin, Q.T. Facile preparation of Ce doped TiO2/diatomite granular composite with enhanced photocatalytic activity. Adv. Powder Technol. 2018, 29, 106–116. [Google Scholar]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. J. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar]
- Xu, W.J.; Zhang, T.J.; Bai, R.S.; Zhang, P.; Yu, J. A one-step rapid synthesis of TS-1 zeolites with highly catalytically active mononuclear TiO6 species. J. Mater. Chem. A 2020, 8, 9677–9683. [Google Scholar]
- Zhang, G.X.; Liu, Y.Y.; Zheng, S.L.; Sun, Z. Efficient removal of formaldehyde by diatomite decorated with BiOCl/TiO2 under visible-light irradiation: Effects of key preparation parameters. Adv. Powder Technol. 2021, 32, 4364–4372. [Google Scholar] [CrossRef]
- Li, C.Q.; Sun, Z.M.; Ma, R.X.; Xue, Y.; Zheng, S. Fluorine doped anatase TiO2 with exposed reactive (001) facets supported on porous diatomite for enhanced visible-light photocatalytic activity. Microporous Mesoporous Mater. 2017, 243, 281–290. [Google Scholar]
- Chen, Y.; Liu, K. Fabrication of Ce/N co-doped TiO2/diatomite granule catalyst and its improved visible-light-driven photoactivity. J. Hazard. Mater. 2017, 324, 139–150. [Google Scholar] [CrossRef]
- Yamanaka, K.; Ohwaki, T.; Morikawa, T. Charge-carrier dynamics in Cu-or Fe-loaded nitrogen-doped TiO2 powder studied by femtosecond diffuse reflectance spectroscopy. J. Phys. Chem. C 2013, 117, 16448–16456. [Google Scholar] [CrossRef]
- Dong, X.B.; Chen, Z.T.; Tang, A.D.; Dionysiou, D.D.; Yang, H. Mineral Modulated Single Atom Catalyst for Effective Water Treatment. Adv. Funct. Mater. 2022, 32, 2111565–2111575. [Google Scholar] [CrossRef]
- Choi, H.; Khan, S.; Choi, J.; Dinh, D.T.; Lee, S.Y.; Paik, U.; Cho, S.-H.; Kim, S. Synergetic control of band gap and structural transformation for optimizing TiO2 photocatalysts. Appl. Catal. B Environ. 2017, 210, 513–521. [Google Scholar]
- Gao, B.J.; Jiang, P.F.; An, F.Q.; Zhao, S.; Ge, Z. Studies on the surface modification of diatomite with polyethyleneimine and trapping effect of the modified diatomite for phenol. Appl. Surf. Sci. 2005, 250, 273–281. [Google Scholar] [CrossRef]




| Sample | BET Surface Area (m2/g) | Pore Volume (cc/g) | Pore Size (nm) |
|---|---|---|---|
| Diatomite | 42.456 | 0.069 | 2.516 |
| Cu/TiO2/Diatomite | 207.759 | 0.305 | 3.938 |
| Cu/TiO2 | 13.361 | 0.020 | 1.356 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Yang, Y.; Miao, Y.; Liu, K.; Lv, F.; Zhou, L.; Tang, X.; Liu, Y.; Guo, X. Photocatalytic Bacterial Inactivation of Acinetobacter baumannli on Cu/TiO2/Diatomite. Catalysts 2022, 12, 1217. https://doi.org/10.3390/catal12101217
Xu X, Yang Y, Miao Y, Liu K, Lv F, Zhou L, Tang X, Liu Y, Guo X. Photocatalytic Bacterial Inactivation of Acinetobacter baumannli on Cu/TiO2/Diatomite. Catalysts. 2022; 12(10):1217. https://doi.org/10.3390/catal12101217
Chicago/Turabian StyleXu, Xiaolin, Yacong Yang, Yingchun Miao, Kaiquan Liu, Fujian Lv, Liping Zhou, Xuqi Tang, Yanmi Liu, and Xinchun Guo. 2022. "Photocatalytic Bacterial Inactivation of Acinetobacter baumannli on Cu/TiO2/Diatomite" Catalysts 12, no. 10: 1217. https://doi.org/10.3390/catal12101217





