Photocatalytic Reduction of Hexavalent Chromium Using Cu3.21Bi4.79S9/g-C3N4 Nanocomposite
Abstract
:1. Introduction
2. Results and Discussion
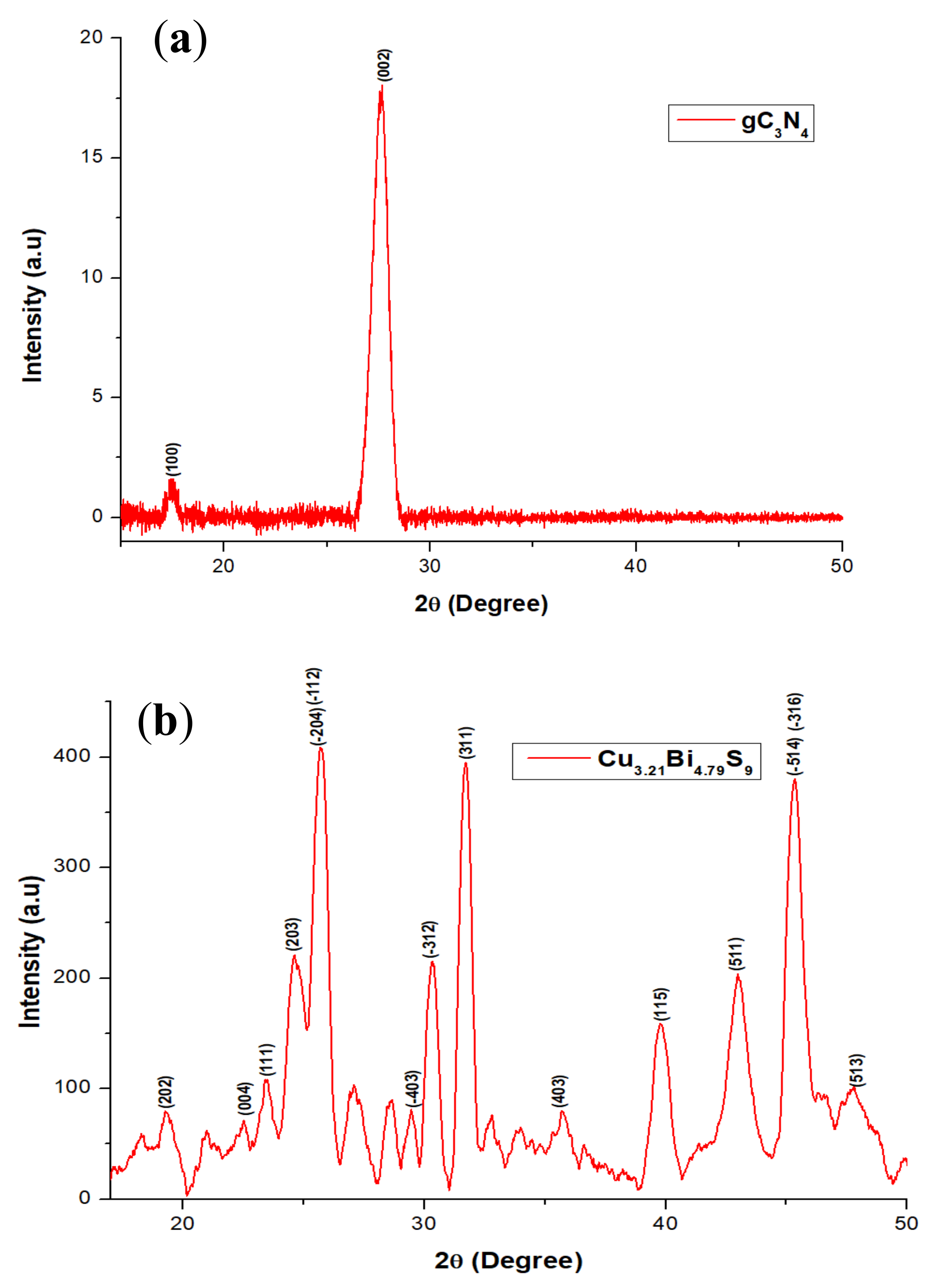
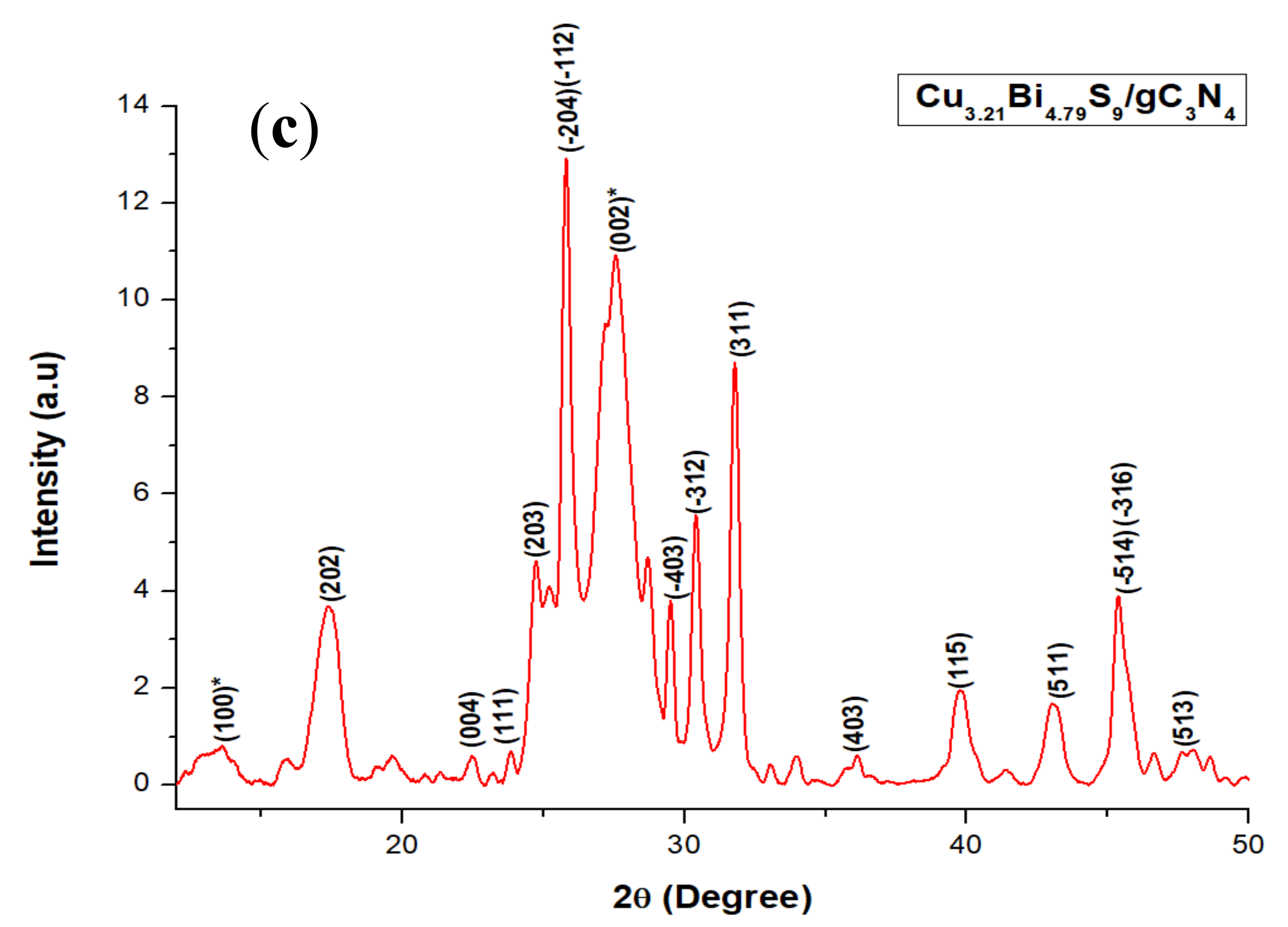
2.1. X-ray Diffraction (XRD) Studies
2.2. FTIR Studies
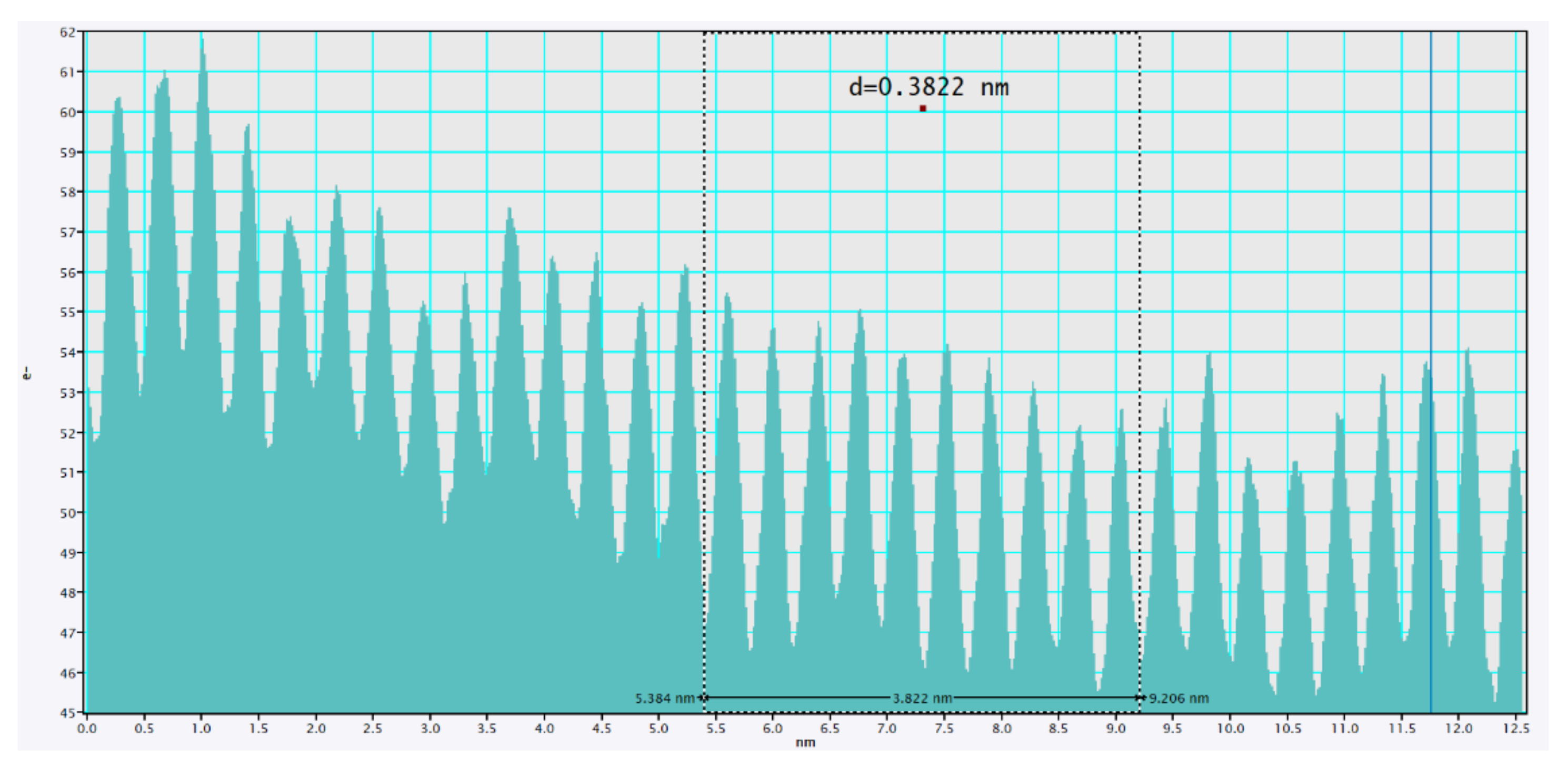
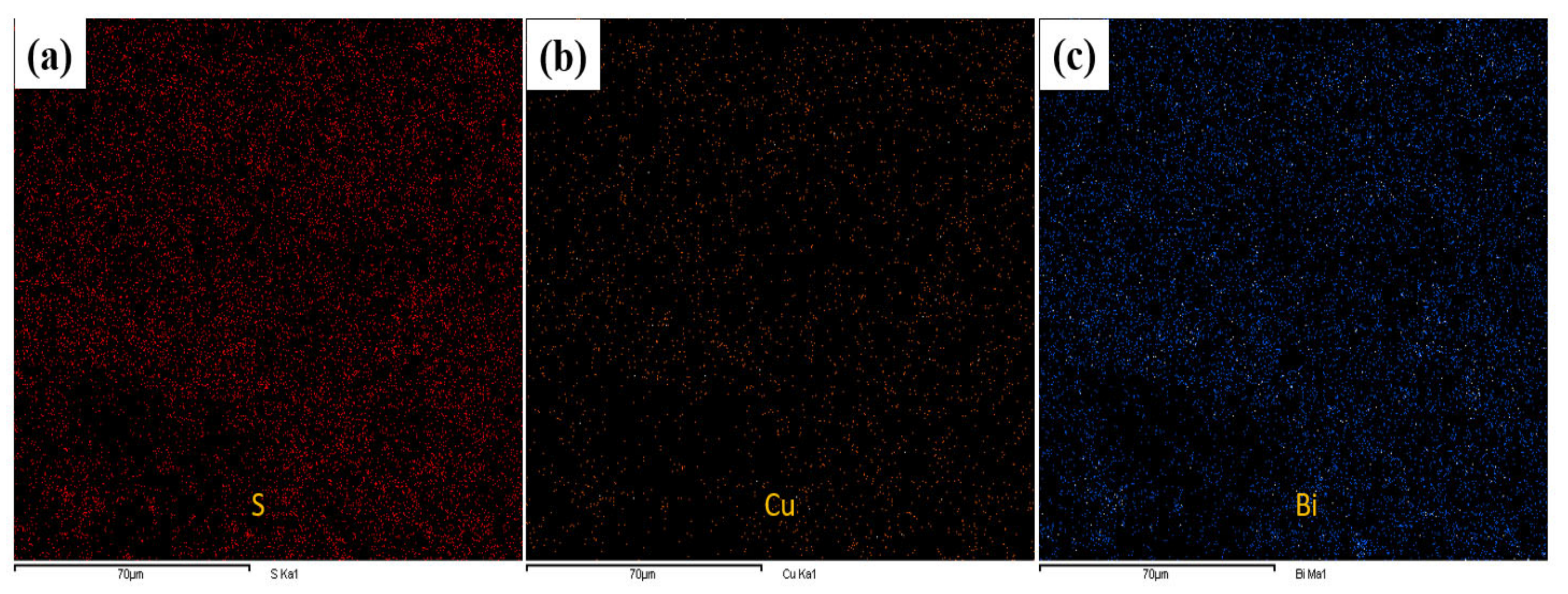
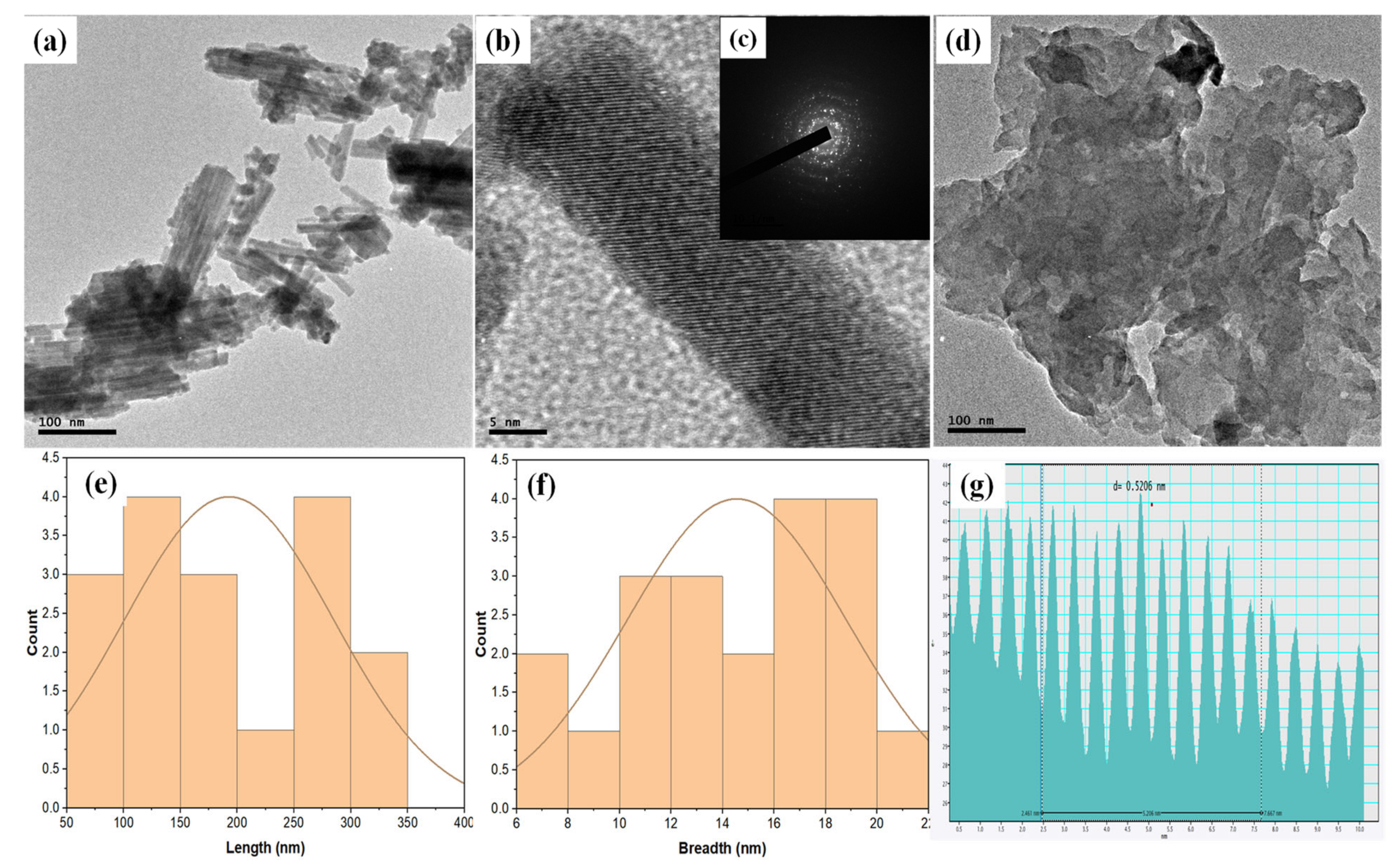
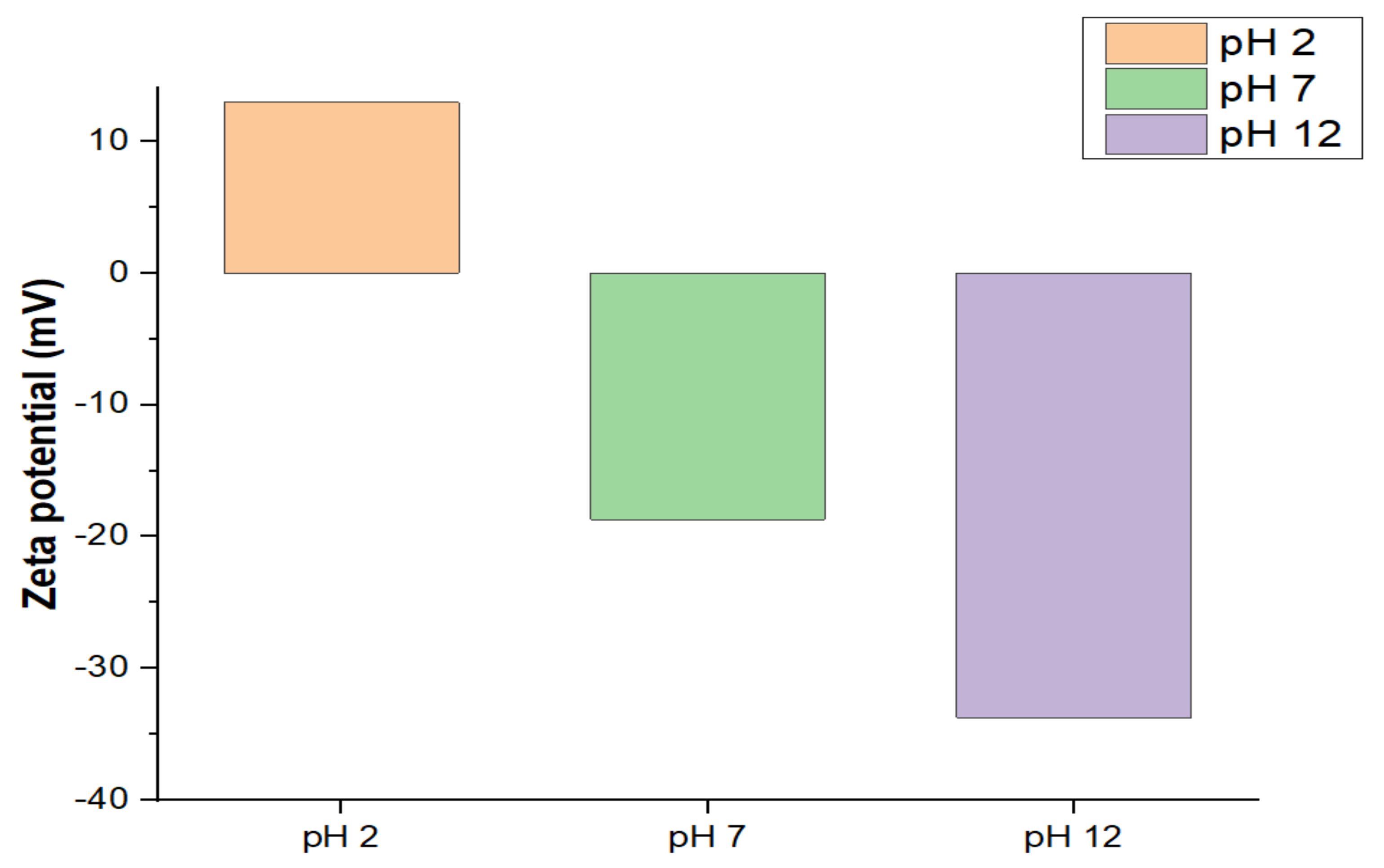
2.3. Morphological Properties of Cu3.21Bi4.79S9 and Cu3.21Bi4.79S9/gC3N4
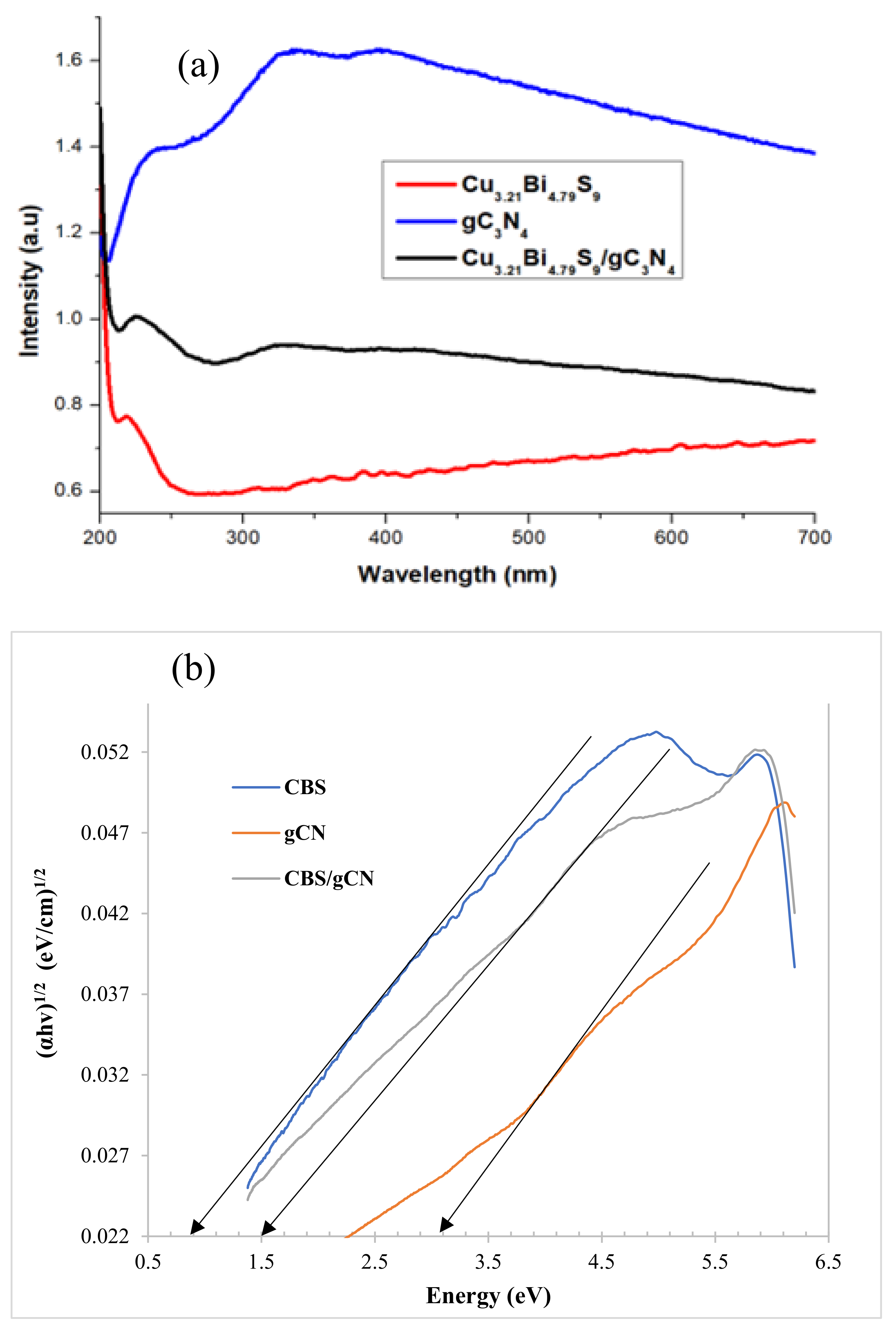
2.4. Optical Properties
2.5. Thermal Studies of Cu3.21Bi4.79S9/gC3N4 Composite
3. Optimum Conditions for the Photocatalytic Investigations
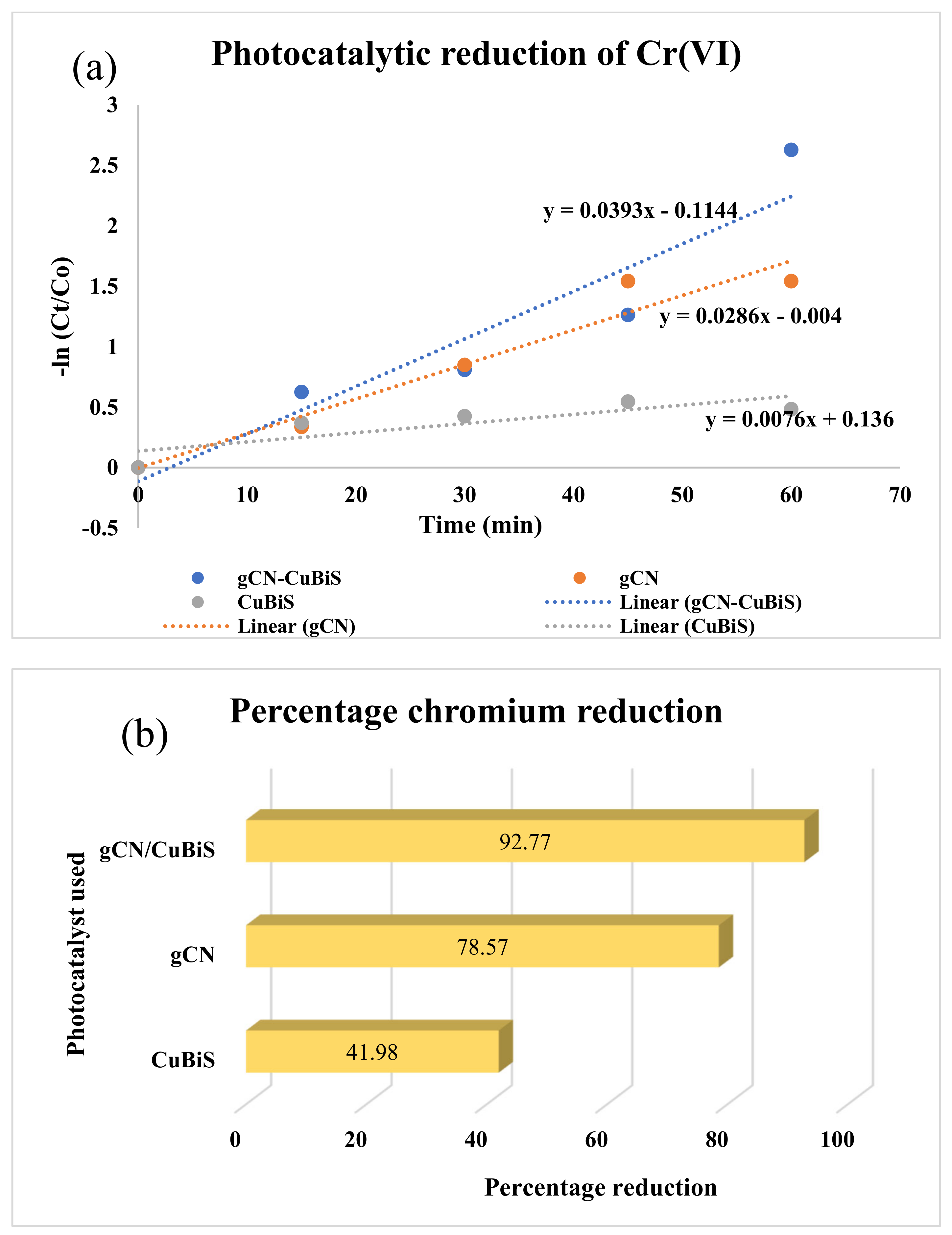
3.1. Photocatalytic Investigations
3.2. Effect of Initial Temperature
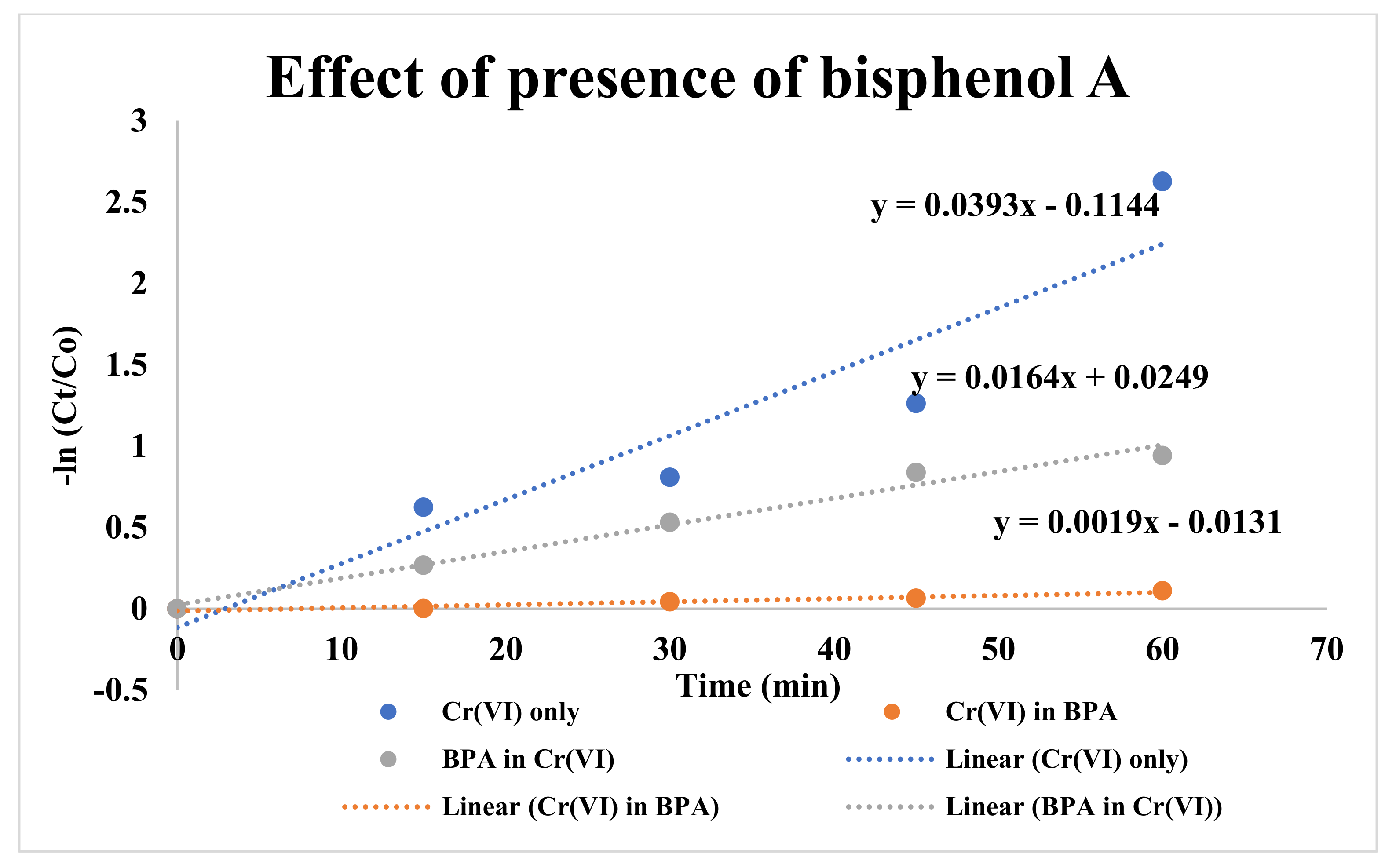
3.3. Effect of the Presence of Bisphenol A
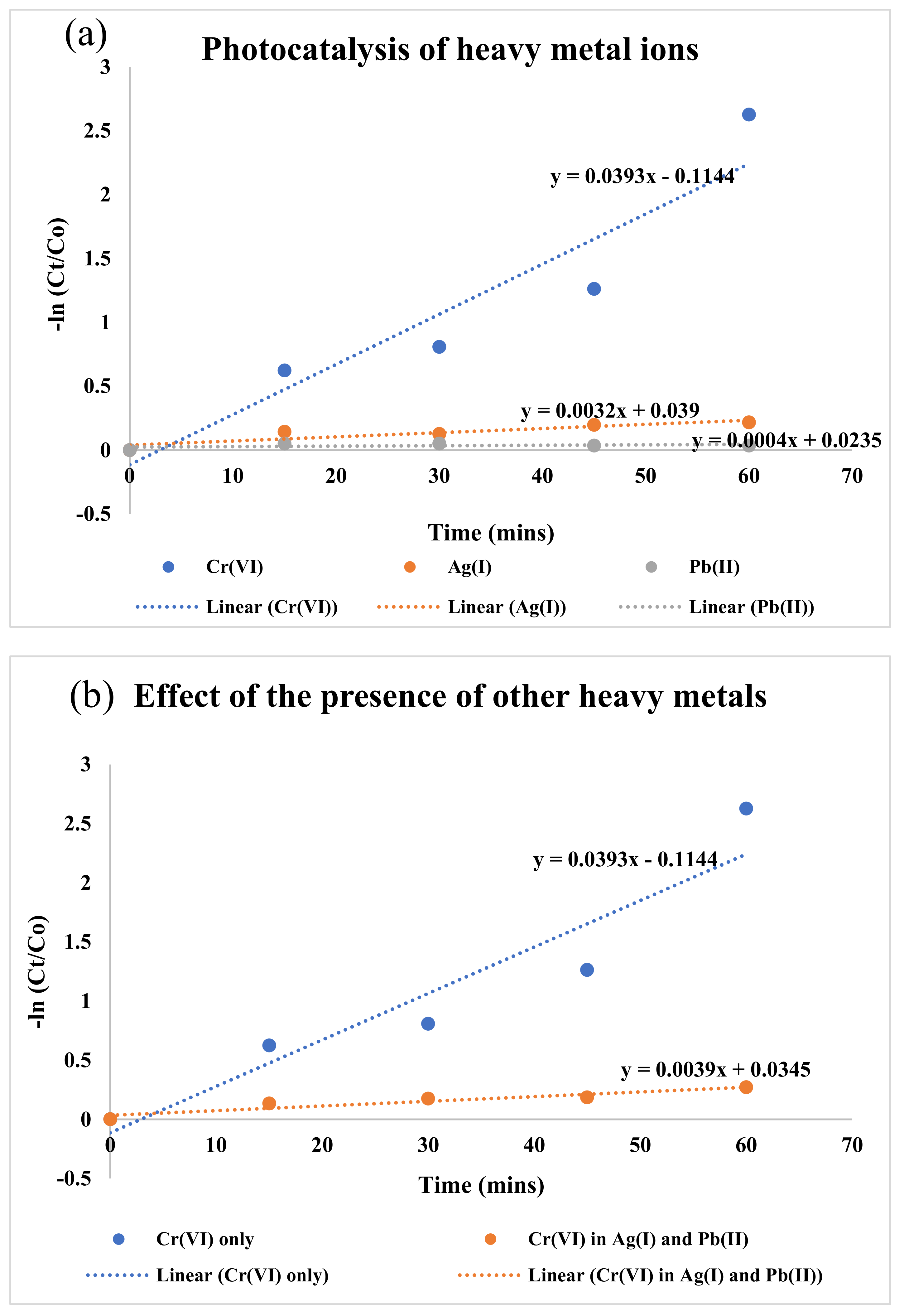
3.4. Effects of the Presence of Other Heavy-Metal Ions
3.5. Radical Scavenging Experiment
4. Experimental
4.1. Materials
4.2. Synthesis of Graphitic Carbon Nitride (gC3N4)
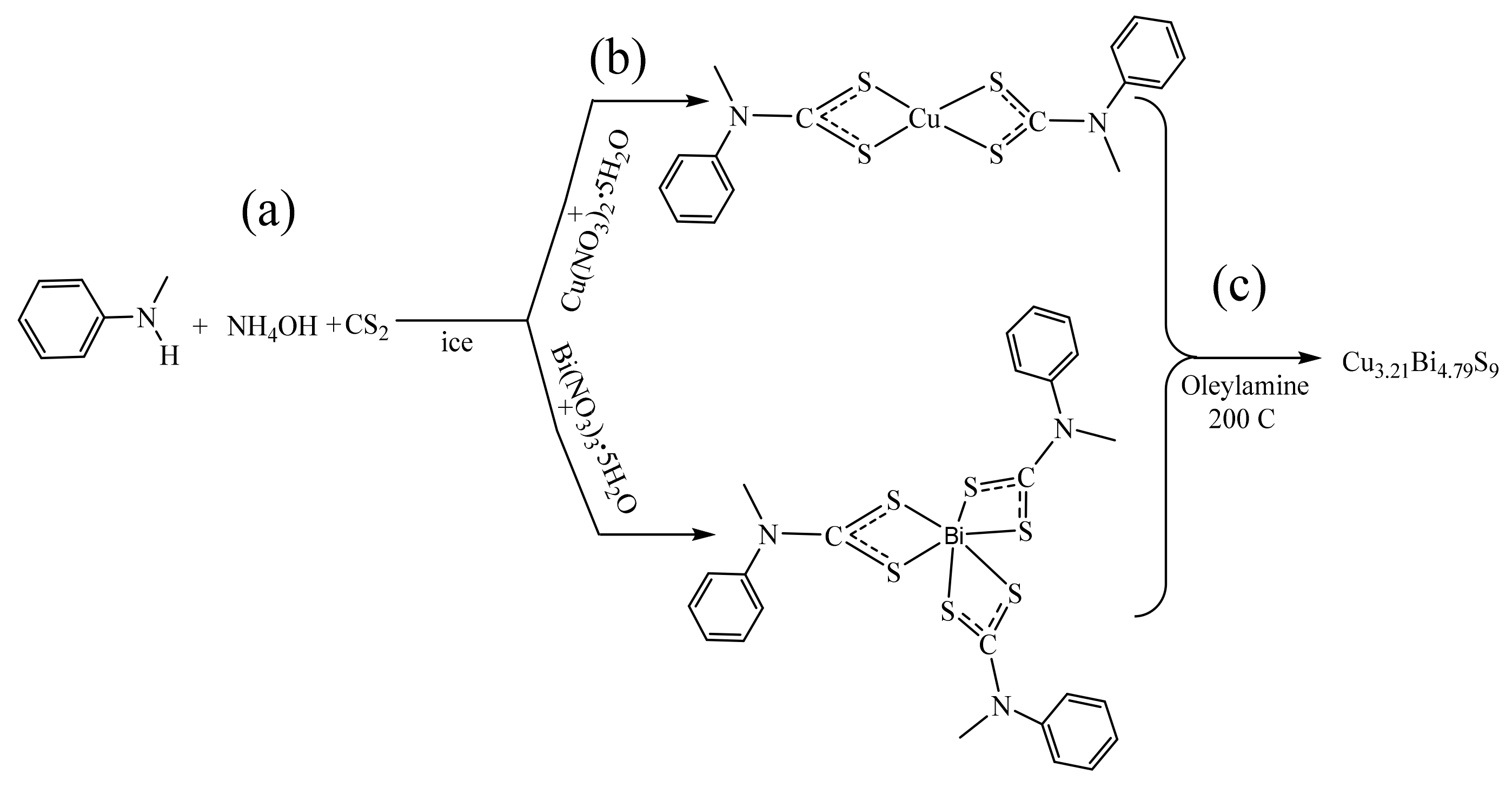
4.3. Synthesis of N-Methyl-N-phenyl Dithiocarbamate Ligand and Complexes
4.4. Synthesis of Cu3.21Bi4.79S9
4.5. Synthesis of Cu3.21Bi4.79S9/gC3N4
4.6. Photocatalytic Investigations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Türkmen, D.; Bakhshpour, M.; Akgönüllü, S.; Aşır, S.; Denizli, A. Heavy Metal Ions Removal From Wastewater Using Cryogels: A Review. Front. Sustain. 2022, 3, 765592. [Google Scholar] [CrossRef]
- Zhao, Z.; An, H.; Lin, J.; Feng, M.; Murugadoss, V.; Ding, T.; Liu, H.; Shao, Q.; Mai, X.; Wang, N.; et al. Progress on the Photocatalytic Reduction Removal of Chromium Contamination. Chem. Rec. 2019, 19, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Conventional and Current Methods of Toxic Metals Removal from Water Using g-C3N4-Based Materials. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1419–1442. [Google Scholar] [CrossRef]
- Viti, C.; Giovannetti, L. Bioremediation of soils polluted with hexavalent chromium using bacteria: A challenge. In Environmental Bioremediation Technologies; Springer: Berlin/Heidelberg, Germany, 2007; pp. 57–76. [Google Scholar]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250–251, 272–291. [Google Scholar] [CrossRef]
- Koutavarapu, R.; Reddy, C.V.; Syed, K.; Reddy, K.R.; Saleh, T.A.; Lee, D.-Y.; Shim, J.; Aminabhavi, T.M. Novel Z-scheme binary zinc tungsten oxide/nickel ferrite nanohybrids for photocatalytic reduction of chromium (Cr(VI)), photoelectrochemical water splitting and degradation of toxic organic pollutants. J. Hazard. Mater. 2022, 423, 127044. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Kuvarega, A.T.; Onwudiwe, D.C. Graphitic carbon nitride-based catalysts and their applications: A review. Nano-Struct. Nano-Objects 2020, 24, 100577. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. The performance of bismuth-based compounds in photocatalytic applications. Surf. Interfaces 2021, 23, 100927. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, G. A review on the factors affecting the photocatalytic degradation of hazardous materials. Mater. Sci. Eng. Int. J. 2017, 1, 106–114. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, X.; Xie, K.; Zhang, X.-F.; Feng, Y.; Jia, M.; Yao, J. Noble metal nanoparticle-functionalized Zr-metal organic frameworks with excellent photocatalytic performance. J. Colloid Interface Sci. 2019, 538, 569–577. [Google Scholar] [CrossRef]
- Rahman, A.; Khan, M.M. Chalcogenides as photocatalysts. New J. Chem. 2021, 45, 19622–19635. [Google Scholar] [CrossRef]
- Sun, Y.; Tan, R.; Jin, Z.; Zhang, Y.; Li, X.; Cai, Q.; Nan, J.; Liu, D.; Yu, H.; Gui, J. Template-free synthesis of Mn2+ doped hierarchical CuS yolk–shell microspheres for photocatalytic reduction of Cr(vi). CrystEngComm 2022, 24, 2171–2178. [Google Scholar] [CrossRef]
- Okla, M.K.; Kokilavani, S.; Mohebaldin, A.; Thomas, A.M.; Soufan, W.; Abdel-Maksoud, M.A.; AbdElgawad, H.; Raju, L.L.; Khan, S.S. Ag decorated CoO NPs supported on chitosan matrix for colorimetric detection of L-cysteine, antibacterial application and photocatalytic reduction of hexavalent chromium ions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128318. [Google Scholar] [CrossRef]
- Kenfoud, H.; Nasrallah, N.; Baaloudj, O.; Derridj, F.; Trari, M. Enhanced photocatalytic reduction of Cr(VI) by the novel hetero-system BaFe2O4/SnO2. J. Phys. Chem. Solids 2022, 160, 110315. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.; Liu, W.; Luo, T.; Jin, Z.; Zhang, Y.; Huang, J.; Zhang, H.; Wang, J.; Peng, F. Rapid photocatalytic reduction of Cr(VI) with high concentration in wastewater by In2S3-ZnIn2S4 heterostructure hierarchical microtubes under visible light. J. Solid State Chem. 2022, 306, 122721. [Google Scholar] [CrossRef]
- Shawky, A.; Mohamed, R.M.; Alahmadi, N.; Zaki, Z.I. Enhanced photocatalytic reduction of hexavalent chromium ions over S-Scheme based 2D MoS2-supported TiO2 heterojunctions under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128564. [Google Scholar] [CrossRef]
- Hua, Y.; Hu, C.; Arif, M.; Chen, S.-m.; Zhang, M.; Liu, X. Direct Z-scheme WO3/In2S3 heterostructures for enhanced photocatalytic reduction Cr(VI). J. Alloy. Compd. 2022, 908, 164488. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Li, Y.; Zhu, R.; Pang, H. Applications of Metal–Organic-Framework-Derived Carbon Materials. Adv. Mater. 2019, 31, 1804740. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Ai, Q.; Gao, G.; Yuan, L.; Fang, Q.; Tian, X.; Zhang, X.; Egap, E.; Ajayan, P.M.; et al. In Situ Synthesis of Lead-Free Halide Perovskite–COF Nanocomposites as Photocatalysts for Photoinduced Polymerization in Both Organic and Aqueous Phases. ACS Mater. Lett. 2022, 4, 464–471. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhang, H.; Xiang, Q. Porous graphitic carbon nitride for solar photocatalytic applications. Nanoscale Horiz. 2020, 5, 765–786. [Google Scholar] [CrossRef]
- Wang, D.; Cao, M.; Feng, Y.; Yao, J. Self-assembly of ZnIn2S4 nanosheets on g-C3N4 nanotubes for efficient photocatalytic reduction of Cr(VI). Microporous Mesoporous Mater. 2022, 330, 111598. [Google Scholar] [CrossRef]
- Ren, Y.; Gong, T.; Tan, S.; Chen, M.; Zhou, F.; Lin, Y.; Yang, L.; Peng, Q. Photocatalytic activities of g-C3N4, Bi3NbO7 and g-C3N4/Bi3NbO7 in photocatalytic reduction of Cr(VI). J. Alloy. Compd. 2022, 902, 163752. [Google Scholar] [CrossRef]
- Sarker, J.C.; Hogarth, G. Dithiocarbamate Complexes as Single Source Precursors to Nanoscale Binary, Ternary and Quaternary Metal Sulfides. Chem. Rev. 2021, 121, 6057–6123. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Ajiboye, T.T.; Marzouki, R.; Onwudiwe, D.C. The Versatility in the Applications of Dithiocarbamates. Int. J. Mol. Sci. 2022, 23, 1317. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Shen, S.; Zhang, Y.; Xu, H.; Wang, Q. A generalized strategy for controlled synthesis of ternary metal sulfide nanocrystals. New J. Chem. 2014, 38, 77–83. [Google Scholar] [CrossRef]
- Olatunde, O.C.; Onwudiwe, D.C. UV-light assisted activation of persulfate by rGO-Cu3BiS3 for the degradation of diclofenac. Results Chem. 2022, 4, 100273. [Google Scholar] [CrossRef]
- Yan, C.; Gu, E.; Liu, F.; Lai, Y.; Li, J.; Liu, Y. Colloidal synthesis and characterizations of wittichenite copper bismuth sulphide nanocrystals. Nanoscale 2013, 5, 1789–1792. [Google Scholar] [CrossRef]
- Sugaki, A.; Shima, H. Phase relations of the Cu2S-Bi2S3 system. Yamaguchi Univ. Tech. Rep. 1972, 1, 45–70. [Google Scholar]
- Balasubramanian, V.; Kumar, P.N.; Sengottaiyan, D. Effect of deposition temperature on structural, optical and electrical properties of copper bismuth sulphide (CuBiS2) thin films deposited by chemical bath deposition. Mater. Sci. 2017, 35, 329–334. [Google Scholar] [CrossRef]
- Barma, M.C.; Long, B.D.; Sabri, M.F.M.; Ramesh, S.; Saidur, R.; Said, S.M.; Kimura, K.; Hai, N.H.; Huy, T.D.; Trung, T.B. Synthesis of Cu3.21Bi4.79S9 bismuth chalcogenide by mechanical alloying. Powder Technol. 2016, 294, 348–352. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Ajibade, P.A. Synthesis, Characterization and Thermal Studies of Zn(II), Cd(II) and Hg(II) Complexes of N-Methyl-N-Phenyldithiocarbamate: The Single Crystal Structure of [(C6H5)(CH3)NCS2]4Hg2. Int. J. Mol. Sci. 2011, 12, 1964–1978. [Google Scholar] [CrossRef]
- Hasanvandian, F.; Shokri, A.; Moradi, M.; Kakavandi, B.; Rahman Setayesh, S. Encapsulation of spinel CuCo2O4 hollow sphere in V2O5-decorated graphitic carbon nitride as high-efficiency double Z-type nanocomposite for levofloxacin photodegradation. J. Hazard. Mater. 2022, 423, 127090. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Imade, E.E.; Oyewo, O.A.; Onwudiwe, D.C. Silver functionalized gC3N4: Photocatalytic potency for chromium(VI) reduction, and evaluation of the antioxidant and antimicrobial properties. J. Photochem. Photobiol. A Chem. 2022, 432, 114107. [Google Scholar] [CrossRef]
- Zhong, J.; Ni, T.; Huang, J.; Li, D.; Tan, C.; Liu, Y.; Chen, P.; Wen, C.; Liu, H.; Wang, Z.; et al. Directional utilization disorder charge via In-plane driving force of functionalized graphite carbon nitride for the robust photocatalytic degradation of fluoroquinolone. Chem. Eng. J. 2022, 442, 135943. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Fereshteh, Z.; Davar, F. Synthesis of oleylamine capped copper nanocrystals via thermal reduction of a new precursor. Polyhedron 2009, 28, 126–130. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Shi, R.; Zhu, Y. Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J. Mater. Chem. A 2013, 1, 14766–14772. [Google Scholar] [CrossRef]
- Riyaz, S.; Parveen, A.; Azam, A. Microstructural and Optical Properties of CuS Nanoparticles prepared by Sol Gel route. Perspect. Sci. 2016, 8, 632–635. [Google Scholar] [CrossRef]
- Veerakumar, P.; Jaysiva, G.; Chen, S.-M.; Lin, K.-C. Development of Palladium on Bismuth Sulfide Nanorods as a Bifunctional Nanomaterial for Efficient Electrochemical Detection and Photoreduction of Hg(II) Ions. ACS Appl. Mater. Interfaces 2022, 14, 5908–5920. [Google Scholar] [CrossRef]
- Esmati, M.; Allahresani, A.; Naghizadeh, A. Synthesis and characterization of Graphitic Carbon Nitride/Mesoporous Nano-Silica (g-C3N4/KCC-1) nanocomposite as a novel highly efficient and recyclable photocatalyst for degradation of antibiotic in aqueous solution. Res. Chem. Intermed. 2021, 47, 1447–1469. [Google Scholar] [CrossRef]
- Miller, T.S.; Jorge, A.B.; Suter, T.M.; Sella, A.; Corà, F.; McMillan, P.F. Carbon nitrides: Synthesis and characterization of a new class of functional materials. Phys. Chem. Chem. Phys. 2017, 19, 15613–15638. [Google Scholar] [CrossRef]
- Haryński, Ł.; Olejnik, A.; Grochowska, K.; Siuzdak, K. A facile method for Tauc exponent and corresponding electronic transitions determination in semiconductors directly from UV–Vis spectroscopy data. Opt. Mater. 2022, 127, 112205. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Chen, X.; Leng, L.; Li, H. Synthesis and applications of novel graphitic carbon nitride/metal-organic frameworks mesoporous photocatalyst for dyes removal. Appl. Catal. B Environ. 2015, 174–175, 445–454. [Google Scholar] [CrossRef]
- Guan, B.; Shan, Q.Y.; Chen, H.; Xue, D.; Chen, K.; Zhang, Y.X. Morphology Dependent Supercapacitance of Nanostructured NiCo2O4 on Graphitic Carbon Nitride. Electrochim. Acta 2016, 200, 239–246. [Google Scholar] [CrossRef]
- Gan, L.; Geng, A.; Song, C.; Xu, L.; Wang, L.; Fang, X.; Han, S.; Cui, J.; Mei, C. Simultaneous removal of rhodamine B and Cr(VI) from water using cellulose carbon nanofiber incorporated with bismuth oxybromide: The effect of cellulose pyrolysis temperature on photocatalytic performance. Environ. Res. 2020, 185, 109414. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-K.; Lee, S.-M. Removal of Cr(VI) and humic acid by using TiO2 photocatalysis. Chemosphere 2006, 63, 1677–1684. [Google Scholar] [CrossRef]
- Abinaya, M.; Govindan, K.; Kalpana, M.; Saravanakumar, K.; Prabavathi, S.L.; Muthuraj, V.; Jang, A. Reduction of hexavalent chromium and degradation of tetracycline using a novel indium-doped Mn2O3 nanorod photocatalyst. J. Hazard. Mater. 2020, 397, 122885. [Google Scholar] [CrossRef]
- Hasija, V.; Raizada, P.; Singh, P.; Verma, N.; Khan, A.A.P.; Singh, A.; Selvasembian, R.; Kim, S.Y.; Hussain, C.M.; Nguyen, V.-H.; et al. Progress on the photocatalytic reduction of hexavalent Cr(VI) using engineered graphitic carbon nitride. Process Saf. Environ. Prot. 2021, 152, 663–678. [Google Scholar] [CrossRef]
- Qi, K.; Liu, S.-Y.; Zada, A. Graphitic carbon nitride, a polymer photocatalyst. J. Taiwan Inst. Chem. Eng. 2020, 109, 111–123. [Google Scholar] [CrossRef]
- Saha, D.; Hoinkis, T.J.; Van Bramer, S.E. Electrospun, flexible and reusable nanofiber mat of graphitic carbon nitride: Photocatalytic reduction of hexavalent chromium. J. Colloid Interface Sci. 2020, 575, 433–442. [Google Scholar] [CrossRef]
- Niu, B.; Xiao, J.; Xu, Z. Recycling Spent LiCoO2 Battery as a High-efficient Lithium-doped Graphitic Carbon Nitride/Co3O4 Composite Photocatalyst and Its Synergistic Photocatalytic Mechanism. Energy Environ. Mater. 2022. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Wang, P.; Yin, L.; Tian, Y.; Li, J. Bifunctional copper modified graphitic carbon nitride catalysts for efficient tetracycline removal: Synergy of adsorption and photocatalytic degradation. Chin. Chem. Lett. 2020, 31, 2789–2794. [Google Scholar] [CrossRef]
- Wu, Q.; Lu, D.; Zhang, B.; Kondamareddy, K.K.; Zeng, Y.; Zhang, Y.; Wang, J.; Zhou, M.; Neena, D.; Hao, H.; et al. Interfacial optimization of CeO2 nanoparticles loaded two-dimensional graphite carbon nitride toward synergistic enhancement of visible-light-driven photoelectric and photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 2313–2326. [Google Scholar] [CrossRef]
- Meng, F.; Liu, Y.; Wang, J.; Tan, X.; Sun, H.; Liu, S.; Wang, S. Temperature dependent photocatalysis of g-C3N4, TiO2 and ZnO: Differences in photoactive mechanism. J. Colloid Interface Sci. 2018, 532, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, X.; Terashima, C.; Fujishima, A.; Nakata, K. Thermodynamic and kinetic analysis of heterogeneous photocatalysis for semiconductor systems. Phys. Chem. Chem. Phys. 2014, 16, 8751–8760. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Joo, H.; Her, N.; Yoon, Y.; Sohn, J.; Kim, S.; Yoon, J. Simultaneously photocatalytic treatment of hexavalent chromium (Cr(VI)) and endocrine disrupting compounds (EDCs) using rotating reactor under solar irradiation. J. Hazard. Mater. 2015, 288, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Zhang, H.; Cai, P.; Qiu, R.; Xiong, Y. Simultaneous photocatalytic reduction of Cr(VI) and oxidation of phenol over monoclinic BiVO4 under visible light irradiation. Chemosphere 2006, 63, 956–963. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhu, L.; Yu, H.; Tang, H. Photocatalytic reduction of Cr(VI) over different TiO2 photocatalysts and the effects of dissolved organic species. J. Hazard. Mater. 2008, 152, 93–99. [Google Scholar] [CrossRef]
- Peller, J.; Wiest, O.; Kamat, P.V. Synergy of Combining Sonolysis and Photocatalysis in the Degradation and Mineralization of Chlorinated Aromatic Compounds. Environ. Sci. Technol. 2003, 37, 1926–1932. [Google Scholar] [CrossRef]
- Yang, J.K.; Lee, S.M.; Siboni, M.S. Effect of different types of organic compounds on the photocatalytic reduction of Cr(VI). Environ. Technol. 2012, 33, 2027–2032. [Google Scholar] [CrossRef]
- Song, J.; Klein, E.L.; Neese, F.; Ye, S. The Mechanism of Homogeneous CO2 Reduction by Ni(cyclam): Product Selectivity, Concerted Proton–Electron Transfer and C–O Bond Cleavage. Inorg. Chem. 2014, 53, 7500–7507. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Tian, F.; Bao, S.; Sun, C.; Yang, W.; Yu, Y. MnIn2S4 nanosheets growing on rods-like β-MnO2 via covalent bonds as high-performance photocatalyst for boosting Cr(VI) photocatalytic reduction under visible light irradiation: Behavior and mechanism study. J. Colloid Interface Sci. 2022, 625, 264–277. [Google Scholar] [CrossRef]
- Shen, X.; Zheng, T.; Yang, J.; Shi, Z.; Xue, Q.; Liu, W.; Shan, S.; Wong, M.H. Removal of Cr(VI) from Acid Wastewater by BC/ZnFe2O4 Magnetic Nanocomposite via the Synergy of Absorption-Photocatalysis. ChemCatChem 2020, 12, 4121–4131. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajiboye, T.O.; Oyewo, O.A.; Marzouki, R.; Onwudiwe, D.C. Photocatalytic Reduction of Hexavalent Chromium Using Cu3.21Bi4.79S9/g-C3N4 Nanocomposite. Catalysts 2022, 12, 1075. https://doi.org/10.3390/catal12101075
Ajiboye TO, Oyewo OA, Marzouki R, Onwudiwe DC. Photocatalytic Reduction of Hexavalent Chromium Using Cu3.21Bi4.79S9/g-C3N4 Nanocomposite. Catalysts. 2022; 12(10):1075. https://doi.org/10.3390/catal12101075
Chicago/Turabian StyleAjiboye, Timothy O., Opeyemi A. Oyewo, Riadh Marzouki, and Damian C. Onwudiwe. 2022. "Photocatalytic Reduction of Hexavalent Chromium Using Cu3.21Bi4.79S9/g-C3N4 Nanocomposite" Catalysts 12, no. 10: 1075. https://doi.org/10.3390/catal12101075






