Biocomposites Using Whole or Valuable Component-Extracted Microalgae Blended with Polymers: A Review
Abstract
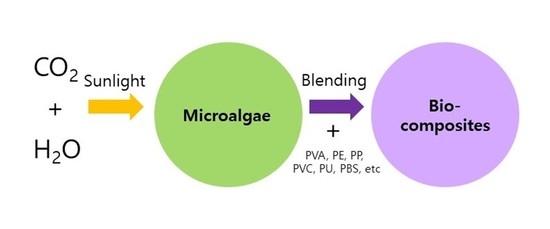
:1. Introduction
2. Major Components in Microalgae
2.1. Proteins
2.2. Carbohydrates
2.3. Lipids
3. Microalgal Biomass-polymer Blends
3.1. Composites with Poly(vinyl alcohol)
3.2. Composites with Polyethylene or Polypropylene
3.3. Composites with Poly(vinyl chloride)
3.4. Composites with Polyurethane
3.5. Biodegradable Bioplastics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aliyu, A.; Lee, J.; Harvey, A. Microalgae for biofuels via thermochemical conversion processes: A review of cultivation, harvesting and drying processes, and the associated opportunities for integrated production. Bioresour. Technol. Rep. 2021, 14, 100676. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Wu, N.; Lan, C.Q. CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 2008, 79, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Phwan, C.K.; Ong, H.C.; Chen, W.-H.; Ling, T.C.; Ng, E.P.; Show, P.L. Overview: Comparison of pretreatment technologies and fermentation processes of bioethanol from microalgae. Energy Convers. Manag. 2018, 173, 81–94. [Google Scholar] [CrossRef]
- Sambusiti, C.; Bellucci, M.; Zabaniotou, A.; Beneduce, L.; Monlau, F. Algae as promising feedstocks for fermentative biohydrogen production according to a biorefinery approach: A comprehensive review. Renew. Sust. Energ. Rev. 2015, 44, 20–36. [Google Scholar] [CrossRef]
- Moroney, J.V.; Ynalvez, R.A. Algal Photosynthesis. Encycl. Life Sci. 2018. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Abdelnour, S.; Alagawany, M.; Abdo, M.; Sakr, M.A.; Khafaga, A.F.; Mahgoub, S.A.; Elnesr, S.S.; Gebriel, M.G. Microalgae in modern cancer therapy: Current knowledge. Biomed. Pharmacother. 2019, 111, 42–50. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Kao, P.-C.; Tsai, C.-J.; Lee, D.-J.; Chang, J.-S. Engineering strategies for simultaneous enhancement of C-phycocyanin production and CO2 fixation with Spirulina platensis. Bioresour. Technol. 2013, 145, 307–312. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chan, M.-C.; Liu, C.-C.; Chen, C.-Y.; Lee, W.-L.; Lee, D.-J.; Chang, J.-S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef]
- Irshad, M.; Hong, M.E.; Myint, A.A.; Kim, J.; Sim, S.J. Safe and Complete Extraction of Astaxanthin from Haematococcus pluvialis by Efficient Mechanical Disruption of Cyst Cell Wall. Int. J. Food Eng. 2019, 15, 20190128. [Google Scholar] [CrossRef]
- De Carvalho, J.C.; Magalhaes, A.I., Jr.; de Melo Pereira, G.V.; Medeiros, A.B.P.; Sydney, E.B.; Rodrigues, C.; Aulestia, D.T.M.; de Souza Vandenberghe, L.P.; Soccol, V.T.; Soccol, C.R. Microalgal biomass pretreatment for integrated processing into biofuels, food, and feed. Bioresour. Technol. 2020, 300, 122719. [Google Scholar] [CrossRef]
- Vigani, M.; Parisi, C.; Rodríguez-Cerezo, E.; Barbosa, M.J.; Sijtsma, L.; Ploeg, M.; Enzing, C. Food and feed products from micro-algae: Market opportunities and challenges for the EU. Trends Food Sci. Technol. 2015, 42, 81–92. [Google Scholar] [CrossRef]
- Zhu, L.; Nugroho, Y.; Shakeel, S.; Li, Z.; Martinkauppi, B.; Hiltunen, E. Using microalgae to produce liquid transportation biodiesel: What is next? Renew. Sust. Energ. Rev. 2017, 78, 391–400. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sust. Energ. Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Pierobon, S.; Cheng, X.; Graham, P.; Nguyen, B.; Karakolis, E.; Sinton, D. Emerging microalgae technology: A review. Sustain. Energy Fuels 2018, 2, 13–38. [Google Scholar] [CrossRef]
- Lee, D.-J.; Liao, G.-Y.; Chang, Y.-R.; Chang, J.-S. Coagulation-membrane filtration of Chlorella vulgaris. Bioresour. Technol. 2012, 108, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, R.; Kannan, K.; Deshkar, A.; Yadav, R.; Chakrabarti, T. Enhanced algal CO2 sequestration through calcite deposition by Chlorella sp. and Spirulina platensis in a mini-raceway pond. Bioresour. Technol. 2010, 101, 2616–2622. [Google Scholar] [CrossRef]
- Zheng, H.; Gao, Z.; Yin, J.; Tang, X.; Ji, X.; Huang, H. Harvesting of microalgae by flocculation with poly (γ-glutamic acid). Bioresour. Technol. 2012, 112, 212–220. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yeh, K.-L.; Aisyah, R.; Lee, D.-J.; Chang, J.-S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef]
- Greenwell, H.C.; Laurens, L.; Shields, R.; Lovitt, R.; Flynn, K. Placing microalgae on the biofuels priority list: A review of the technological challenges. J. R. Soc. Interface 2010, 7, 703–726. [Google Scholar] [CrossRef] [Green Version]
- Doucha, J.; Lívanský, K. Influence of processing parameters on disintegration of Chlorella cells in various types of homogenizers. Appl. Microbiol. Biotechnol. 2008, 81, 431–440. [Google Scholar] [CrossRef]
- William, P.; Laurens, L. Microalgae as biodiesel and biomass feedstock: Review and analysis of the biochemistry, energetic and economics. Energy Environ. Sci 2010, 3, 554–590. [Google Scholar] [CrossRef]
- Halim, R.; Rupasinghe, T.W.; Tull, D.L.; Webley, P.A. Mechanical cell disruption for lipid extraction from microalgal biomass. Bioresour. Technol. 2013, 140, 53–63. [Google Scholar] [CrossRef]
- Cooney, M.; Young, G.; Nagle, N. Extraction of bio-oils from microalgae. Sep. Purif. Rev. 2009, 38, 291–325. [Google Scholar] [CrossRef]
- Mercer, P.; Armenta, R.E. Developments in oil extraction from microalgae. Eur. J. Lipid Sci. Technol. 2011, 113, 539–547. [Google Scholar] [CrossRef]
- Mendes, R.L.; Reis, A.D.; Palavra, A.F. Supercritical CO2 extraction of γ-linolenic acid and other lipids from Arthrospira (Spirulina) maxima: Comparison with organic solvent extraction. Food Chem. 2006, 99, 57–63. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Hu, X.; Su, W.; Zhong, M. Combined enzymatic and mechanical cell disruption and lipid extraction of green alga Neochloris oleoabundans. Int. J. Mol. Sci. 2015, 16, 7707–7722. [Google Scholar] [CrossRef]
- Harris, J.; Viner, K.; Champagne, P.; Jessop, P.G. Advances in microalgal lipid extraction for biofuel production: A review. Biofuel Bioprod. Biorefin. 2018, 12, 1118–1135. [Google Scholar] [CrossRef]
- European Bioplastics. Bioplastics market development update 2020. In Proceedings of the European Bioplastics Conference, Berlin, Germany, 2 December 2020; p. 10117. [Google Scholar]
- Zeller, M.A.; Hunt, R.; Jones, A.; Sharma, S. Bioplastics and their thermoplastic blends from Spirulina and Chlorella microalgae. J. Appl. Polym. Sci. 2013, 130, 3263–3275. [Google Scholar] [CrossRef]
- Dixon, C.; Wilken, L.R. Green microalgae biomolecule separations and recovery. Bioresour. Bioprocess. 2018, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Brown, R.C.; Homsy, S.; Martinez, L.; Sidhu, S.S. Fast pyrolysis of microalgae remnants in a fluidized bed reactor for bio-oil and biochar production. Bioresour. Technol. 2013, 127, 494–499. [Google Scholar] [CrossRef]
- Xu, L.; Brilman, D.W.W.; Withag, J.A.; Brem, G.; Kersten, S. Assessment of a dry and a wet route for the production of biofuels from microalgae: Energy balance analysis. Bioresour. Technol. 2011, 102, 5113–5122. [Google Scholar] [CrossRef] [PubMed]
- Jena, U.; Das, K.; Kastner, J. Effect of operating conditions of thermochemical liquefaction on biocrude production from Spirulina platensis. Bioresour. Technol. 2011, 102, 6221–6229. [Google Scholar] [CrossRef]
- Chen, W.-H.; Huang, M.-Y.; Chang, J.-S.; Chen, C.-Y. Thermal decomposition dynamics and severity of microalgae residues in torrefaction. Bioresour. Technol. 2014, 169, 258–264. [Google Scholar] [CrossRef]
- Chen, W.-H.; Wu, Z.-Y.; Chang, J.-S. Isothermal and non-isothermal torrefaction characteristics and kinetics of microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2014, 155, 245–251. [Google Scholar] [CrossRef]
- Shuping, Z.; Yulong, W.; Mingde, Y.; Chun, L.; Junmao, T. Pyrolysis characteristics and kinetics of the marine microalgae Dunaliella tertiolecta using thermogravimetric analyzer. Bioresour. Technol. 2010, 101, 359–365. [Google Scholar] [CrossRef]
- Mahdy, A.; Mendez, L.; Ballesteros, M.; González-Fernández, C. Enhanced methane production of Chlorella vulgaris and Chlamydomonas reinhardtii by hydrolytic enzymes addition. Energy Convers. Manag. 2014, 85, 551–557. [Google Scholar] [CrossRef]
- Du, Z.; Mohr, M.; Ma, X.; Cheng, Y.; Lin, X.; Liu, Y.; Zhou, W.; Chen, P.; Ruan, R. Hydrothermal pretreatment of microalgae for production of pyrolytic bio-oil with a low nitrogen content. Bioresour. Technol. 2012, 120, 13–18. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, R.; Yu, T.; Li, T.; Zhou, J.; Cen, K. Biodiesel production from lipids in wet microalgae with microwave irradiation and bio-crude production from algal residue through hydrothermal liquefaction. Bioresour. Technol. 2014, 151, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Al Rey, C.V.; Mayol, A.P.; Ubando, A.T.; Biona, J.B.M.M.; Arboleda, N.B.; David, M.Y.; Tumlos, R.B.; Lee, H.; Lin, O.H.; Espiritu, R.A. Microwave drying characteristics of microalgae (Chlorella vulgaris) for biofuel production. Clean Technol. Environ. Policy 2016, 18, 2441–2451. [Google Scholar]
- Phong, W.N.; Show, P.L.; Ling, T.C.; Juan, J.C.; Ng, E.-P.; Chang, J.-S. Mild cell disruption methods for bio-functional proteins recovery from microalgae—Recent developments and future perspectives. Algal Res. 2018, 31, 506–516. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Guldhe, A.; Ansari, F.A.; Singh, P.; Bux, F. Heterotrophic cultivation of microalgae using aquaculture wastewater: A biorefinery concept for biomass production and nutrient remediation. Ecol. Eng. 2017, 99, 47–53. [Google Scholar] [CrossRef]
- Kannah, R.Y.; Kavitha, S.; Karthikeyan, O.P.; Rene, E.R.; Kumar, G.; Banu, J.R. A review on anaerobic digestion of energy and cost effective microalgae pretreatment for biogas production. Bioresour. Technol. 2021, 332, 125055. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.; Gayen, K.; Bhowmick, T.K. Downstream processing of microalgae for pigments, protein and carbohydrate in industrial application: A review. Food Bioprod. Process. 2018, 110, 60–84. [Google Scholar] [CrossRef]
- Ahn, B.; Park, S.E.; Oh, B.K.; Kim, Y.K. Effect of nanoparticle on cellular growth and lipid production in Chlorella vulgaris culture. Biotechnol. Prog. 2018, 34, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Gourmelon, G. Global plastic production rises, recycling lags. Vital Signs 2015, 22, 91–95. [Google Scholar]
- Venkatachalam, H.; Palaniswamy, R. Bioplastic world: A review. J. Adv. Sci. Res. 2020, 11, 43–53. [Google Scholar]
- Rahman, A.; Miller, C. Microalgae as a source of bioplastics. In Algal Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 121–138. [Google Scholar]
- Tharani, D.; Ananthasubramanian, M. Microalgae as sustainable producers of bioplastic. In Microalgae Biotechnology for Food, Health and High Value Products; Springer: Berlin/Heidelberg, Germany, 2020; pp. 373–396. [Google Scholar]
- Shu, Y.; Maruyama, J.; Iwasaki, S.; Maruyama, S.; Shen, Y.; Uyama, H. Nitrogen-doped biomass/polymer composite porous carbons for high performance supercapacitor. J. Power Sources 2017, 364, 374–382. [Google Scholar] [CrossRef]
- Zhu, N.; Ye, M.; Shi, D.; Chen, M. Reactive compatibilization of biodegradable poly (butylene succinate)/Spirulina microalgae composites. Macromol. Res. 2017, 25, 165–171. [Google Scholar] [CrossRef]
- Tran, D.-T.; Lee, H.R.; Jung, S.; Park, M.S.; Yang, J.-W. Lipid-extracted algal biomass based biocomposites fabrication with poly (vinyl alcohol). Algal Res. 2018, 31, 525–533. [Google Scholar] [CrossRef]
- Roja, K.; Sudhakar, D.R.; Anto, S.; Mathimani, T. Extraction and characterization of polyhydroxyalkanoates from marine green alga and cyanobacteria. Biocatal. Agric. Biotechnol. 2019, 22, 101358. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yoo, C.; Jun, S.-Y.; Ahn, C.-Y.; Oh, H.-M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-A.; Cheng, Y.-M.; Huang, J.-W.; Jen, J.-F.; Huang, Y.-S.; Yu, C.-C. Effects of ultrasonic and microwave pretreatments on lipid extraction of microalgae. Bioprocess. Biosyst. Eng. 2014, 37, 1543–1549. [Google Scholar] [CrossRef]
- Neto, A.M.P.; de Souza, R.A.S.; Leon-Nino, A.D.; da Costa, J.D.a.A.; Tiburcio, R.S.; Nunes, T.A.; de Mello, T.C.S.; Kanemoto, F.T.; Saldanha-Corrêa, F.M.P.; Gianesella, S.M.F. Improvement in microalgae lipid extraction using a sonication-assisted method. Renew. Energ. 2013, 55, 525–531. [Google Scholar] [CrossRef]
- Marudhupandi, T.; Sathishkumar, R.; Kumar, T.T.A. Heterotrophic cultivation of Nannochloropsis salina for enhancing biomass and lipid production. Biotechnol. Rep. 2016, 10, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Mirsiaghi, M.; Reardon, K.F. Conversion of lipid-extracted Nannochloropsis salina biomass into fermentable sugars. Algal Res. 2015, 8, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Gozan, M.; Noviasari, C. The effect of glycerol addition as plasticizer in Spirulina platensis based bioplastic. In Proceedings of the 3rd International Tropical Renewable Energy Conference “Sustainable Development of Tropical Renewable Energy”, Kuta, Bali, Indonesia, 6–8 September 2018; p. 03048. [Google Scholar]
- Khalis, S. The Effect of Compatibilizer Addition on Chlorella vulgaris Microalgae Utilization as a Mixture for Bioplastic. In Proceedings of the 3rd International Tropical Renewable Energy Conference “Sustainable Development of Tropical Renewable Energy”, Kuta, Bali, Indonesia, 6–8 September 2018; p. 03047. [Google Scholar]
- Sabathini, H.; Windiani, L.; Gozan, M. Mechanical Physicial Properties of Chlorella-PVA based Bioplastic with Ultrasonic Homogenizer. In Proceedings of the 3rd International Tropical Renewable Energy Conference “Sustainable Development of Tropical Renewable Energy”, Kuta, Bali, Indonesia, 6–8 September 2018; p. 03046. [Google Scholar]
- Otsuki, T.; Zhang, F.; Kabeya, H.; Hirotsu, T. Synthesis and tensile properties of a novel composite of Chlorella and polyethylene. J. Appl. Polym. Sci. 2004, 92, 812–816. [Google Scholar] [CrossRef]
- Mateescu, C.; Zaharescu, T.; Mariş, M. Chemiluminescence study on the radiochemical stability of polypropylene modified with microalgal extracts. Radiat. Phys. Chem. 2021, 183, 109401. [Google Scholar] [CrossRef]
- Hosseini Tafreshi, S.A.; Aghaie, P.; Toghyani, M.A.; Ramazani-Moghaddam-Arani, A. Improvement of ionizing gamma irradiation tolerance of Chlorella vulgaris by pretreatment with polyethylene glycol. Int. J. Radiat. Biol. 2020, 96, 919–928. [Google Scholar] [CrossRef]
- Zhang, F.; Kabeya, H.; Kitagawa, R.; Hirotsu, T.; Yamashita, M.; Otsuki, T. An exploratory research of PVC-Chlorella composite material (PCCM) as effective utilization of Chlorella biologically fixing CO2. J. Mater. Sci. 2000, 35, 2603–2609. [Google Scholar] [CrossRef]
- De Haro, J.C.; Allegretti, C.; Smit, A.T.; Turri, S.; D’Arrigo, P.; Griffini, G. Biobased polyurethane coatings with high biomass content: Tailored properties by lignin selection. ACS Sustain. Chem. Eng. 2019, 7, 11700–11711. [Google Scholar] [CrossRef]
- Saha, P.; Aloui, H.; Yun, J.H.; Kim, H.S.; Kim, B.S. Development of a novel composite film based on polyurethane and defatted Chlorella biomass: Physical and functional characterization. J. Appl. Polym. Sci. 2021, 138, 50152. [Google Scholar] [CrossRef]
- Monshupanee, T.; Nimdach, P.; Incharoensakdi, A. Two-stage (photoautotrophy and heterotrophy) cultivation enables efficient production of bioplastic poly-3-hydroxybutyrate in auto-sedimenting cyanobacterium. Sci. Rep. 2016, 6, 37121. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, C.K.; Costa, J.A.V.; de Morais, M.G. Polyhydroxybutyrate (PHB) synthesis by Spirulina sp. LEB 18 using biopolymer extraction waste. Appl. Biochem. Biotechnol. 2018, 185, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Abdo, S.M.; Ali, G.H. Analysis of polyhydroxybutrate and bioplastic production from microalgae. Bull. Natl. Res. Cen. 2019, 43, 97. [Google Scholar] [CrossRef]


| Species | Composition (%) | Ref. | ||
|---|---|---|---|---|
| Proteins | Carbohydrates | Lipids | ||
| Chlorella vulgaris | 41.51 | 20.99 | 15.67 | [31] |
| Chlorella vulgaris | 29.0 | 49.5 | 19.7 | [32] |
| Spirulina platensis | 49.23 | 31.20 | 11.20 | [33] |
| Chlorella sorokiniana | 18.81 | 35.67 | 9.90 | [34] |
| Scenedesmus obliquus | 30.38 | 13.41 | 4.66 | [35] |
| Dunaliella tertiolecta | 61.32 | 21.69 | 2.87 | [36] |
| Chlamydomonas reinhardtii | 64.76 | 22.64 | 12.60 | [37] |
| Nannocloropsis oculata | 39 | 20 | 17 | [38] |
| Nannochloropsis oceanica | 19.1 | 22.7 | 24.8 | [39] |
| Microalgae | Blended Polymer | Pros | Cons | Ref. |
|---|---|---|---|---|
| Lipid extracted Nannochloropsis salina | PVA |
|
| [54] |
| Chlorella vulgaris | PVA-g-MAH | - Enhanced tensile strength and elongation properties | - Complex compatibilizer required | [62] |
| Chlorella vulgaris and Spirulina platensis | PP, PE |
| - Incompatibility especially with Chlorella | [29] |
| Chlorella | PVC | - High tensile strength due to rigidity of PVC | - High sensitivity to water content in microalgae | [67] |
| Chlorella | PU | - Superior mechanical properties due to interactions with isocyanate group | - Complex process requiring a coupling reagent | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.M.; Chang, W.-S.; Kim, Y.-K. Biocomposites Using Whole or Valuable Component-Extracted Microalgae Blended with Polymers: A Review. Catalysts 2022, 12, 25. https://doi.org/10.3390/catal12010025
Kim GM, Chang W-S, Kim Y-K. Biocomposites Using Whole or Valuable Component-Extracted Microalgae Blended with Polymers: A Review. Catalysts. 2022; 12(1):25. https://doi.org/10.3390/catal12010025
Chicago/Turabian StyleKim, Gyu Min, Won-Seok Chang, and Young-Kee Kim. 2022. "Biocomposites Using Whole or Valuable Component-Extracted Microalgae Blended with Polymers: A Review" Catalysts 12, no. 1: 25. https://doi.org/10.3390/catal12010025