Photocatalytic Degradation of Thiacloprid Using Tri-Doped TiO2 Photocatalysts: A Preliminary Comparative Study
Abstract
:1. Introduction
2. Results
2.1. Characterization of Photocatalysts
2.2. Photocatalytic Activity Results
2.3. Kinetics Evaluation of THI Degradation
- C
- = concentration of THI (mg L−1) at the generic irradiation time;
- C0
- = concentration of THI (mg L−1) after the dark period;
- T
- = irradiation time (min);
- k
- = apparent kinetic constant (min−1).
3. Materials and Methods
3.1. Materials
3.2. Preparation of Photocatalysts
3.3. Photocatalytic Tests
3.4. Chemical-Physical Characterization Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, Y.-J.; Kang, J.-K.; Park, S.-J.; Lee, C.-G.; Moon, J.-K.; Alvarez, P.J.J. Photocatalytic degradation of neonicotinoid insecticides using sulfate-doped Ag3PO4 with enhanced visible light activity. Chem. Eng. J. 2020, 402, 126183. [Google Scholar] [CrossRef]
- Hladik, M.L.; Kolpin, D.W.; Kuivila, K.M. Widespread occurrence of neonicotinoid insecticides in streams in a high corn and soybean producing region, USA. Environ. Pollut. 2014, 193, 189–196. [Google Scholar] [CrossRef]
- Todey, S.A.; Fallon, A.M.; Arnold, W.A. Neonicotinoid insecticide hydrolysis and photolysis: Rates and residual toxicity. Environ. Toxicol. Chem. 2018, 37, 2797–2809. [Google Scholar] [CrossRef]
- Goulson, D. REVIEW: An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Elbert, A.; Haas, M.; Springer, B.; Thielert, W.; Nauen, R. Applied aspects of neonicotinoid uses in crop protection. Pest Manag. Sci. 2008, 64, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Moriya, K.; Lantzsch, R.; Seifert, H.; Lindner, W.; Jelich, K.; Göhrt, A.; Beck, M.E.; Etzel, W. Thiacloprid (Bay YRC 2894)-A new member of the Chloronicotinyl Insecticide (CNI) family. Pflanzenschutz-Nachr. Bayer 2001, 54, 147–160. [Google Scholar]
- Yin, K.; Deng, Y.; Liu, C.; He, Q.; Wei, Y.; Chen, S.; Liu, T.; Luo, S. Kinetics, pathways and toxicity evaluation of neonicotinoid insecticides degradation via UV/Chlorine process. Chem. Eng. J. 2018, 346, 298–306. [Google Scholar] [CrossRef]
- Cernigoj, U.; Stangar, U.L.; Jirkovský, J. Effect of dissolved ozone or ferric ions on photodegradation of thiacloprid in presence of different TiO2 catalysts. J. Hazard. Mater. 2010, 177, 399–406. [Google Scholar] [CrossRef]
- Abramović, B.F.; Banić, N.D.; Šojić, D.V. Degradation of thiacloprid in aqueous solution by UV and UV/H2O2 treatments. Chemosphere 2010, 81, 114–119. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 photocatalysis: Concepts, mechanisms, and challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Park, M.; Park, S.-J. Recent advances in TiO2 films prepared by Sol-Gel methods for photocatalytic degradation of organic pollutants and antibacterial activities. Coatings 2019, 9, 613. [Google Scholar] [CrossRef] [Green Version]
- Pant, B.; Ojha, G.P.; Kuk, Y.-S.; Kwon, O.H.; Park, Y.W.; Park, M. Synthesis and characterization of ZnO-TiO2/carbon fiber composite with enhanced photocatalytic properties. Nanomaterials 2020, 10, 1960. [Google Scholar] [CrossRef] [PubMed]
- Anas, M.; Han, D.; Mahmoud, K.; Park, H.; Abdel-Wahab, A. Photocatalytic degradation of organic dye using titanium dioxide modified with metal and non-metal deposition. Mater. Sci. Semicond. Process. 2015, 41, 209–218. [Google Scholar] [CrossRef]
- Yu, J.C.; Ho, W.; Yu, J.; Yip, H.; Wong, P.K.; Zhao, J. Efficient visible-light-induced photocatalytic disinfection on sulfur-doped nanocrystalline titania. Environ. Sci. Technol. 2005, 39, 1175–1179. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Wu, C.-H.; Wu, J.-T.; Chen, Y.-R. Synthesis and characterization of a phosphorus-doped TiO2 immobilized bed for the photodegradation of bisphenol a under UV and sunlight irradiation. React. Kinet. Mech. Catal. 2015, 114, 753–766. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Q.; Li, S.; Li, J.; Wang, Q. Pr, N, and P tri-doped anatase TiO2 nanosheets with enhanced photocatalytic activity under sunlight. Chin. J. Catal. 2014, 35, 1068–1077. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, J.; Xiang, Q.; Li, Y.; Liu, M.; Liao, Y. Transition-Metal-Ion (Fe, Co, Cr, Mn, Etc.) doping of TiO2 nanotubes: A general approach. Inorg. Chem. 2019, 58, 12511–12515. [Google Scholar] [CrossRef]
- Shen, X.-Z.; Guo, J.; Liu, Z.-C.; Xie, S.-M. Visible-light-driven Titania photocatalyst Co-Doped with nitrogen and Ferrum. Appl. Surf. Sci. 2008, 254, 4726–4731. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, X.; Zhang, L.; Xu, L.; Ma, L.; Luo, J.; Li, M.; Zeng, L. Photoredox degradation of different water pollutants (MO, RhB, MB, and Cr(VI)) using Fe–N–S-Tri-Doped TiO2 nanophotocatalyst prepared by novel chemical method. Mater. Res. Bull. 2015, 70, 106–113. [Google Scholar] [CrossRef]
- Mancuso, A.; Sacco, O.; Sannino, D.; Pragliola, S.; Vaiano, V. Enhanced visible-light-driven photodegradation of acid orange 7 azo dye in aqueous solution using Fe-N Co-Doped TiO2. Arab. J. Chem. 2020, 13, 8347–8360. [Google Scholar] [CrossRef]
- Khaledi Maki, L.; Maleki, A.; Rezaee, R.; Daraei, H.; Yetilmezsoy, K. LED-activated immobilized Fe-Ce-N Tri-Doped TiO2 nanocatalyst on glass bed for photocatalytic degradation organic dye from aqueous solutions. Environ. Technol. Innov. 2019, 15, 100411. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Hyne, R.V. Detection and analysis of neonicotinoids in river waters–development of a passive sampler for three commonly used insecticides. Chemosphere 2014, 99, 143–151. [Google Scholar] [CrossRef]
- Schmuck, R. Ecotoxicological profile of the insecticide thiacloprid. Pflanzenschutz Nachr.-Bayer-Engl. Ed. 2001, 54, 161–184. [Google Scholar]
- Khlyustova, A.; Sirotkin, N.; Kusova, T.; Kraev, A.; Titov, V.; Agafonov, A. Doped TiO2: Effect of doping element on the photocatalytic activity. Mater. Adv. 2020, 1. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Xing, Z. One-step synthesis of Fe–N–S-Tri-Doped TiO2 catalyst and its enhanced visible light photocatalytic activity. Mater. Res. Bull. 2012, 47, 3804–3809. [Google Scholar] [CrossRef]
- Xing, M.; Wu, Y.; Zhang, J.; Chen, F. Effect of synergy on the visible light activity of B, N and Fe Co-Doped TiO2 for the degradation of MO. Nanoscale 2010, 2, 1233–1239. [Google Scholar] [CrossRef]
- de la Osa, R.A.; Iparragirre, I.; Ortiz, D.; Saiz, J.M. The extended Kubelka–Munk theory and its application to spectroscopy. ChemTexts 2019, 6, 2. [Google Scholar] [CrossRef]
- Ricci, P.C.; Carbonaro, C.M.; Stagi, L.; Salis, M.; Casu, A.; Enzo, S.; Delogu, F. Anatase-to-rutile phase transition in TiO2 nanoparticles irradiated by visible light. J. Phys. Chem. C 2013, 117, 7850–7857. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Antonopoulou, M.; Daikopoulos, C.; Deligiannakis, Y.; Konstantinou, I. Characterization and catalytic performance of B-Doped, B–N Co-Doped and B–N–F Tri-Doped TiO2 towards simultaneous Cr(VI) reduction and benzoic acid oxidation. Appl. Catal. B Environ. 2016, 184, 44–54. [Google Scholar] [CrossRef]
- Adyani, S.M.; Ghorbani, M. A comparative study of physicochemical and photocatalytic Properties of visible light responsive Fe, Gd and P Single and Tri-Doped TiO2 nanomaterials. J. Rare Earths 2018, 36, 72–85. [Google Scholar] [CrossRef]
- Akshay, V.R.; Arun, B.; Mandal, G.; Mutta, G.R.; Chanda, A.; Vasundhara, M. Observation of optical Band-Gap narrowing and enhanced magnetic moment in Co-Doped Sol–Gel-Derived anatase TiO2 nanocrystals. J. Phys. Chem. C 2018, 122, 26592–26604. [Google Scholar] [CrossRef]
- Ramandi, S.; Entezari, M.; Ghows, N. Sono-synthesis of solar light responsive S–N–C–Tri doped TiO2 Photo-Catalyst under optimized conditions for degradation and mineralization of diclofenac. Ultrason. Sonochem. 2017, 38, 234–245. [Google Scholar] [CrossRef]
- Wang, J.A.; Limas-Ballesteros, R.; López, T.; Moreno, A.; Gómez, R.; Novaro, O.; Bokhimi, X. Quantitative determination of titanium lattice defects and solid-state reaction mechanism in iron-doped TiO2 photocatalysts. J. Phys. Chem. B 2001, 105, 9692–9698. [Google Scholar] [CrossRef]
- Gaur, L.K.; Kumar, P.; Kushavah, D.; Khiangte, K.R.; Mathpal, M.C.; Agrahari, V.; Gairola, S.P.; Soler, M.A.G.; Swart, H.C.; Agarwal, A. Laser induced phase transformation influenced by co doping in TiO2 nanoparticles. J. Alloys Compd. 2019, 780, 25–34. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Jiang, J.; Rong, Y.; Wang, Y.; Wu, Y.; Pan, C. Characterization of oxygen vacancy associates within hydrogenated TiO2: A positron annihilation study. J. Phys. Chem. C 2012, 116, 22619–22624. [Google Scholar] [CrossRef]
- Decomposition and Detoxification of the Insecticide Thiacloprid by TiO2-Mediated Photocatalysis: Kinetics, Intermediate Products and Transformation Pathways-Berberidou-2019-Journal of Chemical Technology & Biotechnology-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/jctb.6034?casa_token=mlM5yFvrjRkAAAAA%3AqjvoJlIhUQ_zQmYIRRpXJG94RegEqrQWiH9QZaKrcgQ3wAgZVLhyxtgjZKsfd_vzqTH8s8Tw6XgHLg (accessed on 2 February 2021).
- Zhong, Z.; Li, M.; Fu, J.; Wang, Y.; Muhammad, Y.; Li, S.; Wang, J.; Zhao, Z.; Zhao, Z. Construction of cu-bridged Cu2O/MIL(Fe/Cu) catalyst with enhanced interfacial contact for the synergistic photo-Fenton degradation of thiacloprid. Chem. Eng. J. 2020, 395, 125184. [Google Scholar] [CrossRef]
- Banić, N.; Abramović, B.; Krstić, J.; Šojić, D.; Lončarević, D.; Cherkezova-Zheleva, Z.; Guzsvány, V. Photodegradation of thiacloprid using Fe/TiO2 as a heterogeneous photo-Fenton catalyst. Appl. Catal. B Environ. 2011, 107, 363–371. [Google Scholar] [CrossRef]
- Banić, N.D.; Abramović, B.F.; Šojić, D.V.; Krstić, J.B.; Finčur, N.L.; Bočković, I.P. Efficiency of neonicotinoids photocatalytic degradation by using annular slurry reactor. Chem. Eng. J. 2016, 286, 184–190. [Google Scholar] [CrossRef]
- Rózsa, G.; Kozmér, Z.; Alapi, T.; Schrantz, K.; Takács, E.; Wojnárovits, L. Transformation of Z-thiacloprid by three advanced oxidation processes: Kinetics, intermediates and the role of reactive species. Catal. Today 2017, 284, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Abramović, B.F.; Banić, N.D.; Krstić, J.B. Degradation of thiacloprid by ZnO in a laminar falling film slurry photocatalytic reactor. Ind. Eng. Chem. Res. 2013, 52, 5040–5047. [Google Scholar] [CrossRef]
- Mendiola-Alvarez, S.; Hernandez-Ramírez, A.; Guzmán Mar, J.; Garza-Tovar, L.L.; Reyes, L. Phosphorous-doped TiO2 nanoparticles: Synthesis, characterization, and visible photocatalytic evaluation on sulfamethazine degradation. Environ. Sci. Pollut. Res. 2019, 26. [Google Scholar] [CrossRef] [PubMed]
- Mayoufi, A.; Nsib, M.F.; Ahmed, O.; Houas, A. Synthesis, characterization and photocatalytic performance of W, N, S-Tri-Doped TiO2 under visible light irradiation. Comptes Rendus Chim. 2015, 18, 875–882. [Google Scholar] [CrossRef]
- Mancuso, A.; Sacco, O.; Vaiano, V.; Sannino, D.; Pragliola, S.; Venditto, V.; Morante, N. Visible light active Fe-Pr Co-doped TiO2 for water pollutants degradation. Catal. Today 2021. [Google Scholar] [CrossRef]
- Freeda, M.; Suresh, G. Structural and luminescent properties of Eu-doped CaAl2O4 nanophosphor by Sol-Gel method. Mater. Today Proc. 2017, 4, 4260–4265. [Google Scholar] [CrossRef]
- Ganesh, I.; Gupta, A.K.; Kumar, P.P.; Sekhar, P.S.C.; Radha, K.; Padmanabham, G.; Sundararajan, G. Preparation and characterization of Ni-Doped TiO2 materials for photocurrent and photocatalytic applications. Sci. World J. 2012, 2012, 127326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
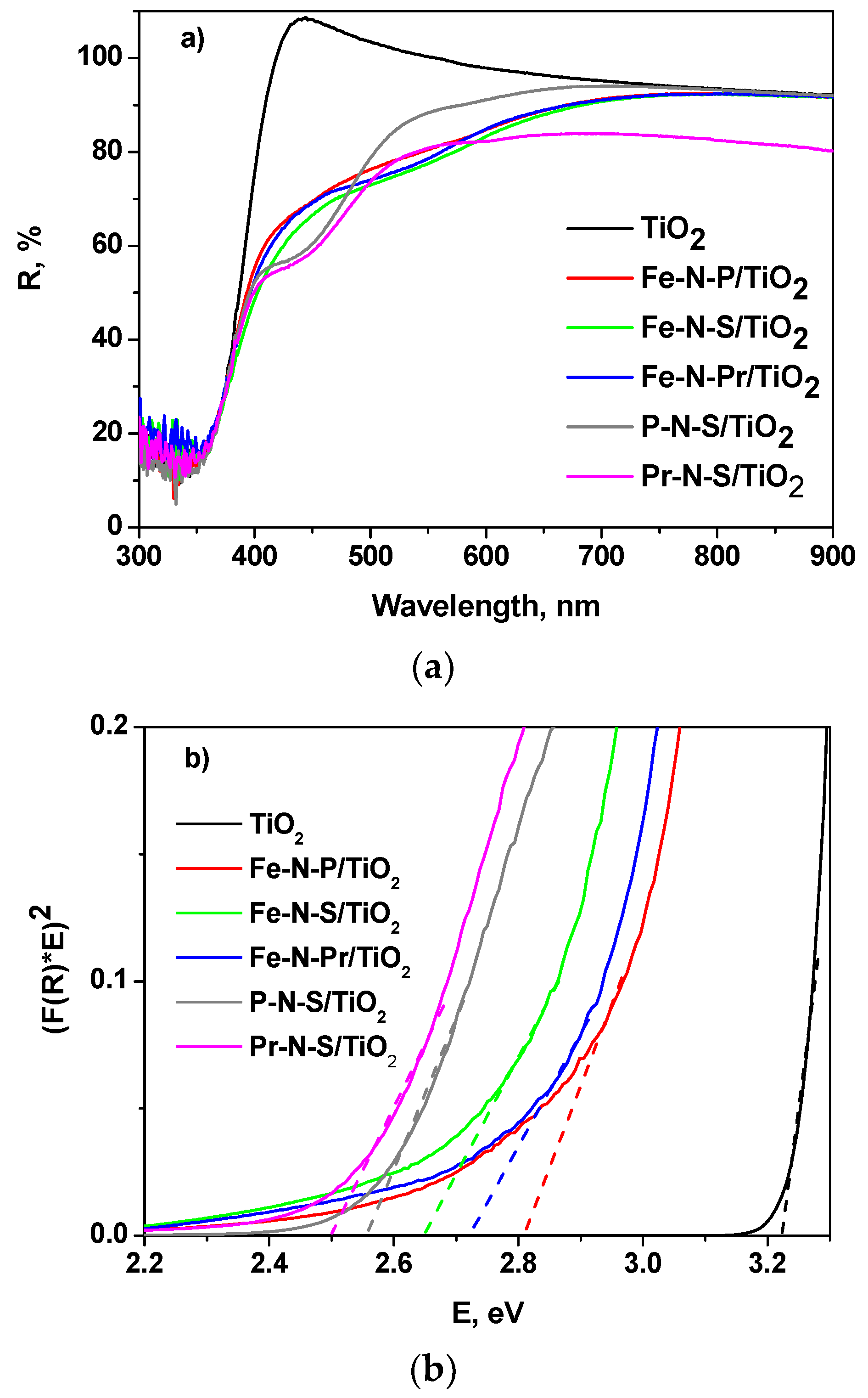
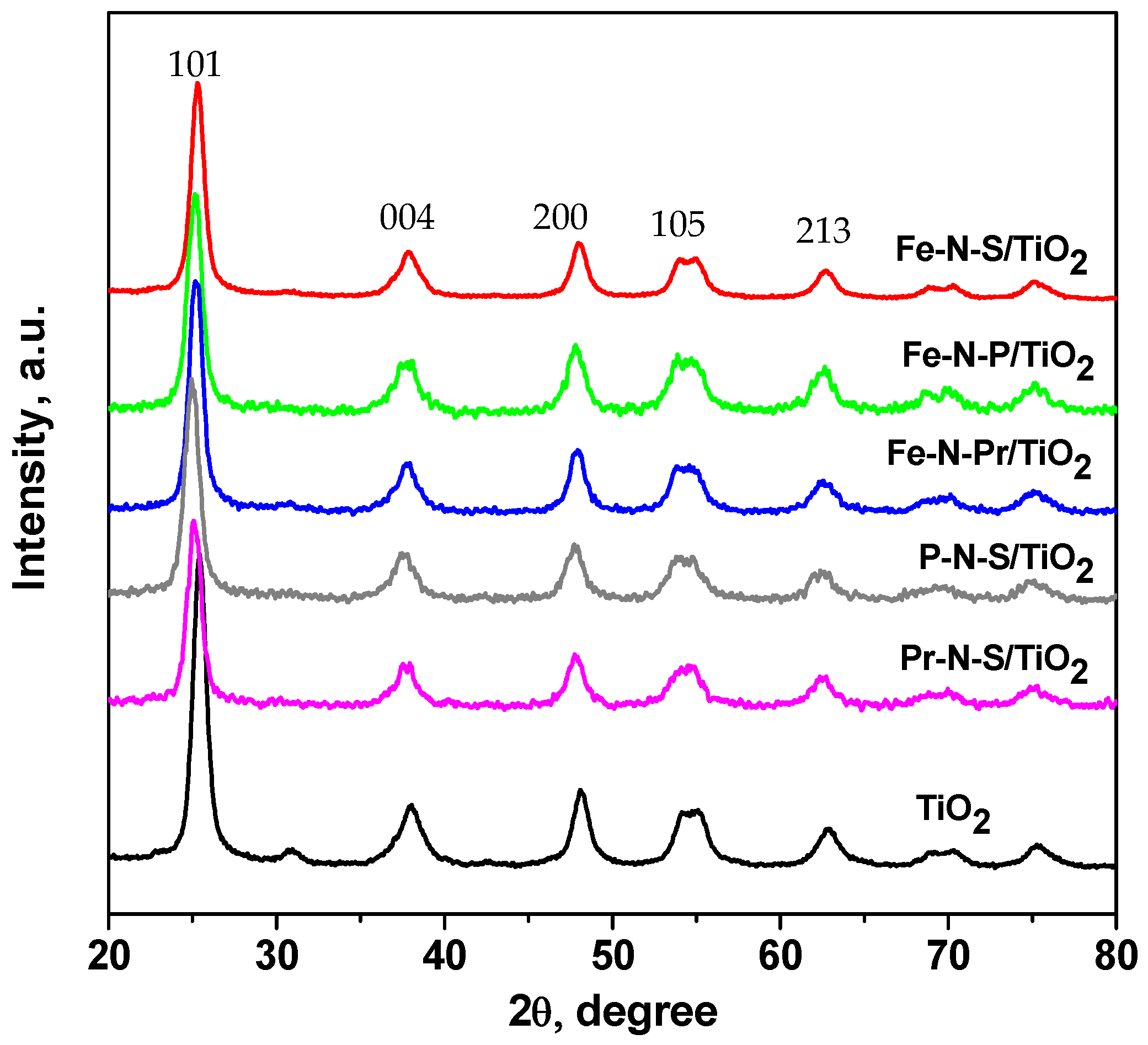
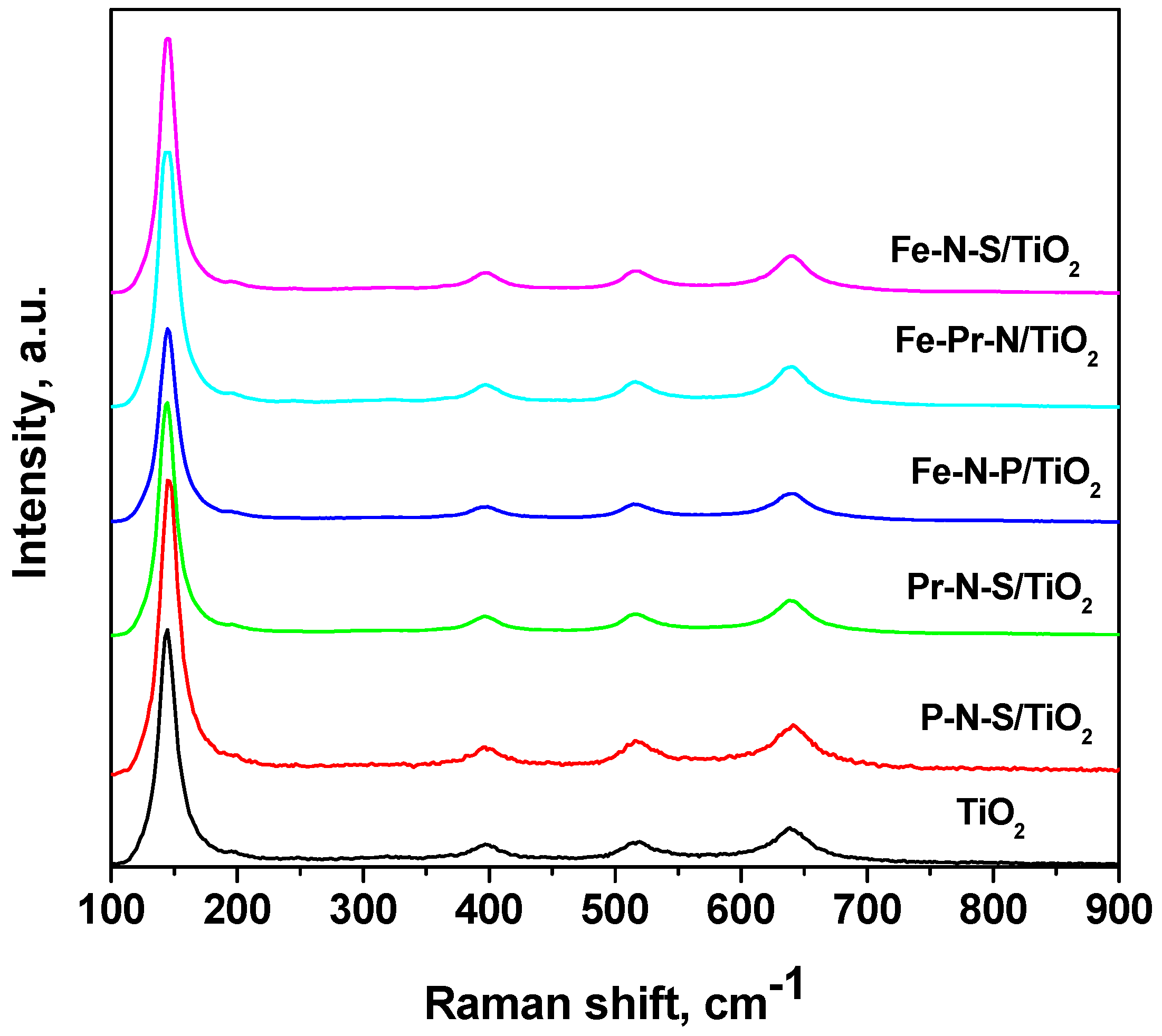



| Sample | Crystallite Size (nm) | Lattice Parameter a = b | (Ǻ) c | SBET (m2 g−1) | Ebg (eV) |
|---|---|---|---|---|---|
| TiO2 | 10 | 3.74 | 8.68 | 107 | 3.2 |
| Fe-N-P/TiO2 | 8 | 3.80 | 10.00 | 105 | 2.8 |
| Fe-N-S/TiO2 | 9 | 3.79 | 9.40 | 81 | 2.65 |
| Fe-N-Pr/TiO2 | 10 | 3.81 | 9.92 | 91 | 2.73 |
| P-N-S/TiO2 | 8 | 3.80 | 10.09 | 109 | 2.55 |
| Pr-N-S/TiO2 | 9 | 3.81 | 9.86 | 85 | 2.5 |
| Catalyst | Catalyst Dosage | THI Concentration | Light Source | Photocatalytic Degradation Efficiency (%) | Reference |
|---|---|---|---|---|---|
| Fe-TiO2 | 1.67 g L−1 | ~80 mg L−1 | UV light | ~45% | [38] |
| ZnO | 2.0 g L−1 | ~100 mg L−1 | UV-A light | ~100% | [39] |
| ZnO | 2.0 g L−1 | ~100 mg L−1 | visible light | ~59% | [39] |
| TiO2 | 1.0 g L−1 | ~25 mg L−1 | UV-A light | ~100% | [40] |
| ZnO | 2.0 g L−1 | ~100 mg L−1 | UV light | ~86% | [41] |
| Ag3PO4 | 0.8 g L−1 | ~5 mg L−1 | visible light | ~30% | [1] |
| Fe-N-P-TiO2 | 0.5 g L−1 | ~0.5 mg L−1 | UV-A light | ~64% | [this paper] |
| Fe-N-P-TiO2 | 0.5 g L−1 | ~0.5 mg L−1 | visible light | ~29% | [this paper] |
| Fe-N-P-TiO2 | 0.5 g L−1 | ~0.5 mg L−1 | solar light | ~73% | [this paper] |
| Sample | kUV (min−1) | kVis (min−1) | ksolar (min−1) |
|---|---|---|---|
| TiO2 | 1.7 × 10−3 | 7.58 × 10−6 | 1.8 × 10−3 |
| Fe-N-P/TiO2 | 6.0 × 10−3 | 2.1 × 10−3 | 7.2 × 10−3 |
| Fe-N-S/TiO2 | 2.2 × 10−3 | 7.88 × 10−4 | 3.9 × 10−3 |
| Fe-N-Pr/TiO2 | 4.9 × 10−3 | 1.5 × 10−3 | 5.5 × 10−3 |
| P-N-S/TiO2 | 2.5 × 10−3 | 1.0 × 10−3 | 4.8 × 10−3 |
| Pr-N-S/TiO2 | 2.8 × 10−3 | 2.02 × 10−4 | 2.9 × 10−3 |
| Sample | T (mL) | Ur (g) | F (mg) | Pac (μL) | Ss (mg) | PrN (μL) | N/Ti | Fe/Ti | P/Ti | S/Ti | Pr/Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TiO2 | 12.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fe-N-P/TiO2 | 12.5 | 1.2 | 25 | 17 | 0 | 0 | 0.97 | 0.0017 | 0.01 | 0 | 0 |
| Fe-N-S/TiO2 | 12.5 | 1.2 | 25 | 0 | 25 | 0 | 0.97 | 0.0017 | 0 | 0.005 | 0 |
| Fe-N-Pr/TiO2 | 12.5 | 1.2 | 0 | 0 | 0 | 8.5 | 0.97 | 0.0017 | 0 | 0 | 0.0069 |
| P-N-S/TiO2 | 12.5 | 1.2 | 0 | 17 | 25 | 0 | 0.97 | 0 | 0.01 | 0.005 | 0 |
| Pr-N-S/TiO2 | 12.5 | 1.2 | 0 | 17 | 0 | 8.5 | 0.97 | 0 | 0.01 | 0 | 0.0069 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, A.; Navarra, W.; Sacco, O.; Pragliola, S.; Vaiano, V.; Venditto, V. Photocatalytic Degradation of Thiacloprid Using Tri-Doped TiO2 Photocatalysts: A Preliminary Comparative Study. Catalysts 2021, 11, 927. https://doi.org/10.3390/catal11080927
Mancuso A, Navarra W, Sacco O, Pragliola S, Vaiano V, Venditto V. Photocatalytic Degradation of Thiacloprid Using Tri-Doped TiO2 Photocatalysts: A Preliminary Comparative Study. Catalysts. 2021; 11(8):927. https://doi.org/10.3390/catal11080927
Chicago/Turabian StyleMancuso, Antonietta, Wanda Navarra, Olga Sacco, Stefania Pragliola, Vincenzo Vaiano, and Vincenzo Venditto. 2021. "Photocatalytic Degradation of Thiacloprid Using Tri-Doped TiO2 Photocatalysts: A Preliminary Comparative Study" Catalysts 11, no. 8: 927. https://doi.org/10.3390/catal11080927






