Photocatalytic Study of Cyanide Oxidation Using Titanium Dioxide (TiO2)-Activated Carbon Composites in a Continuous Flow Photo-Reactor
Abstract
:1. Introduction
2. Results
2.1. Physical and Chemical Characterization of GCR-20 Activated Carbon and TiO2-AC Composite
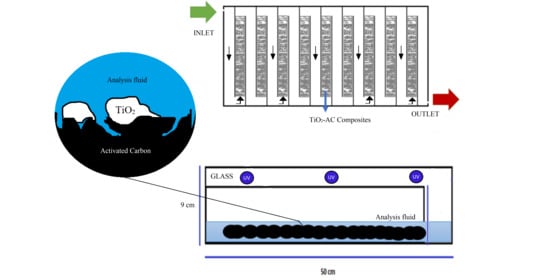
2.2. Photo-Reactor Construction
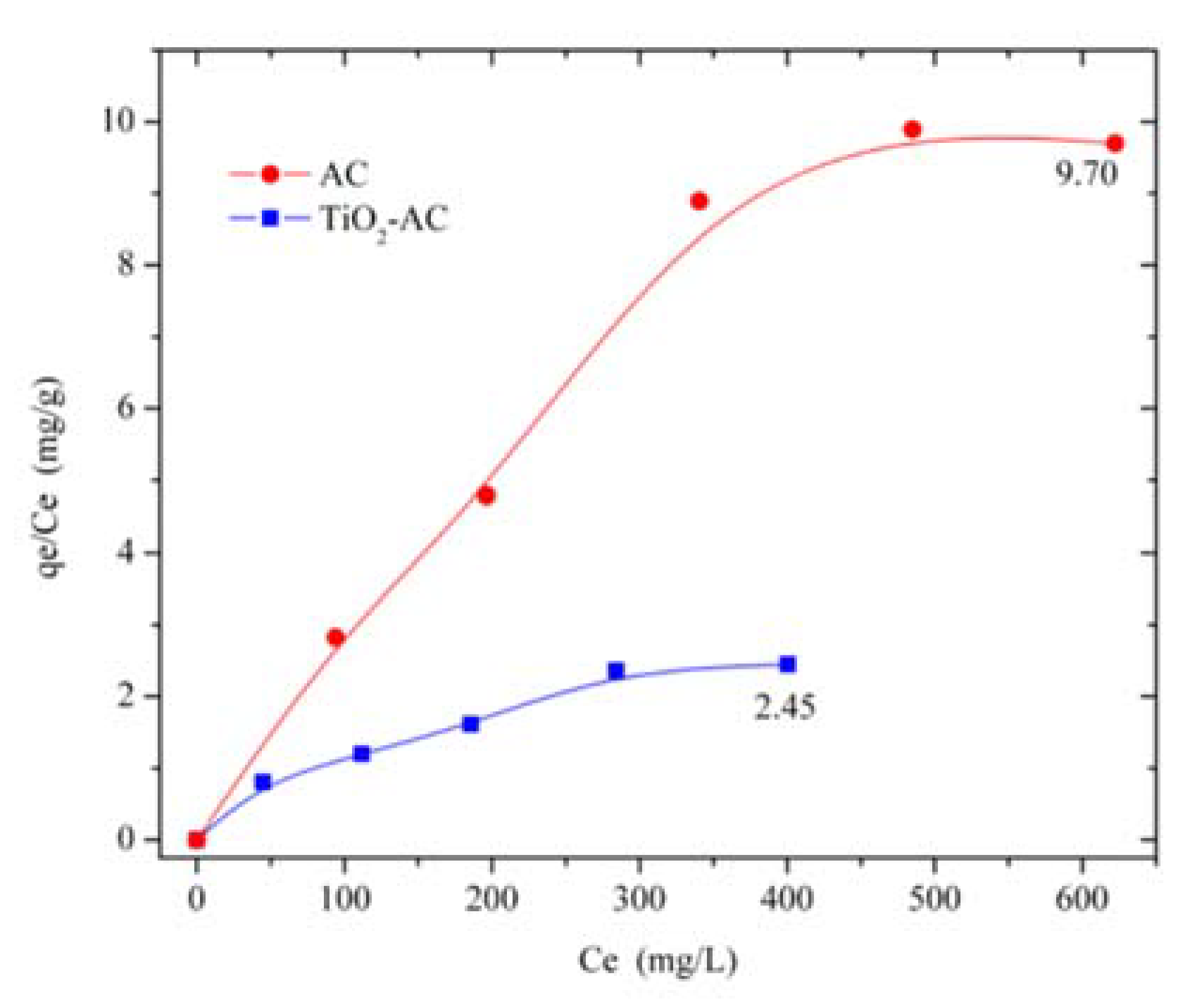
2.3. Cyanide Adsorption Tests
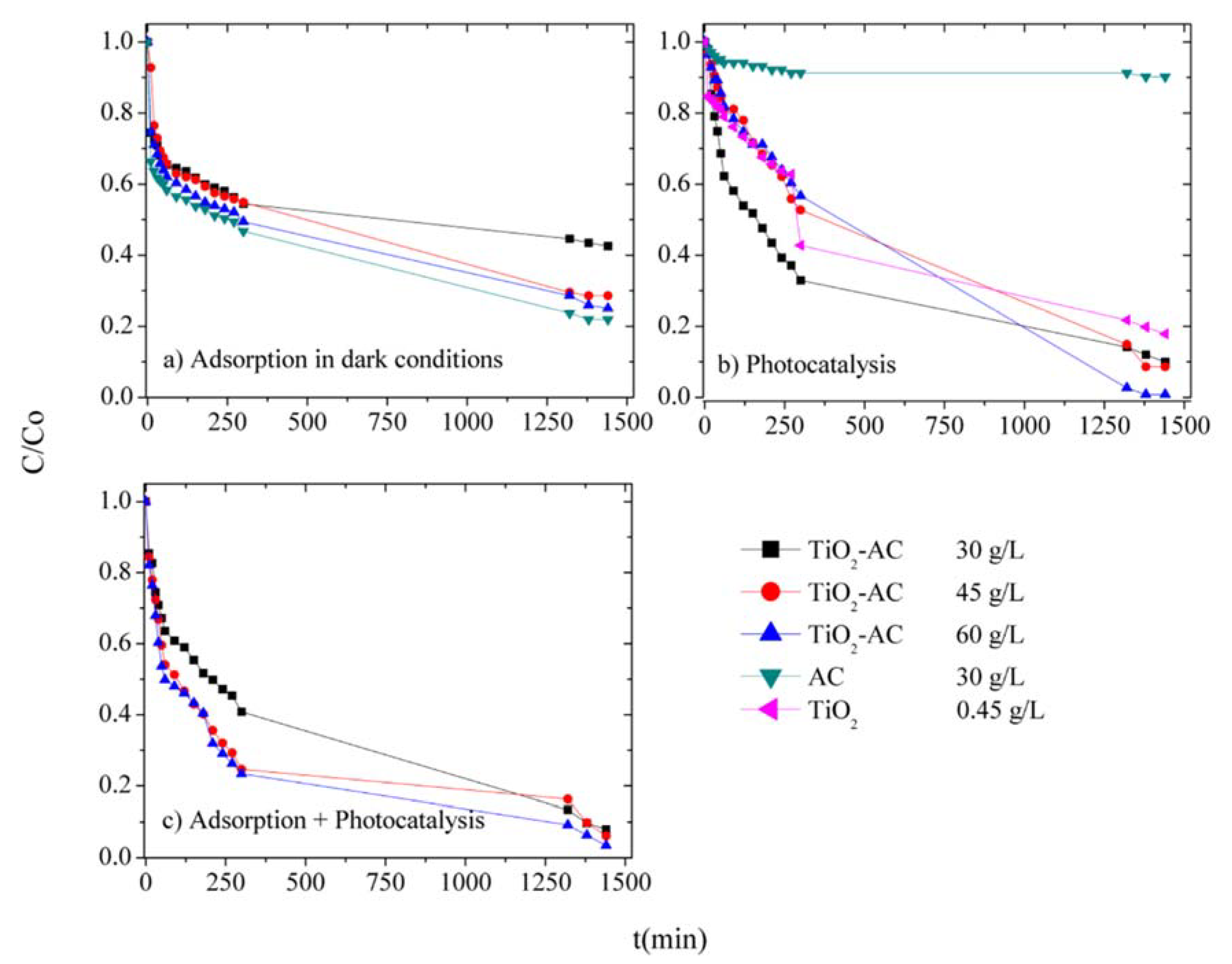
2.4. Photocatalytic Cyanide Ion Degradation
3. Discussion
4. Materials and Methods
4.1. TiO2-AC Composites Preparation
4.2. Physical and Chemical Characterization of GCR-20 Activated Carbon and TiO2-AC Composite
4.3. Photo-Reactor Construction
4.4. Cyanide Adsorption Study
4.5. Adsorption Study
4.5.1. Adsorption with AC
4.5.2. Adsorption with TiO2-AC Composite and Photodegradation Process
4.6. Photocatalytic Cyanide Ion Degradation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, M.; Gu, Q.; Cui, X.; Liu, X. Cyanide Containing Wastewater Treatment by Ozone Enhanced Catalytic Oxidation over Diatomite Catalysts. MATEC Web Conf. 2018, 142, 4–10. [Google Scholar] [CrossRef]
- Safavi, B.; Asadollahfardi, G.; Darban, A.K. Cyanide removal simulation from wastewater in the presence of Titanium dioxide nanoparticles. Adv. Nano Res. 2017, 5, 27–34. [Google Scholar] [CrossRef]
- Mishra, J.; Pattanayak, D.S.; Das, A.A.; Mishra, D.K.; Rath, D.; Sahoo, N.K. Enhanced photocatalytic degradation of cyanide employing Fe-porphyrin sensitizer with hydroxyapatite palladium doped TiO2nano-composite system. J. Mol. Liq. 2019, 287, 110821. [Google Scholar] [CrossRef]
- Koohestani, H.; Sadrnezhaad, S.K. Photocatalytic degradation of methyl orange and cyanide by using TiO2/CuO composite, Desalin. Water Treat. 2016, 57, 22029–22038. [Google Scholar] [CrossRef]
- Tarras-Wahlberg, N.H. Environmental management of small-scale and artisanal mining: The Portovelo-Zaruma goldmining area, southern Ecuador. J. Environ. Manag. 2002, 65, 165–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vangsnes, G.F. The meanings of mining: A perspective on the regulation of artisanal and small-scale gold mining in southern Ecuador. Extr. Ind. Soc. 2018, 5, 317–326. [Google Scholar] [CrossRef]
- MAE (Ministerio del Ambiente). Texto Unificado Legislación Secundaria, Medio Ambiente, Libro VI. 2015, p. 187. Available online: http://www.ambiente.gob.ec/wp-content/uploads/downloads/2015/02/TEXTO-UNIFICADO-LEGISLACION-SECUNDARIA-MEDIO-AMBIENTE.pdf (accessed on 15 September 2019).
- Pan, Y.; Zhang, Y.; Huang, Y.; Jia, Y.; Chen, L.; Cui, H. Synergistic effect of adsorptive photocatalytic oxidation and degradation mechanism of cyanides and Cu/Zn complexes over TiO2/ZSM-5 in real wastewater. J. Hazard. Mater. 2021, 416, 125802. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Y.; Zhao, S.; Liu, Z.; Chang, H.; Zhao, X. Photocatalytic oxidation of free cyanide over graphitic carbon nitride nanosheets under visible light. Chem. Eng. J. 2019, 369, 553–562. [Google Scholar] [CrossRef]
- Pueyo, N.; Miguel, N.; Mosteo, R.; Ovelleiro, J.L.; Ormad, M.P. Synergistic effect of the presence of suspended and dissolved matter on the removal of cyanide from coking wastewater by TiO2 photocatalysis. J. Environ. Sci. Health Part A 2017, 52, 182–188. [Google Scholar] [CrossRef]
- Núñez-Salas, R.E.; Hernández-Ramírez, A.; Hinojosa-Reyes, L.; Guzmán-Mar, J.L.; Villanueva-Rodríguez, M.; Maya-Treviño, M.d.L. Cyanide degradation in aqueous solution by heterogeneous photocatalysis using boron-doped zinc oxide. Catal. Today 2019, 328, 202–209. [Google Scholar] [CrossRef]
- Betancourt-Buitrago, L.A.; Hernandez-Ramirez, A.; Colina-Marquez, J.A.; Bustillo-Lecompte, C.F.; Rehmann, L.; Machuca-Martinez, F. Recent developments in the photocatalytic treatment of cyanide wastewater: An approach to remediation and recovery of metals. Processes 2019, 7, 225. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Alonso, M.D.; Coronado, J.M.; Maira, A.J.; Soria, J.; Loddo, V.; Augugliaro, V. Ozone enhanced activity of aqueous titanium dioxide suspensions for photocatalytic oxidation of free cyanide ions. Appl. Catal. B Environ. 2002, 39, 257–267. [Google Scholar] [CrossRef]
- Ijadpanah-saravi, H.; Dehestaniathar, S.; Khodadadi-Darban, A.; Zolfaghari, M.; Saeedzadeh, S. Photocatalytic decomposition of cyanide in pure water by biphasic titanium dioxide nanoparticles. Desalin. Water Treat. 2015, 57, 20503–20510. [Google Scholar] [CrossRef]
- Baeissa, E.S. Synthesis and characterization of sulfur-titanium dioxide nanocomposites for photocatalytic oxidation of cyanide using visible light irradiation. Chin. J. Catal. 2015, 36, 698–704. [Google Scholar] [CrossRef]
- Chiang, K.; Amal, R.; Tran, T. Photocatalytic degradation of cyanide using titanium dioxide modified with copper oxide. Adv. Environ. Res. 2002, 6, 471–485. [Google Scholar] [CrossRef]
- Omri, A.; Lambert, S.D.; Geens, J.; Bennour, F.; Benzina, M. Synthesis, Surface Characterization and Photocatalytic Activity of TiO2 Supported on Almond Shell Activated Carbon. J. Mater. Sci. Technol. 2014, 30, 894–902. [Google Scholar] [CrossRef]
- Harraz, F.A.; Abdel-Salam, O.E.; Mostafa, A.A.; Mohamed, R.M.; Hanafy, M. Rapid synthesis of titania-silica nanoparticles photocatalyst by a modified sol-gel method for cyanide degradation and heavy metals removal. J. Alloys Compd. 2013, 551, 1–7. [Google Scholar] [CrossRef]
- Koohestani, H.; Hassanabadi, M.; Mansouri, H.; Pirmoradian, A. Investigation of photocatalytic performance of natural zeolite/TiO2 composites. Micro Nano Lett. 2019, 14, 669–673. [Google Scholar] [CrossRef]
- De la Torre Chauvin, E.H. Préparation de Charbon Actif à Partir de Coques de Noix de Palmier à Huile Pour la Récupération D’or et le Traitement D’effluents Cyanurés. Ph.D. Thesis, Université catholique de Louvain, Louvain-La-Neuve, Belgium, 2015. [Google Scholar]
- De La Torre, E.; Adatty, M.; Gámez, S. Activated Carbon-Spinels Composites for Waste Water Treatment. Metals 2018, 8, 1070. [Google Scholar] [CrossRef] [Green Version]
- Pilco, Y. Estudio de la Oxidación de Efluentes Cianurados en Presencia de Aire y Carbón Activado. Bachelor’s Thesis, Escuela Politécnica Nacional, Quito, Ecuador, 2008. [Google Scholar]
- Compagnoni, M.; Ramis, G.; Freyria, F.S.; Armandi, M.; Bonelli, B.; Rossetti, I. Innovative photoreactors for unconventional photocatalytic processes: The photoreduction of CO2 and the photo-oxidation of ammonia. Rend. Lincei 2017, 28, 151–158. [Google Scholar] [CrossRef]
- Velasco, L.F.; Carmona, R.J.; Matos, J.; Ania, C.O. Performance of activated carbons in consecutive phenol photooxidation cycles. Carbon 2014, 73, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Marugán, J.; van Grieken, R.; Cassano, A.E.; Alfano, O.M. Intrinsic kinetic modeling with explicit radiation absorption effects of the photocatalytic oxidation of cyanide with TiO2 and silica-supported TiO2 suspensions. Appl. Catal. B Environ. 2008, 85, 48–60. [Google Scholar] [CrossRef]
- Royaee, S.J.; Sohrabi, M.; Barjesteh, P.J. Performance evaluation of a continuous flow Photo-Impinging Streams Cyclone Reactor for phenol degradation. Chem. Eng. Res. Des. 2012, 90, 1923–1929. [Google Scholar] [CrossRef]
- Motegh, M.; van Ommen, J.R.; Appel, P.W.; Kreutzer, M.T. Scale-Up Study of a Multiphase Photocatalytic Reactor—Degradation of Cyanide in Water over TiO2. Environ. Sci. Technol. 2014, 48, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Laine, J.; Herrmann, J.-M.; Uzcategui, D.; Brito, J.L. Influence of activated carbon upon titania on aqueous photocatalytic consecutive runs of phenol photodegradation. Appl. Catal. B Environ. 2007, 70, 461–469. [Google Scholar] [CrossRef]
- Eskandari, P.; Farhadian, M.; Nazar, A.R.S.; Goshadrou, A. Cyanide adsorption on activated carbon impregnated with ZnO, Fe2O3, TiO2 nanometal oxides: A comparative study. Int. J. Environ. Sci. Technol. 2020, 18, 297–316. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, M.; Cai, J.; Liang, L.; Ren, G.; Jiang, L. Ultrahigh yield of hydrogen peroxide on graphite felt cathode modified with electrochemically exfoliated graphene. J. Mater. Chem. A. 2017, 5, 8070–8080. [Google Scholar] [CrossRef]
- Moreira, J.; Lima, V.B.; Goulart, L.A.; Lanza, M.R.V. Electrosynthesis of hydrogen peroxide using modified gas diffusion electrodes (MGDE) for environmental applications: Quinones and azo compounds employed as redox modifiers. Appl. Catal. B Environ. 2019, 248, 95–107. [Google Scholar] [CrossRef]
- Mashuri, S.I.S.; Ibrahim, M.L.; Kasim, M.F.; Mastuli, M.S.; Rashid, U.; Abdullah, A.H.; Islam, A.; Asikin-Mijan, N.; Tan, Y.H.; Mansir, N.; et al. Photocatalysis for organic wastewater treatment: From the basis to current challenges for society. Catalysts 2020, 10, 1260. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Vidal, A.; Fernández, P.; Cáceres, J.; Trincado, P.; Oliveira, J.C.; Vincent, M. New large solar photocatalytic plant: Set-up and preliminary results. Chemosphere 2002, 47, 235–240. [Google Scholar] [CrossRef]
- Tanveer, M.; Guyer, G.T. Solar assisted photo degradation of wastewater by compound parabolic collectors: Review of design and operational parameters. Renew. Sustain. Energy Rev. 2013, 24, 534–543. [Google Scholar] [CrossRef]
- Coronel, S.; Pauker, C.S.; Jentzsch, P.E.V.; de la Torre, E.; Endara, D.; Muñoz-Bisesti, F. Titanium dioxide/copper/carbon composites for the photocatalytic degradation of phenol. Chem. Chem. Technol. 2020, 14, 161–168. [Google Scholar] [CrossRef]
- Kim, H.W.; Ross, M.B.; Kornienko, N.; Zhang, L.; Guo, J.; Yang, P.; McCloskey, B.D. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts. Nat. Catal. 2018, 1, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Richald, M. Étude et Optimisation de Composites à Base de Charbon Actif et D’oxyde de Titane Pour L’oxydation Photocatalytique des ion Cyanures. Master’s Thesis, Haute École Lucia De Brouckère, Bruselas, Bélgica, 2015. [Google Scholar]
- Murillo, H. Obtención de un Compósito de Dióxido de Titanio y Carbón Activado Aplicado a la Oxidación Fotocatalítica del Ión Cianuro. Bachelor’s Thesis, Escuela Politécnica Nacional, Quito, Ecuador, 2014. [Google Scholar]

| Material | SBET (m2/g) | Pore Volume (cm3/g) | Φ (Å) |
|---|---|---|---|
| AC | 1336 | 0.618 | 58.92 |
| TiO2-AC | 902 | 0.504 | 33.97 |
| Parameter | Value |
|---|---|
| Particle size d80 (mm) | 3.10 |
| Humidity (%) | 6.82 |
| Volatile (%) | 5.79 |
| Ashes (%) | 7.85 |
| Fixed Carbon (%) | 79.55 |
| Parameter | AC | TiO2-AC |
|---|---|---|
| qmax (mg·g−1) | 155.17 | 52.33 |
| b (L·mg−1) | 0.013 | 0.015 |
| R2 | 0.99 | 0.95 |
| Parameter | AC 60 g/L | TiO2-AC 30 g/L | TiO2-AC 45 g/L | TiO2-AC 60 g/L |
|---|---|---|---|---|
| Kapp (g·g−1min−1) | 5.42 × 10−6 | 1.66 × 10−6 | 3.98 × 10−6 | 4.43 × 10−6 |
| R2 | 0.98 | 0.83 | 0.98 | 0.98 |
| CN− Degradation due to adsorption (%) | 78.06 | 57.41 | 71.34 | 74.95 |
| Parameter | AC 60 g/L | TiO2 0.45 g/L | TiO2-AC 30 g/L | TiO2-AC 45 g/L | TiO2-AC 60 g/L |
|---|---|---|---|---|---|
| Individual photocatalytic degradation | |||||
| Kapp (min−1) | 4.02 × 10−5 | 1.06 × 10−3 | 1.34 × 10−3 | 1.60 × 10−3 | 4.90 × 10−2 |
| R2 | 0.97 | 0.97 | 0.91 | 0.99 | 0.98 |
| CN− Degradation (%) | 9.73 | 82.11 | 90.08 | 91.39 | 99.16 |
| Simultaneous adsorption and photocatalytic degradation | |||||
| Kapp (min−1) | - | - | 1.47 × 10−3 | 1.39 × 10−3 | 1.75 × 10−3 |
| R2 | - | - | 0.83 | 0.98 | 0.98 |
| CN− Degradation (%) | - | - | 92.04 | 93.86 | 96.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coronel, S.; Endara, D.; Lozada, A.B.; Manangón-Perugachi, L.E.; de la Torre, E. Photocatalytic Study of Cyanide Oxidation Using Titanium Dioxide (TiO2)-Activated Carbon Composites in a Continuous Flow Photo-Reactor. Catalysts 2021, 11, 924. https://doi.org/10.3390/catal11080924
Coronel S, Endara D, Lozada AB, Manangón-Perugachi LE, de la Torre E. Photocatalytic Study of Cyanide Oxidation Using Titanium Dioxide (TiO2)-Activated Carbon Composites in a Continuous Flow Photo-Reactor. Catalysts. 2021; 11(8):924. https://doi.org/10.3390/catal11080924
Chicago/Turabian StyleCoronel, Stalin, Diana Endara, Ana Belén Lozada, Lucía E. Manangón-Perugachi, and Ernesto de la Torre. 2021. "Photocatalytic Study of Cyanide Oxidation Using Titanium Dioxide (TiO2)-Activated Carbon Composites in a Continuous Flow Photo-Reactor" Catalysts 11, no. 8: 924. https://doi.org/10.3390/catal11080924